Synergetic Catalytic Effect between Ni and Co in Bimetallic Phosphide Boosting Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Synthesis
2.2.1. Preparation of NiCoP/NF
2.2.2. Synthesis of Other NF-Based Catalysts with Ni, CoP, 20% Pt/C, or RuO2
2.3. Electrochemical Measurements
2.4. Exchange Current Density
2.5. Active Site Number
2.6. TOF Calculation
3. Results and Discussion
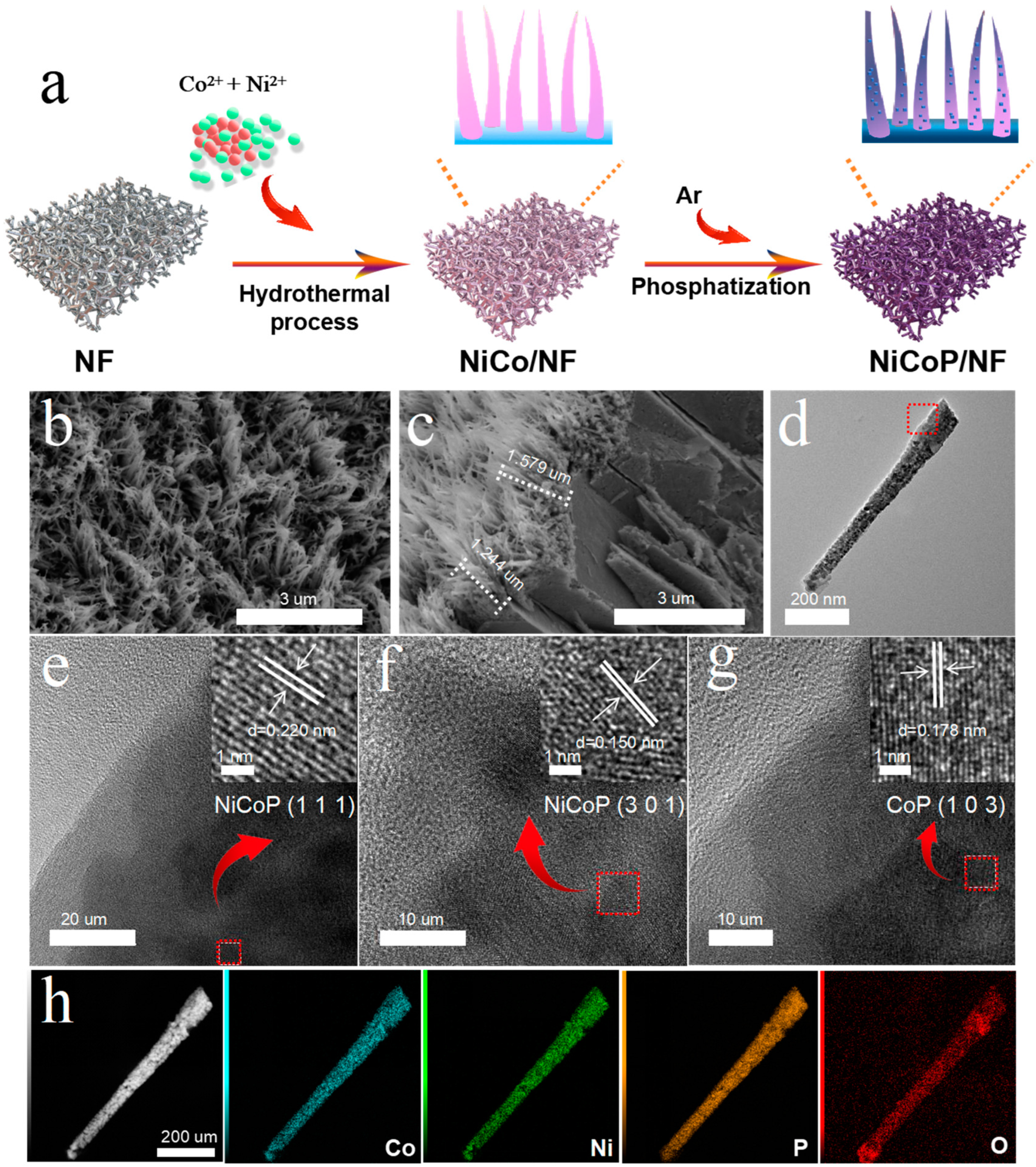
3.1. Synthesis and Characterizations
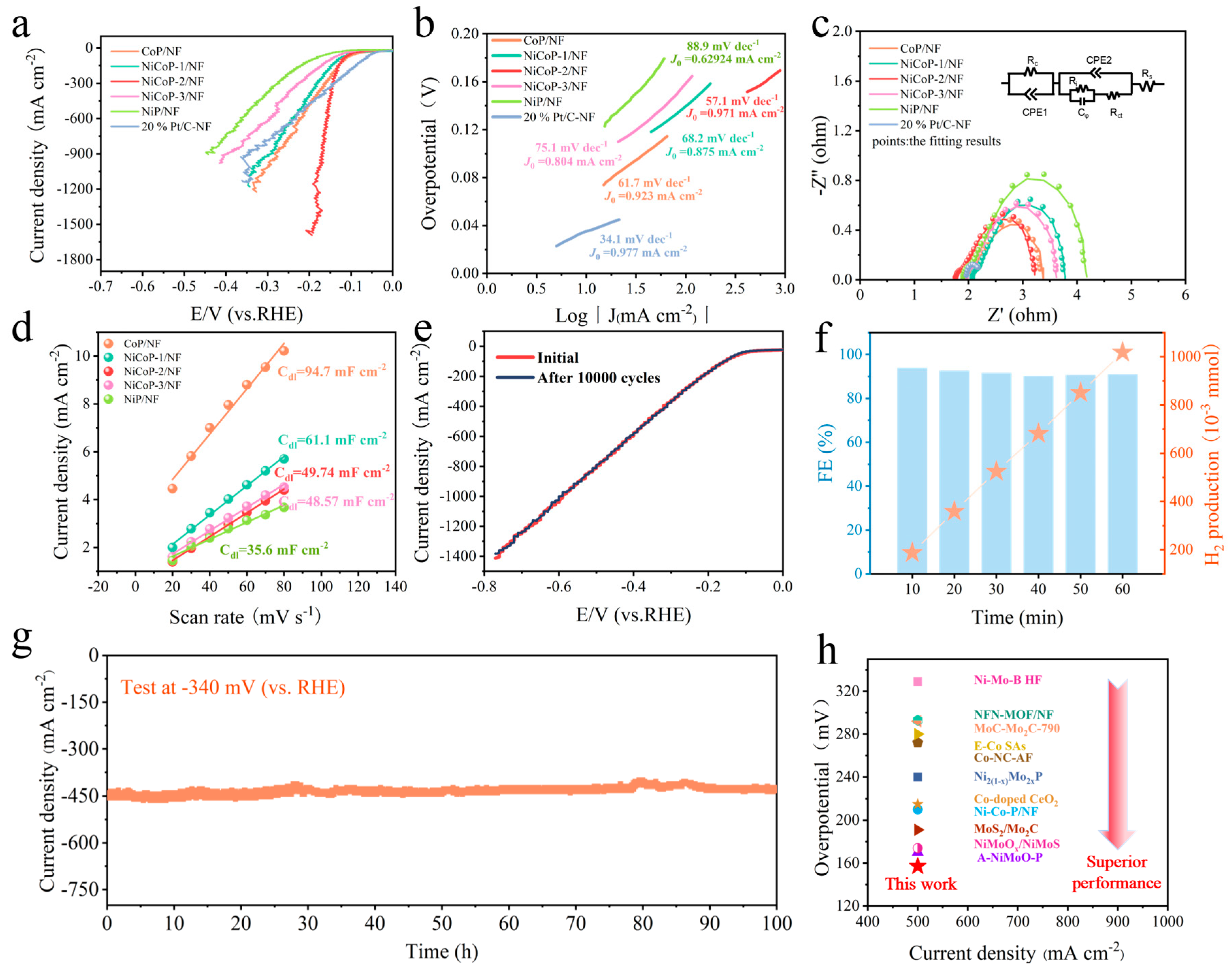
3.2. Electrocatalytic HER Performance Evaluation
3.3. Insight into the HER Mechanism
3.4. Evaluation of the Overall Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Yang, H.; Jiang, Y.; Liu, Z.; Zhang, S.; Zhang, Z.; Qiao, Z.; Lu, Y.; Cheng, T.; Terasaki, O.; et al. Coherent hexagonal platinum skin on nickel nanocrystals for enhanced hydrogen evolution activity. Nat. Commun. 2023, 14, 2424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shi, X.; Ning, H.; Yang, R.; Lu, B.; Luo, Q.; Mao, S.; Xi, L.; Wang, Y. Tailoring a local acid-like microenvironment for efficient neutral hydrogen evolution. Nat. Commun. 2023, 14, 4209. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Bellani, S.; Ferri, M.; Saleh, G.; Shinde, D.V.; Zappia, M.I.; Brescia, R.; Prato, M.; De Trizio, L.; Infante, I.; et al. High-performance alkaline water electrolyzers based on Ru-perturbed Cu nanoplatelets cathode. Nat. Commun. 2023, 14, 4680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, J.; Meng, G.; Wu, T.; Chen, C.; Tian, H.; Chen, Y.; Kong, F.; Chang, Z.; Cui, X.; et al. Active site recovery and N-N bond breakage during hydrazine oxidation boosting the electrochemical hydrogen production. Nat. Commun. 2023, 14, 1997. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, C.; Qin, M.; Li, B.; Lin, B.; Mao, Q.; Yang, H.; Liu, B.; Wang, Y. Reversible hydrogen spillover in Ru-WO3−x enhances hydrogen evolution activity in neutral pH water splitting. Nat. Commun. 2022, 13, 5382. [Google Scholar] [CrossRef]
- Gu, J.; Li, L.; Xie, Y.; Chen, B.; Tian, F.; Wang, Y.; Zhong, J.; Shen, J.; Lu, J. Turing structuring with multiple nanotwins to engineer efficient and stable catalysts for hydrogen evolution reaction. Nat. Commun. 2023, 14, 5389. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef]
- Slobodkin, I.; Davydova, E.; Sananis, M.; Breytus, A.; Rothschild, A. Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte. Nat. Mater. 2024, 23, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Fang, K.; Wang, H.; Wang, Y.; Huang, H.; Mo, C.; Ning, J.; Hu, Y. Lattice oxygen activation and local electric field enhancement by Co-doping Fe and F in CoO nanoneedle arrays for industrial electrocatalytic water oxidation. Nat. Commun. 2024, 15, 1012. [Google Scholar] [CrossRef]
- Shit, S.C.; Mondal, I.; Pendem, S.; Bai, L.; Park, J.Y.; Mondal, J. MOF-Derived Bifunctional Iron Oxide and Iron Phosphide Nanoarchitecture Photoelectrode for Neutral Water Splitting. ChemElectroChem 2018, 5, 2842–2849. [Google Scholar] [CrossRef]
- Mondal, I.; Lee, H.; Kim, H.; Park, J.Y. Plasmonic–Catalytic Nanomaterials: Plasmon-Induced Hot Carrier Separation across Dual Interface in Gold–Nickel Phosphide Heterojunction for Photocatalytic Water Splitting. Adv. Funct. Mater. 2020, 30, 2070068. [Google Scholar] [CrossRef]
- Shuai, C.; Mo, Z.; Niu, X.; Zhao, P.; Dong, Q.; Chen, Y.; Liu, N.; Guo, R. Nickel/cobalt bimetallic phosphides derived metal-organic frameworks as bifunctional electrocatalyst for oxygen and hydrogen evolution reaction. J. Alloys Compd. 2020, 847, 156514. [Google Scholar] [CrossRef]
- Qiu, B.; Cai, L.; Wang, Y.; Lin, Z.; Zuo, Y.; Wang, M.; Chai, Y. Fabrication of Nickel–Cobalt Bimetal Phosphide Nanocages for Enhanced Oxygen Evolution Catalysis. Adv. Funct. Mater. 2018, 28, 1706008. [Google Scholar] [CrossRef]
- Sun, J.; Ren, M.; Yu, L.; Yang, Z.; Xie, L.; Tian, F.; Yu, Y.; Ren, Z.; Chen, S.; Zhou, H. Highly efficient hydrogen evolution from a mesoporous hybrid of nickel phosphide nanoparticles anchored on cobalt phosphosulfide/phosphide nanosheet arrays. Small 2019, 15, 1804272. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Y.; Shi, J.; Hao, X.; Ma, Z.; Yang, K.; Zhang, T.; Li, C.; Zhang, D.; Huang, X.; et al. Corrosion-resistant cobalt phosphide electrocatalysts for salinity tolerance hydrogen evolution. Nat. Commun. 2023, 14, 7708. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wong, L.W.; Zheng, F.; Zheng, X.; Tsang, C.S.; Lai, K.H.; Shen, W.; Ly, T.H.; Deng, Q.; Zhao, J. Unraveling and leveraging in situ surface amorphization for enhanced hydrogen evolution reaction in alkaline media. Nat. Commun. 2023, 14, 6462. [Google Scholar] [CrossRef] [PubMed]
- Bodhankar, P.M.; Sarawade, P.B.; Kumar, P.; Vinu, A.; Kulkarni, A.P.; Lokhande, C.D.; Dhawale, D.S. Nanostructured metal phosphide based catalysts for electrochemical water splitting: A review. Small 2022, 18, e2107572. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.R.; Pan, U.N.; Dhakal, P.P.; Ghising, R.B.; Sidra, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Manganese-doped bimetallic (Co,Ni)2P integrated CoP in N,S Co-doped carbon: Unveiling a compatible hybrid electrocatalyst for overall water splitting. Small 2023, 20, 2307241. [Google Scholar] [CrossRef] [PubMed]
- Pakhira, S.; Kumar, V.; Ghosh, S. Revealing the superior electrocatalytic performance of 2D monolayer WSe2 transition metal dichalcogenide for efficient H2 evolution reaction. Adv. Mater. Interfaces 2023, 10, 2202075. [Google Scholar] [CrossRef]
- Jose, V.; Do, V.H.; Prabhu, P.; Peng, C.K.; Chen, S.Y.; Zhou, Y.; Lin, Y.G.; Lee, J.M. Activating amorphous Ru metallenes through Co integration for enhanced water electrolysis. Adv. Energy Mater. 2023, 13, 2301119. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, K.; Liu, S.; Chen, X.; Cheng, Y.; Cheong, W.C.; Chen, Z.; Zheng, L.; Zhang, J.; Li, X.; et al. Construction of CoP/NiCoP nanotadpoles heterojunction interface for wide pH hydrogen evolution electrocatalysis and supercapacitor. Adv. Energy Mater. 2019, 9, 1901213. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Sivanantham, A.; Shanmugam, S.; Kalai Selvan, R. Electrospun carbon nanofibers encapsulated with NiCoP: A multifunctional electrode for supercapattery and oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Adv. Energy Mater. 2018, 8, 1800555. [Google Scholar] [CrossRef]
- Boppella, R.; Tan, J.; Yang, W.; Moon, J. homologous CoP/NiCoP heterostructure on N-doped carbon for highly efficient and pH-universal hydrogen evolution electrocatalysis. Adv. Funct. Mater. 2018, 29, 1807976. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, G.; Zhang, Y.; Pan, L.; Zhang, X.; Zou, J.-J. Visible-light-induced unbalanced charge on NiCoP/TiO2 sensitized system for rapid H2 generation from hydrolysis of ammonia borane. Appl. Catal. B Environ. 2020, 260, 118183. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on nicop nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhang, X.; Yan, F.; Zhu, C.; Chen, Y. Self-supported tripod-like nickel phosphide nanowire arrays for hydrogen evolution. J. Mater. Chem. A 2019, 7, 22412–22419. [Google Scholar] [CrossRef]
- Tian, H.; Yu, X.; Huang, W.; Chang, Z.; Pei, F.; Zhou, J.; Dai, N.; Meng, G.; Chen, C.; Cui, X.; et al. WO3-assisted synergetic effect catalyzes efficient and CO-tolerant hydrogen oxidation for PEMFCs. Small 2023, 19, e2303061. [Google Scholar] [CrossRef]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-organic frameworks derived nanotube of Nickel-Cobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Lin, F.; Dong, Z.; Yao, Y.; Yang, L.; Fang, F.; Jiao, L. Electrocatalytic hydrogen evolution of ultrathin CoMo5N6 heterojunction with interfacial electron redistribution. Adv. Energy Mater. 2020, 10, 2002176. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.-X.; Gou, W.; Zhang, M.; Xia, Z.; Zhang, S.; Chang, C.-R.; Ma, Y.; Qu, Y. Ethylene-glycol ligand environment facilitates highly efficient hydrogen evolution of Pt/CoP through proton concentration and hydrogen spillover. Energy Environ. Sci. 2019, 12, 2298–2304. [Google Scholar] [CrossRef]
- Huang, Y.; Song, X.; Chen, S.; Zhang, J.; Gao, H.; Liao, J.; Ge, C.; Sun, W. Multi-layer architecture of novel sea urchin-like Co-hopeite to boosting overall alkaline water splitting. Adv. Mater. Interfaces 2023, 10, 2202349. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, I.K.; Xie, Y.; Zhou, H.; Sun, J.; Zhou, J.; Ni, Y.; Luo, D.; Yu, F.; Yu, Y.; et al. Ternary Ni2(1−x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density. Nano Energy 2018, 53, 492–500. [Google Scholar] [CrossRef]
- Yu, C.; Xu, F.; Luo, L.; Abbo, H.S.; Titinchi, S.J.J.; Shen, P.K.; Tsiakaras, P.; Yin, S. Bimetallic Ni-Co phosphide nanosheets self-supported on nickel foam as high-performance electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2019, 317, 191–198. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Luo, W.; Yu, X.; Chang, Z.; Kong, F.; Zhu, L.; Huang, Y.; Tian, H.; Cui, X.; et al. In situ active site refreshing of electro-catalytic materials for ultra-durable hydrogen evolution at elevated current density. Adv. Energy Mater. 2024, 14, 1614–6832. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 5462. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH-universal self-standing MoC–Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12, 6776. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.-M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, R.; Liu, H.; Rao, Y.; Yu, Y.; Chen, S.; Yue, Q.; Zhang, Y.; Kang, Y. Promoting formation of oxygen vacancies in two-dimensional cobalt-doped ceria nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 6461–6466. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, Z.; Liu, J.; Dong, J.; Liao, J.; Liu, H.; Huang, H.; Liu, J.; Yan, M.; Huang, K.; et al. Design of aligned porous carbon films with single-atomCo-N-C sitesfor high-current-density hydrogen generation. Adv. Mater. 2021, 33, 2103533. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Chuah, X.F.; Lu, S.Y. In situ grown bimetallic mof-based composite as highly efficient bifunctional electrocatalyst for overall water splitting with ultrastability at high current densities. Adv. Energy Mater. 2018, 8, 1801065. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, L.; Han, C.; Zong, H.; Yang, G.; Lin, S.; Kumar, A.; Jadhav, A.R.; Tran, N.Q.; Hwang, Y.; et al. Identifying the activity origin of a cobalt single-atom catalyst for hydrogen evolution using supervised learning. Adv. Funct. Mater. 2021, 31, 2100547. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Chen, L.; Zhu, X.; Dong, P.; Chee, M.O.L.; Ye, M.; Guo, Y.; Shen, J. Monolithic Ni-Mo-B bifunctional electrode for large current water splitting. Adv. Funct. Mater. 2021, 32, 2107308. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tian, H.; Zhu, L.; Li, S.; Cui, X. Synergetic Catalytic Effect between Ni and Co in Bimetallic Phosphide Boosting Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 853. https://doi.org/10.3390/nano14100853
Wang X, Tian H, Zhu L, Li S, Cui X. Synergetic Catalytic Effect between Ni and Co in Bimetallic Phosphide Boosting Hydrogen Evolution Reaction. Nanomaterials. 2024; 14(10):853. https://doi.org/10.3390/nano14100853
Chicago/Turabian StyleWang, Xiaohan, Han Tian, Libo Zhu, Shujing Li, and Xiangzhi Cui. 2024. "Synergetic Catalytic Effect between Ni and Co in Bimetallic Phosphide Boosting Hydrogen Evolution Reaction" Nanomaterials 14, no. 10: 853. https://doi.org/10.3390/nano14100853



