Adverse Effect of Metallic Gold and Silver Nanoparticles on Xenopus laevis Embryogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. AuNP and AgNP Characterization
2.3. Exposure to AuNPs and AgNPs
2.4. Phenotype Analysis
2.5. Transmission Electron Microscopy Analysis
2.6. Confocal Microscopy
2.7. Alcian Blue Staining
2.8. Real-Time PCR
2.9. Oxygen Reactive Species Analysis
3. Results
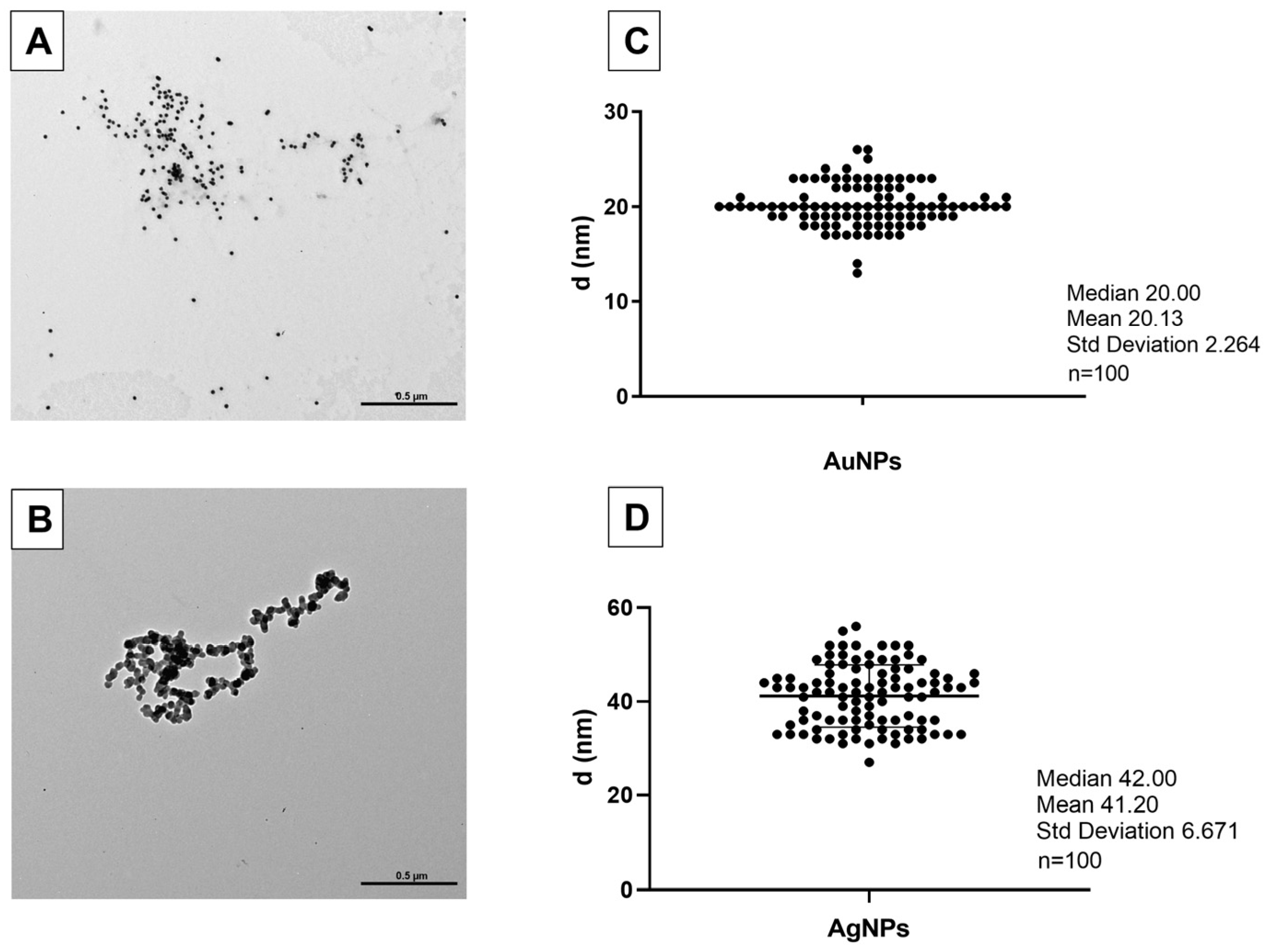
3.1. AuNPs and AgNPs Characterization
3.2. AuNPs and AgNPs Are Not Lethal
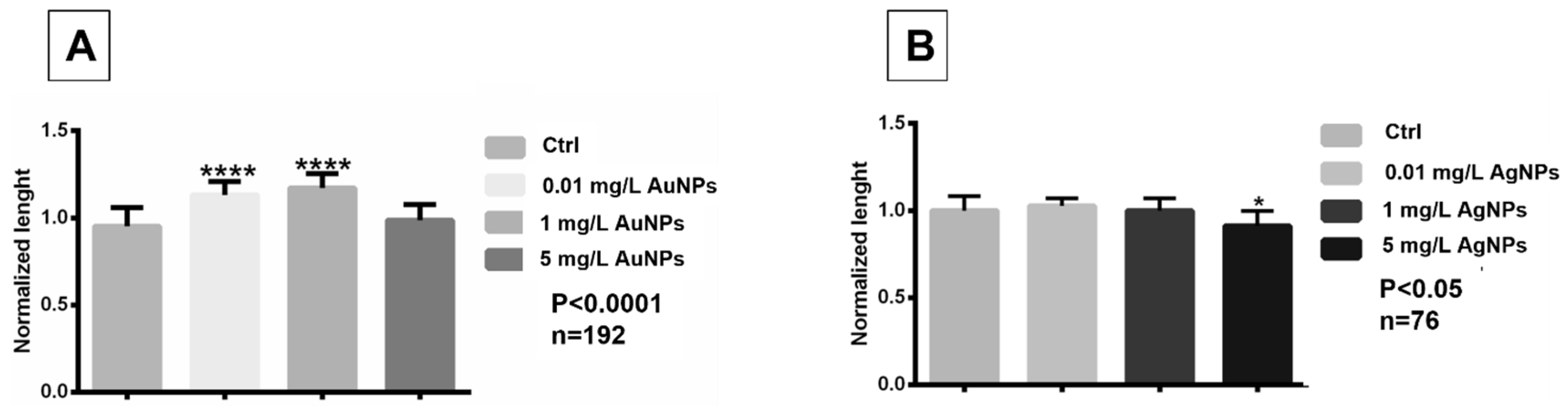
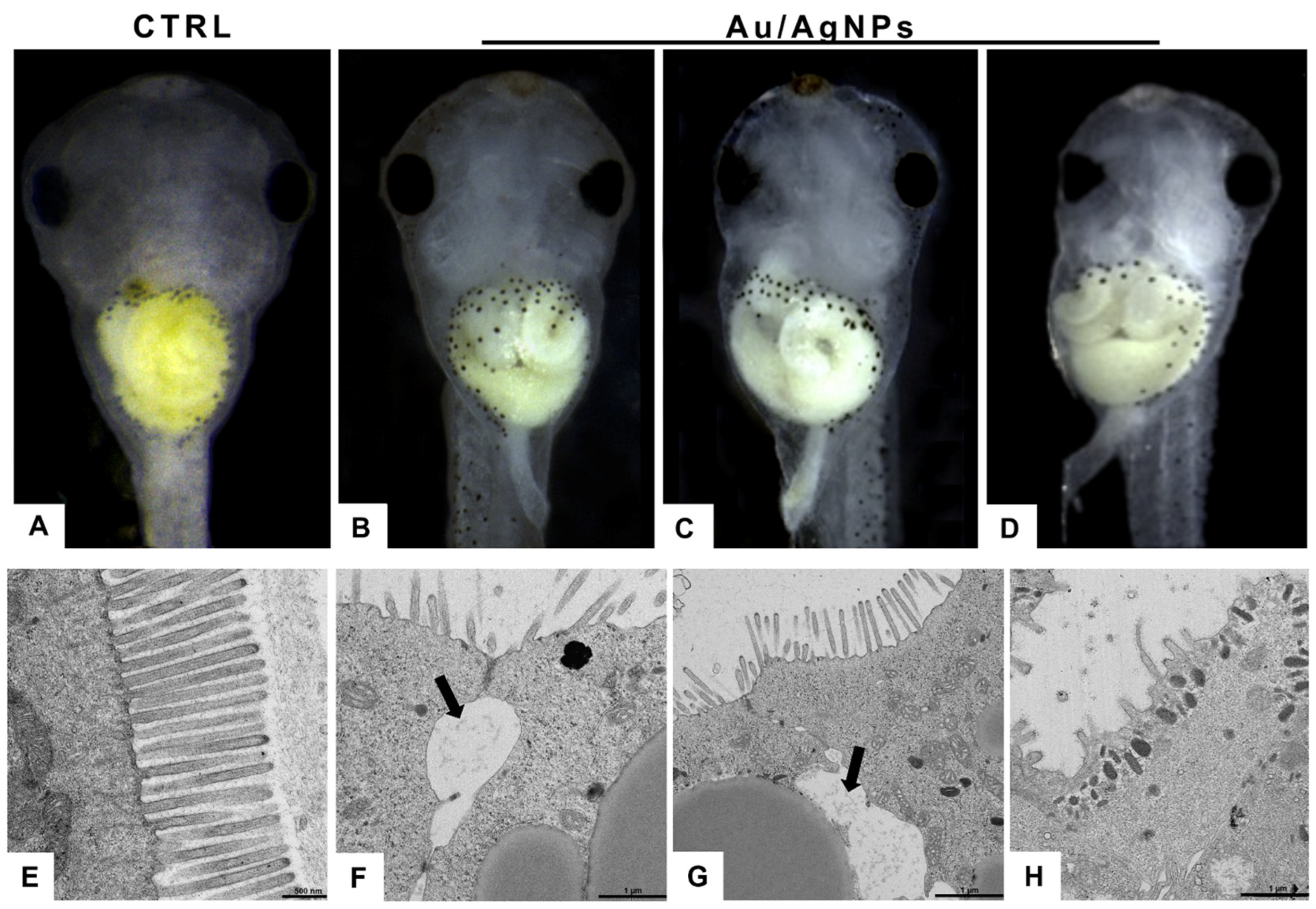
3.3. Exposure to AuNPs and AgNPs Induced Length and Pigment Variation and Heart Rate Alterations
3.4. AuNPs and AgNPs Caused Intestinal Damage
3.5. AuNPs and AgNPs Modified Skull–Facial Cartilages and Branchial Basket Conformation
3.6. No Oxidative Stress Is Induced by AuNPs and AgNPs Exposure
3.7. Exposure to AuNPs and AgNPs Altered Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ASTM 2020ASTM E2996-20; Standard Guide for Workforce Education in Nanotechnology Health and Safety. ASTM International: West Conshohocken, PA, USA, 2020.
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, R.; Tussellino, M.; Ronca, R.; Benvenuto, G.; Fogliano, C.; Fusco, S.; Netti, P.A. Toxic effects of SiO2NPs in early embryogenesis of Xenopus laevis. Chemosphere 2022, 289, 133233. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–general aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Abu-Dief, A.M. Silver nanoparticles (AgNPs) as a metal nano-therapy: Possible mechanisms of antiviral action against COVID-19. Inorg. Nano-Metal Chem. 2022, 1–19. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M.; Qu, Z.G.; He, X.C.; Lin, M.; Sha, B.Y.; Shi, X.H.; Lu, T.J.; Xu, F.; et al. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Ahamed, M.; Alsalhi, M.S.; Siddiqui, M.K. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.A.; Sunitha, S.; Sofi, M.A.; Khadheer Pasha, S.K.; Choi, D. An overview of antimicrobial and anticancer potential of silver nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic transfer and toxicity of silver nanoparticles along a phytoplankton-zooplankton-fish food chain. Sci. Total. Environ. 2022, 842, 156807. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and toxicity of silver nanoparticles in the food chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Tussellino, M.; Ronca, R.; Formiggini, F.; De Marco, N.; Fusco, S.; Netti, P.A.; Carotenuto, R. Polystyrene nanoparticles affect Xenopus laevis development. J. Nanoparticle Res. 2015, 17, 70. [Google Scholar] [CrossRef]
- Kahlon, S.K.; Sharma, G.; Julka, J.M.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Carotenuto, R.; Pallotta, M.M.; Tussellino, M.; Fogliano, C. Xenopus laevis (Daudin, 1802) as a model organism for bioscience: A historic review and perspective. Biology 2023, 12, 890. [Google Scholar] [CrossRef]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental concentrations of a delorazepam-based drug impact on embryonic development of non-target Xenopus laevis. Aquat. Toxicol. 2022, 250, 106244. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, R.; Fogliano, C.; Rienzi, M.; Siciliano, A.; Salvatore, M.M.; De Tommaso, G.; Benvenuto, G.; Galdiero, E.; Guida, M. Comparative Toxicological Evaluation of tattoo inks on two model organisms. Biology 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- York, J.R.; McCauley, D.W. The origin and evolution of vertebrate neural crest cells. Open Biol. 2020, 10, 190285. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, C.; Motta, C.M.; Acloque, H.; Avallone, B.; Carotenuto, R. Water contamination by delorazepam induces epigenetic defects in the embryos of the clawed frog Xenopus laevis. Sci. Total. Environ. 2023, 896, 165300. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, R.; Tussellino, M.; Mettivier, G.; Russo, P. Survival fraction and phenotype alterations of Xenopus laevis embryos at 3 Gy, 150 kV X-ray irradiation. Biochem. Biophys. Res. Commun. 2016, 480, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin): A Systematic and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; North-Holland: Amsterdam, The Netherlands, 1956. [Google Scholar]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef] [PubMed]
- Symens, N.; Walczak, R.; Demeester, J.; Mattaj, I.; De Smedt, S.C.; Remaut, K. Nuclear inclusion of nontargeted and chromatin-targeted polystyrene beads and plasmid DNA containing nanoparticles. Mol. Pharm. 2011, 8, 1757–1766. [Google Scholar] [CrossRef]
- Bantle, J.A.; Fort, D.J.; James, B.L. Identification of developmental toxicants using the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Hydrobiologia 1989, 188, 577–585. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Peltzer, P.M.; Martinuzzi, C.S.; Attademo, A.M.; Colussi, C.L.; Bassó, A. Acute toxicity of colloidal silicon dioxide nanoparticles on Amphibian larvae: Emerging environmental concern. Int. J. Environ. Res. 2018, 12, 269–278. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Tussellino, M.; Ronca, R.; Carotenuto, R.; Pallotta, M.M.; Furia, M.; Capriglione, T. Chlorpyrifos exposure affects fgf8, sox9, and bmp4 expression required for cranial neural crest morphogenesis and chondrogenesis in Xenopus laevis embryos. Environ. Mol. Mutagen. 2016, 57, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Matsuno, Y.-K.; Kameyama, A. A procedure for Alcian blue staining of mucins on polyvinylidene difluoride membranes. Anal. Chem. 2012, 84, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Almroth, B.C.; Sturve, J.; Berglund, Å.; Förlin, L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 2005, 73, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, D.; Falanga, A.; Muscetti, O.; Tarallo, R.; Fusco, S.; Galdiero, M.; Galdiero, S.; Netti, P.A. Shuttle-mediated nanoparticle delivery to the blood-brain barrier. Small 2013, 9, 853–862. [Google Scholar] [CrossRef]
- Maynard, A.D.; Warheit, D.B.; Philbert, M.A. The new toxicology of sophisticated materials: Nanotoxicology and beyond. Toxicol. Sci. 2011, 120 (Suppl. S1), S109–S129. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Cánovas, M.; Tvarijonaviciute, A.; Llorca, M.; Vega, A.; Farré, M.; Pastor, J.; Roher, N.; Teles, M. Nanoplastics are bioaccumulated in fish liver and muscle and cause DNA damage after a chronic exposure. Environ. Res. 2022, 212 Pt A, 113433. [Google Scholar] [CrossRef]
- Hackley, V.A.; Clogston, J.D. Measuring the hydrodynamic size of nanoparticles in aqueous media using batch-mode dynamic light scattering. Methods Mol. Biol. 2011, 697, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Lévy, A.; Villa, M.D.A.; Laurens, G.; Blanchet, V.; Bozek, J.; Gaudin, J.; Lamour, E.; Macé, S.; Mignon, P.; Milosavljević, A.R.; et al. Surface chemistry of gold nanoparticles produced by laser ablation in pure and saline water. Langmuir ACS J. Surf. Colloids 2021, 37, 5783–5794. [Google Scholar] [CrossRef]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of nanoparticle aggregation in human blood serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total. Environ. 2020, 709, 136050. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Morsy, E.A.; Hussien, A.M.; Ibrahim, M.A.; Farroh, K.Y. The effect of different concentrations of gold nanoparticles on growth performance, toxicopathological and immunological parameters of broiler chickens. Biosci. Rep. 2020, 40, BSR20194296. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-S.; So, D.; Lee, M.; Yoon, J.; Reipa, V.; Tona, A.; Yi, F.; Nelson, B.C.; LaVan, D.A.; Hackley, V.A.; et al. Polyethyleneimine/polyethylene glycol–conjugated gold nanoparticles as nanoscale positive/negative controls in nanotoxicology: Testing in frog embryo teratogenesis assay–Xenopus and mammalian tissue culture system. Nanotoxicology 2023, 17, 94–115. [Google Scholar] [CrossRef]
- Bai, C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Makeyev, O.H.; Shur, V.Y.; Beikin, Y.B.; Sutunkova, M.P.; Kireyeva, E.P.; Minigalieva, I.A.; Loginova, N.V.; et al. Comparative in Vivo Assessment of Some Adverse Bioeffects of Equidimensional Gold and Silver Nanoparticles and the Attenuation of Nanosilver’s Effects with a Complex of Innocuous Bioprotectors. Int. J. Mol. Sci. 2013, 14, 2449–2483. [Google Scholar] [CrossRef]
- Colombo, A.; Saibene, M.; Moschini, E.; Bonfanti, P.; Collini, M.; Kasemets, K.; Mantecca, P. Teratogenic hazard of BPEI-coated silver nanoparticles to Xenopus laevis. Nanotoxicology 2017, 11, 405–418. [Google Scholar] [CrossRef]
- Teles, M.; Soares, A.; Tort, L.; Guimarães, L.; Oliveira, M. Linking cortisol response with gene expression in fish exposed to gold nanoparticles. Sci. Total. Environ. 2017, 584–585, 1004–1011. [Google Scholar] [CrossRef]
- Belz, R.G.; O Duke, S. Herbicides and plant hormesis. Pest Manag. Sci. 2014, 70, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, H.; Huang, Q.; Li, J.; Yan, J.; He, D.; Fan, C.; Song, H. Long-term effects of nanoparticles on nutrition and metabolism. Small 2014, 10, 3603–3611. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, R.; Santo, N.; Fascio, U.; Moschini, E.; Freddi, S.; Chirico, G.; Camatini, M.; Mantecca, P. Nano-sized CuO, TiO2 and ZnO affect Xenopus laevis development. Nanotoxicology 2012, 6, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Nations, S.; Long, M.; Wages, M.; Maul, J.D.; Theodorakis, C.W.; Cobb, G.P. Subchronic and chronic developmental effects of copper oxide (CuO) nanoparticles on Xenopus laevis. Chemosphere 2015, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Nielsen, C.; Tabin, C.J.; Roberts, D.J. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut-foregut boundary. Development 2000, 127, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Enerbäck, S.; Carlsson, P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 2001, 128, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-T.; Shah, R.; Jamrich, M. Function and regulation of FoxF1 during Xenopus gut development. Development 2004, 131, 3637–3647. [Google Scholar] [CrossRef]
- Lange, K. Role of microvillar cell surfaces in the regulation of glucose uptake and organization of energy metabolism. Am. J. Physiol. Physiol. 2002, 282, C1–C26. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, G.; Xu, Y.; Zheng, L.; Ling, J. Accumulation and toxicity of multi-walled carbon nanotubes in Xenopus tropicalis tadpoles. Chemosphere 2020, 257, 127205. [Google Scholar] [CrossRef]
- Reifers, F.; Walsh, E.C.; Léger, S.; Stainier, D.Y.R.; Brand, M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar). Development 2000, 127, 225–235. [Google Scholar] [CrossRef]
- Shi, Y.; Katsev, S.; Cai, C.; Evans, S. BMP signalling is required for heart formation in vertebrates. Dev. Biol. 2000, 224, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, J.C.; Yue, M.S.; Martin, N.R.; Elemans, L.M.H.; Lanham, K.A.; Heideman, W.; Rezendes, R.; Baker, T.R.; Taylor, M.R.; Plavicki, J.S. sox9b is required in cardiomyocytes for cardiac morphogenesis and function. Sci. Rep. 2018, 8, 13906. [Google Scholar] [CrossRef]
- Sen, G.T.; Ozkemahli, G.; Shahbazi, R.; Erkekoglu, P.; Ulubayram, K.; Kocer-Gumusel, B. The effects of polymer coating of gold nanoparticles on oxidative stress and DNA damage. Int. J. Toxicol. 2020, 39, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Abalaka, S.E.; Fatihu, M.Y.; Ibrahim, N.D.G.; Kazeem, H.M. Histopathologic changes in the gills and skin of adult Clarias gariepinus exposed to ethanolic extract of Parkia biglobosa pods. Basic Appl. Pathol. 2010, 3, 109–114. [Google Scholar] [CrossRef]
- Karlsson, O.; Lindquist, N.G. Melanin affinity and its possible role in neurodegeneration. J. Neural Transm. 2013, 120, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Abbott, F.S. Endocrine regulation of pigmentation in fish. Am. Zool. 1973, 13, 885–894. [Google Scholar] [CrossRef]
- Barral, D.C.; Seabra, M.C. The melanosome as a model to study organelle motility in mammals. Pigment. Cell Res. 2004, 17, 111–118. [Google Scholar] [CrossRef]
- Jiang, M.; Volland, S.; Paniagua, A.E.; Wang, H.; Balaji, A.; Li, D.G.; Lopes, V.S.; Burgess, B.L.; Williams, D.S. Microtubule motor transport in the delivery of melanosomes to the actin-rich, apical domain of in the retinal pigment epithelium. J. Cell Sci. 2020, 133, jcs242214. [Google Scholar] [CrossRef]
- El Mir, J.; Fedou, S.; Thézé, N.; Morice-Picard, F.; Cario, M.; Fayyad-Kazan, H.; Thiébaud, P.; Rezvani, H. Xenopus: An in vivo model for studying skin response to ultraviolet B irradiation. Dev. Growth Differ. 2023, 65, 194–202. [Google Scholar] [CrossRef]
- Mancilla, A.; Mayor, R. Neural crest formation in Xenopus laevis: Mechanisms of Xslug induction. Dev. Biol. 1996, 177, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Brito, J.M.; Creuzet, S. Role of the neural crest in face and brain development. Brain Res. Rev. 2007, 55, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.A.; Hu, D.; Rubenstein, J.L.R.; Maden, M.; Helms, J.A. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development 2001, 128, 2755–2767. [Google Scholar] [CrossRef] [PubMed]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Wang, Y.-H.; Ivanisevic, A.-M.; Upholt, W.B.; Rodgers, B. Region- and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev. Dyn. 2002, 223, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, F.; Chen, L.; Hu, D.; Wang, X.; Cui, Y.; Suo, Y.; Zhang, H.; He, J.; Yin, Z.; et al. Bisphenol A exposure induces apoptosis and impairs early embryonic development in Xenopus laevis. Environ. Pollut. 2021, 280, 116901. [Google Scholar] [CrossRef] [PubMed]
- Heasman, J. Patterning the early Xenopus embryo. Development 2006, 133, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Sokol, S.Y. Axis determination by inhibition of Wnt signalling in Xenopus. Genes Dev. 1999, 13, 2328–2336. [Google Scholar] [CrossRef]
- Yamaguchi, T.P. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 2001, 11, R713–R724. [Google Scholar] [CrossRef]






| Gene Name | Oligo Forward Sequence | Oligo Reverse Sequence |
|---|---|---|
| bmp4 | CCTCAGCAGCATTCCAGAGAA | TCCGGTGGAAACCCTCATCC |
| fgf8 | CGTTTGGAAGCAGAGTTCGC | GTTGCCTTGTCTTCGACCCT |
| sox9 | ACGGCGCAGAAAGTCTGTTA | GACATCTGTCTTGGGGGTGG |
| egr2 | AGTAAGACCCCAGTCCACGA | GCAGTAATCGCAGGCAAAGG |
| pax6 | CAGAACATCTTTTACCCAGGA | GAATGTGGCTGGGTGTGTTA |
| rax1 | GGAAAGACCTCAAGCGAGTG | ATACCTGCACCCTGACCTCG |
| odc1 | GTGGCAAGGAATCACCCGAA | TCAAAGACACATCGTGCATC |
| AuNPs | Size [nm] | Size [SD] | Z-Potential [mV] | Z-Potential [SD] | PdI | PdI [SD] |
|---|---|---|---|---|---|---|
| H2O | 32.76 ± 4.0.96 a | 1.67 | −35.8 ± 1.36° | 2.35 | 0.35 ± 0.024° | 0.04 |
| FETAX | 62.08 ± 0.35 a | 0.60 | −24.37 ± 1.43° | 2.48 | 0.48 ± 0.003° | 0.006 |
| AgNPs | Size [nm] | Size [SD] | Z-Potential [mV] | Z-Potential [SD] | PdI | PdI [SD] |
| H2O | 66.98 ± 4.198 a | 7.271 | −25.70 ± 3.256° | 5.640 | 0.3840 ± 0.04596° | 0.07961 |
| FETAX | 174.8 ± 42.23 a | 73.14 | −22.43 ± 0.9244° | 1.601 | 0.4297 ± 0.07917° | 0.1371 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carotenuto, R.; Tussellino, M.; Fusco, S.; Benvenuto, G.; Formiggini, F.; Avallone, B.; Motta, C.M.; Fogliano, C.; Netti, P.A. Adverse Effect of Metallic Gold and Silver Nanoparticles on Xenopus laevis Embryogenesis. Nanomaterials 2023, 13, 2488. https://doi.org/10.3390/nano13172488
Carotenuto R, Tussellino M, Fusco S, Benvenuto G, Formiggini F, Avallone B, Motta CM, Fogliano C, Netti PA. Adverse Effect of Metallic Gold and Silver Nanoparticles on Xenopus laevis Embryogenesis. Nanomaterials. 2023; 13(17):2488. https://doi.org/10.3390/nano13172488
Chicago/Turabian StyleCarotenuto, Rosa, Margherita Tussellino, Sabato Fusco, Giovanna Benvenuto, Fabio Formiggini, Bice Avallone, Chiara Maria Motta, Chiara Fogliano, and Paolo Antonio Netti. 2023. "Adverse Effect of Metallic Gold and Silver Nanoparticles on Xenopus laevis Embryogenesis" Nanomaterials 13, no. 17: 2488. https://doi.org/10.3390/nano13172488





