Advances in Nanomaterials Based on Cashew Nut Shell Liquid
Abstract
:1. Introduction
2. CNSL-Based Nanomaterials
2.1. Cardanol-Based Nanomaterials
2.2. Anacardic Acid-Based Nanomaterials
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNSL | Cashew nut shell liquid |
| CA | Cardanol |
| AA | Anacardic acid |
| CD | Cardol |
| CH | Cholesterol |
| O/W | Oil/water |
| tCNSL | Technical CNSL. |
| H2Pp | Metal-free porphyrin. |
| CuPp | Copper porphyrin. |
| Pc | Phthalocyanine. |
| OA | Oleic acid. |
| FTIR | Fourier-transform infrared spectroscopy. |
| UV | Ultraviolet. |
| TGA | Thermogravimetric analysis. |
| DSC | Differential scanning calorimetry. |
| DTG | Derivative thermogravimetry. |
| XRD | X-ray diffraction. |
| TEM | Transmission electron microscopy. |
| SEM | Scanning electron microscopy. |
| RhB | Rhodamine B. |
| VOCs | Volatile organic compounds. |
| MOF | Metal–organic framework. |
| TEM | Transmission electron microscopy. |
| HRTEM | High resolution transmission electron microscopy. |
| 1HNMR | Proton nuclear magnetic resonance. |
| DLS | Dynamic light scattering. |
| PDI | Polydispersity index. |
| ZP | Zeta potential. |
| EE | Encapsulation efficiency. |
| DNase | Deoxyribonuclease I. |
| TDI | Toluene 2,4-diisocyanate. |
References
- Aswathi, V.P.; Meera, S.; Maria, C.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Rizwan, M.A.; Singh, S. Opportunities and potential of green chemistry in nanotechnology. Nanotechnol. Environ. Eng. 2022, 7, 661–673. [Google Scholar] [CrossRef]
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. 2021, 15, 100418. [Google Scholar] [CrossRef]
- Lu, Y.; Ozcan, S. Green nanomaterials: On track for a sustainable future. Nano Today 2015, 10, 417–420. [Google Scholar] [CrossRef]
- Lomonaco, D.; Mele, G.; Mazzetto, S.E. Cashew nutshell liquid (CNSL): From an agro-industrial waste to a sustainable alternative to petrochemical resources. In Cashew Nut Shell Liquid; Springer: Cham, Switzerland, 2017; pp. 19–38. [Google Scholar] [CrossRef]
- Mgaya, J.; Shombe, G.B.; Masikane, S.C.; Mlowe, S.; Mubofu, E.B.; Revaprasadu, N. Cashew nut shell: A potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Chem. 2019, 21, 1186–1201. [Google Scholar] [CrossRef]
- Mubofu, E.B.; Mgaya, J.E. Chemical Valorization of Cashew Nut Shell Waste. Top. Curr. Chem. 2018, 376, 8. [Google Scholar] [CrossRef] [PubMed]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Leite, A.S.; Islam, M.; Júnior, A.L.G.; Sousa, J.M.C.; Alencar, M.O.B.D.; Paz, M.F.C.J.; Rolim, H.; Medeiros, M.G.F.; Melo-Cavalcante, A.A.; Lopes, J. Pharmacological properties of cashew (Anacardium occidentale). Afr. J. Biotechnol. 2016, 35, 1855–1863. [Google Scholar] [CrossRef]
- Al-Hazzani, A.; Periyasamy, V.; Subash-Babu, P.; Alshatwi, A.A. Formulation of cashew nut shell liquid (CSNL) nanoemulsion, a potent inhibitor of human MCF-7 breast cancer cell proliferation. Med. Chem. Res. 2012, 21, 1384–1388. [Google Scholar] [CrossRef]
- Kala, S.; Sogan, N.; Verma, P.; Naik, S.N.; Agarwal, A.; Patanjali, P.K.; Kumar, J. Nanoemulsion of cashew nut shell liquid bio-waste: Mosquito larvicidal activity and insights on possible mode of action. S. Afr. J. Bot. 2019, 127, 293–300. [Google Scholar] [CrossRef]
- Jorge, M.R.; Crispim, B.D.A.; Merey, F.M.; Barufatti, A.; Cabrini, I.; da Silva Dantas, F.G.; de Oliveira, K.M.P.; Kummrow, F.; Beatriz, A.; Santos, T.; et al. Sulphonates’ mixtures and emulsions obtained from technical cashew nut shell liquid and cardanol for control of Aedes aegypti (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2020, 27, 27870–27884. [Google Scholar] [CrossRef]
- TMary, R.N.; Jayavel, R. Fabrication of chitosan/Cashew Nut Shell Liquid/plant extracts-based bio-formulated nanosheets with embedded iron oxide nanoparticles as multi-functional barrier resist eco-packaging material. Appl. Nanosci. 2022, 12, 1719–1730. [Google Scholar] [CrossRef]
- Zafar, F.; Khan, S.; Ghosal, A.; Azam, M.; Sharmin, E.; Haq, Q.M.R.; Nishat, N. Clean synthesis and characterization of green nanostructured polymeric thin films from endogenous Mg (II) ions coordinated methylolated-Cashew nutshell liquid. J. Clean. Prod. 2019, 238, 117716. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Marcelo, A.M.P.; da Silva, K.T.; da Silva, F.L.F.; Mota, J.P.F.; Nascimento, J.P.C.D.; Sombra, A.S.B.; da Silva Clemente, C.; Mele, G.; Carbone, L.; et al. New ZnO@Cardanol Porphyrin Composite Nanomaterials with Enhanced Photocatalytic Capability under Solar Light Irradiation. Materials 2017, 10, 1114. [Google Scholar] [CrossRef]
- Zafar, F.; Khan, S.; Mondal, A.H.; Sharmin, E.; RizwanulHaq, Q.M.; Nishat, N. Application of FTIR-ATR spectroscopy to confirm the microwave assisted synthesis and curing of Cashew nut shell liquid derived nanostructured materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117732. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Mota, J.P.F.; Júnior, A.E.C.; Lima, N.M.A.; Fechine, P.B.A.; Denardin, J.C.; Carbone, L.; Bloise, E.; Mele, G.; Mazzetto, S.E. Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid. Molecules 2019, 24, 3284. [Google Scholar] [CrossRef]
- da Silva, A.L.; da Silva, L.R.R.; de Andrade Camargo, I.; da Silva Agostini, D.L.; Denardin, J.C.; Rosa, D.D.S.; Mele, G.; de Oliveira, D.L.V.; Fechine, P.B.A.; Mazzetto, S.E. Superparamagnetic nano-biocomposites for application as dielectric resonator antennas. Mater. Chem. Phys. 2017, 185, 104–113. [Google Scholar] [CrossRef]
- Nwuzor, I.C.; Chukwuneke, J.L.; Ewulonu, C.M.; Okolie, P.C. Fabrication of cardanol thermosetting resin reinforced with cellulose nanofibril/expanded graphite nano-biocomposites. Ind. Crops Prod. 2022, 187, 115392. [Google Scholar] [CrossRef]
- Balachandran, V.S.; Jadhav, S.R.; Vemula, P.K.; John, G. Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials. Chem. Soc. Rev. 2013, 42, 427. [Google Scholar] [CrossRef]
- John, G.; Vemula, P.K. Design and development of soft nanomaterials from biobased amphiphiles. Soft Matter 2006, 2, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Nagarajan, S. Strongly fluorescent organogels and self-assembled nanostructures from pyrene coupled coumarin derivatives: Application in cell imaging. J. Mater. Chem. B 2015, 3, 5690. [Google Scholar] [CrossRef]
- Lalitha, K.; Prasad, Y.S.; Maheswari, C.U.; Sridharan, V.; John, G.; Nagarajan, S. Stimuli responsive hydrogels derived from a renewable resource: Synthesis, self-assembly in water and application in drug delivery. J. Mater. Chem. B 2015, 3, 5560. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Jayakannan, M. Divergent Nanostructures from Identical Ingredients: Unique Amphiphilic Micelle Template for Polyaniline Nanofibers, Tubes, Rods, and Spheres. Macromolecules 2008, 41, 7706–7715. [Google Scholar] [CrossRef]
- Shinde, S.D.; Jayakannan, M. New Amphiphilic Sulfonic Acid Dopant-Cum-Templates for Diverse Conducting Polyaniline Nanomaterials. J. Appl. Polym. Sci. 2013, 127, 1781–1793. [Google Scholar] [CrossRef]
- Anilkumar, P.; Jayakannan, M. A Novel Supramolecular Organogel Nanotubular Template Approach for Conducting Nanomaterials. J. Phys. Chem. B 2010, 114, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Jayakannan, M. Self-Assembled Cylindrical and Vesicular Molecular Templates for Polyaniline Nanofibers and Nanotapes. J. Phys. Chem. B 2009, 113, 11614–11624. [Google Scholar] [CrossRef]
- Balachandran, V.S.; Jadhav, S.R.; Pradhan, P.; De Carlo, S.; John, G. Adhesive Vesicles through Adaptive Response of a Biobased Surfactant. Angew. Chem. 2010, 122, 9699–9702. [Google Scholar] [CrossRef]
- Bloise, E.; Carbone, L.; Colafemmina, G.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G.; Mele, G. First Example of a Lipophilic Porphyrin-Cardanol Hybrid Embedded in a Cardanol-Based Micellar Nanodispersion. Molecules 2012, 17, 12252–12261. [Google Scholar] [CrossRef]
- Bloise, E.; Becerra-Herrera, M.; Mele, G.; Sayago, A.; Carbone, L.; D’Accolti, L.; Mazzetto, S.E.; Vasapollo, G. Sustainable Preparation of Cardanol-Based Nanocarriers with Embedded Natural Phenolic Compounds. ACS Sustain. Chem. Eng. 2014, 2, 1299–1304. [Google Scholar] [CrossRef]
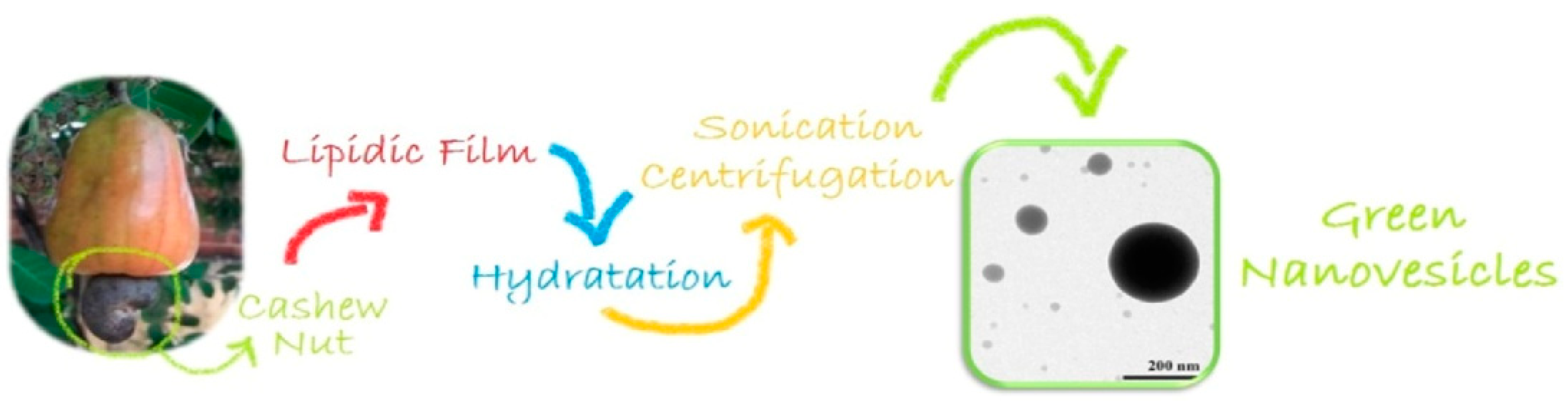
- Behalo, M.S.; Bloise, E.; Carbone, L.; Del Sole, R.; Lomonaco, D.; Mazzetto, S.E.; Mele, G.; Mergola, L. Cardanol-based green nanovesicles with antioxidant and cytotoxic activities. J. Exp. Nanosci. 2016, 11, 1274–1284. [Google Scholar] [CrossRef]
- Di Bello, M.P.; Bloise, E.; Mazzetto, S.E.; Mele, G. Formulation and Chemical Stability in Aqueous Media of Cannabidiol Embedded in Cardanol-Based Nanovesicles. ACS Sustain. Chem. Eng. 2017, 5, 8870–8875. [Google Scholar] [CrossRef]
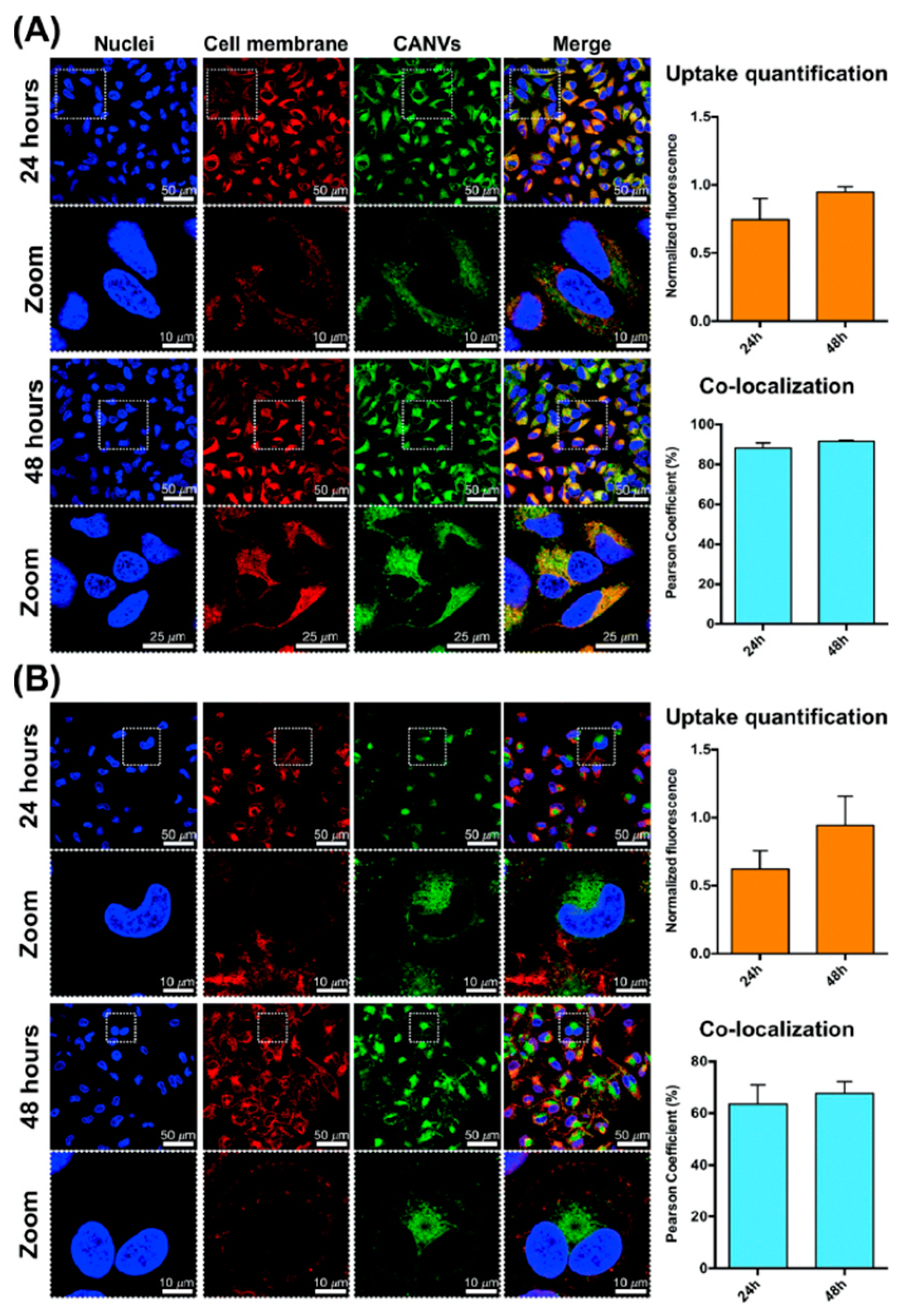
- Bloise, E.; Di Bello, M.P.; Mele, G.; Rizzello, L. A green method for the production of an efficient bioimaging nanotool. Nanoscale Adv. 2019, 1, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bloise, E.; Perrone, E.; Perinelli, D.R.; Schmutz, M.; Arima, V.; Mele, G.; Carbone, L. Environmentally Friendly Method of Assembly of Cardanol and Cholesterol into Nanostructures Using a Continuous Flow Microfluidic Device. ACS Sustain. Chem. Eng. 2022, 10, 8484–8494. [Google Scholar] [CrossRef]
- Behalo, M.S.; Bloise, E.; Mele, G.; Salomone, A.; Messa, F.; Carbone, L.; Mazzetto, S.E.; Lomonaco, D. Bio-based benzoxazines synthesized in a deep eutectic solvent: A greener approach toward vesicular nanosystems. J. Heterocycl. Chem. 2020, 57, 768–773. [Google Scholar] [CrossRef]
- Hamad, F.B.; Mubofu, E.B. Potential Biological Applications of Bio-Based Anacardic Acids and Their Derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. [Google Scholar] [CrossRef]
- Vien, L.T.; Nga, N.T.; Hue, P.T.K.; Kha, T.H.B.; Hoang, N.H.; Hue, P.T.; Thien, P.N.; Huang, C.F.; Kiem, P.V.; Thao, D.T. A New Liposomal Formulation of Hydrogenated Anacardic Acid to Improve Activities Against Cancer Stem Cells. Nat. Prod. Commun. 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Legut, M.; Lipka, D.; Filipczak, N.; Piwoni, A.; Kozubek, A.; Gubernator, J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines–in vitro studies. Int. J. Nanomed. 2014, 9, 653–668. [Google Scholar] [CrossRef]
- Filipczak, N.; Jaromin, A.; Piwoni, A.; Mahmud, M.; Sarisozen, C.; Torchilin, V.; Gubernator, J. A triple co-delivery liposomal carrier that enhances apoptosis via an intrinsic pathway in melanoma cells. Cancers 2019, 11, 1982. [Google Scholar] [CrossRef]
- De Araujo, J.T.C.; Martin-Pastor, M.; Pérez, L.; Pinazo, A.; de Sousa, F.F.O. Development of anacardic acid-loaded zein nanoparticles: Physical chemical characterization, stability and antimicrobial improvement. J. Mol. Liq. 2021, 332, 115808. [Google Scholar] [CrossRef]
- Lima, R.A.; de Souza, S.L.X.; Lima, L.A.; Batista, A.L.X.; Araújo, J.T.C.D.; Sousa, F.F.O.; Rolim, J.P.M.L.; Bandeira, T.D.J.P.G. Antimicrobial effect of anacardic acid–loaded zein nanoparticles loaded on Streptococcus mutans biofilms. Braz. J. Microbiol. 2020, 51, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.J.S.; de Araujo, J.T.C.; Ferreira, R.M.D.A.; Souto, R.N.P.; Lima, L.A.; de Barros Silva, P.G.; Garcia, M.T.; de la Fuente, A.; de Sousa, F.F.O. Larvicidal activity, aquatic and in vivo toxicity of anacardic acid loaded-zein nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102513. [Google Scholar] [CrossRef]
- Anjum, M.M.; Patel, K.K.; Dehari, D.; Pandey, N.; Tilak, R.; Agrawal, A.K.; Singh, S. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: Chitosan and DNase coating improve antimicrobial activity. Drug Deliv. Transl. Res. 2021, 11, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, V.; Katiyar, S.S.; Dora, C.P.; Agrawal, A.K.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomat. 2018, 73, 424–436. [Google Scholar] [CrossRef]
- Kushwah, V.; Jain, D.K.; Agrawal, A.K.; Jain, S. Improved antitumor efficacy and reduced toxicity of docetaxel using anacardic acid functionalized stealth liposomes. Colloids Surf. B Biointerfaces 2018, 172, 213–223. [Google Scholar] [CrossRef]
- Filho, J.C.P.; de Morais, S.M.; Sobrinho, A.C.N.; Cavalcante, G.S.; da Silva, N.A.; da Silva Abreu, F.O.M. Design of chitosan-alginate core-shell nanoparticules loaded with anacardic acid and cardol for drug delivery. Polímeros 2019, 29, e2019060. [Google Scholar] [CrossRef]
- Oliveira, S.N.; Uchoa, A.F.J.; Moreira, D.R.; Petzhold, C.L.; Weiss, C.K.; Landfesterc, K.; Ricardo, N.M.P.S. Design and Evaluation of Dual Release from Anacardic Acid-Based Polyurea Nanocapsules Components. J. Braz. Chem. Soc. 2023, 34, 560–570. [Google Scholar] [CrossRef]
- Shelma, R.; Paul, W.; Sharma, C.P. Development and characterization of self-aggregated nanoparticles from anacardoylated chitosan as a carrier for insulin. Carbohydr. Polym. 2010, 80, 285–290. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic nanoparticles coated with anacardic acid derived from cashew nut shell liquid. J. Mater. Sci. 2013, 48, 7875–7882. [Google Scholar] [CrossRef]
- Bezerra, T.T.; de Almeida, M.O.; de Amorim Lima, N.M.; de Castro Rodrigues, N.L.; Ribeiro, V.G.P.; Teixeira, M.J.; Carbone, L.; Mele, G.; Lomonaco, D.; Mazzetto, S.E. In vitro antileishmanial activity of sustainable anacardic acid and cardol based silver nanoparticles on L. braziliensis. Int. J. Pharm. 2022, 619, 121698. [Google Scholar] [CrossRef]
- Mubofu, E.B.; Mlowe, S.; Revaprasadu, N. Cashew nut shells as source of chemicals for preparation of chalcogenide nanoparticles. Nanosyst. Phys. Chem. Math. 2016, 7, 724–727. [Google Scholar] [CrossRef]
- Mlowe, S.S.; Pullabhotla, R.R.; Mubofu, E.E.; Ngassapa, F.F.; Revaprasadu, N.N. Low temperature synthesis of anacardic-acid-capped cadmium chalcogenide nanoparticles. Int. Nano Lett. 2014, 4, 106. [Google Scholar] [CrossRef]
- Chacko, A.S.; Prasad, V.S. Self–assembly of Anacardic Acid Derived Cation–modified Montmorillonite into ’Arthropodal’ Branched Nanofibers. Chem. Sel. 2017, 2, 2288–2292. [Google Scholar] [CrossRef]
- Bloise, E.; Di Bello, M.P.; Carbone, L.; Mazzetto, S.E.; Mele, G. Anacardic Acid: A Promising Building Block for the Sustainable Preparation of Vesicular Nanosystems. Waste Biomass Valor. 2021, 12, 4367–4374. [Google Scholar] [CrossRef]
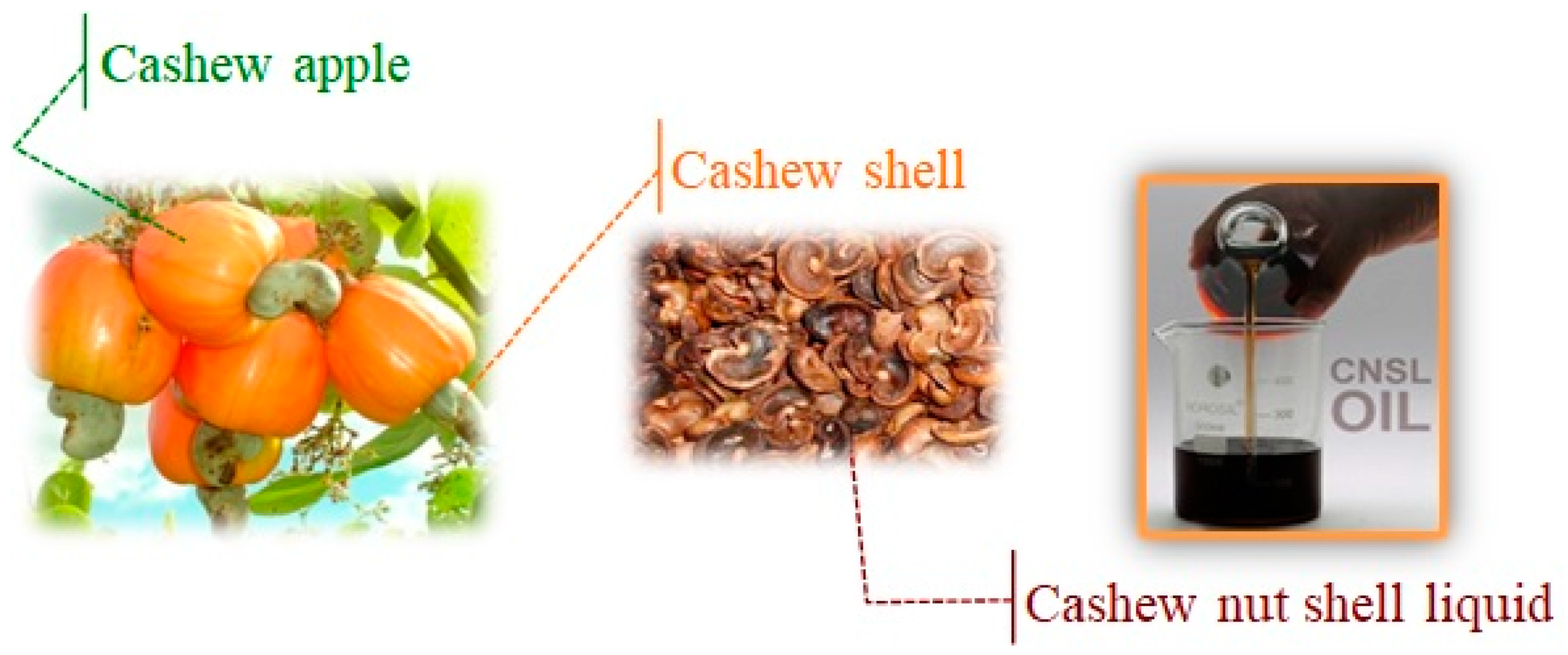
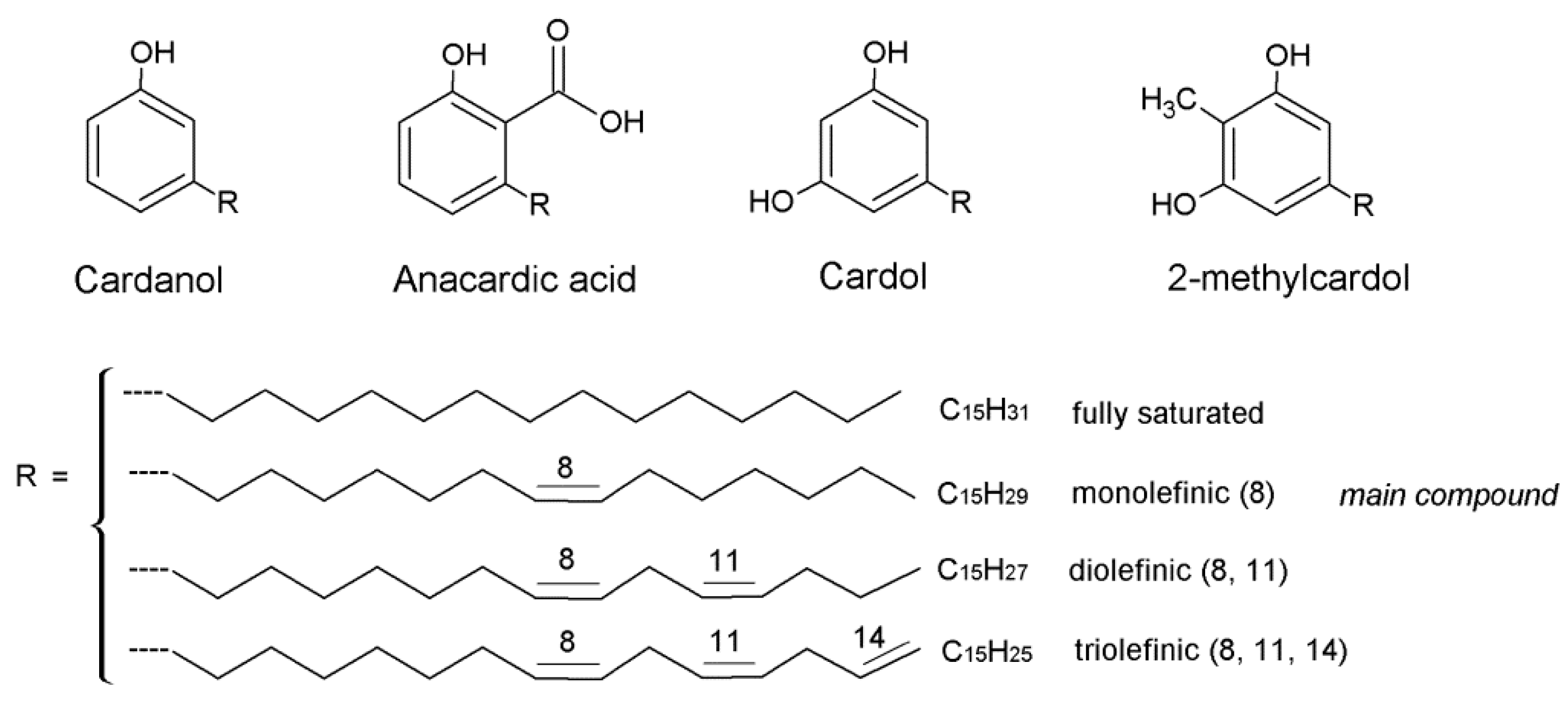




| Cardanol | Anacardic Acid | Cardol | 2-Metylcardol | Polymeric Materials | |
|---|---|---|---|---|---|
| 3–10% | 60–70% | 10–20% | 2–3% | - | Natural CNSL |
| 60–70% | <5% | 10–20% | 2–3% | 7–10% | Technical CNSL |
| >90% | Not detected | <5% | Not detected | - | Distilled CNSL (180–200 °C) |
| Nanoformulation | Technological and Electrochemical Applications | Ref. |
|---|---|---|
| ZnO nanostructures impregnated with CA-H2Pp and CA-CuPp | Heterogeneous photocatalysts for water purification | [16] |
| Nano-biocomposites consisting of CA/formaldehyde/epoxy resin polymer added with spongy gourd fiber/magnetite nanoparticles | Dielectric resonators | [19] |
| Sheet-like nano-biocomposites consisting of CA thermosetting resin/cellulose nanofibrils/expanded graphite nanoplatelets | Coating systems and automotive applications | [20] |
| CA-benzenesulfonic acid as a dopant for polyaniline nanofibers, nanorods, nanospheres, and nanotubes | Electrochemical properties and processability in electronic and optical devices | [25] |
| 4-(3-dodecyl-8-enylphenyloxy) butane sulfonic acid synthesized by CA as a dopant for polyaniline nanofibers and nanotapes | Bio- and chemical sensors as well as optical devices | [28] |
| Nanoformulation | Biomedical Applications | Ref. |
|---|---|---|
| Nanostructured MOF consisting of CA organic ligand and nontoxic endogenous cation Mn(II) bivalent salt (MnIIMicCol) | Thermally stable antimicrobial coating materials Antibacterial activity against E. coli, K. pneumoniae, B. subtilis, and S. aureus | [17] |
| Core/shell/shell hybrid nanomaterials formed by an Fe3O4 core/OA layer/CA-Pc layer | Therapeutic agents for photodynamic therapy and magnetic hyperthermia | [18] |
| Nano-flake fluorescent organogels consisting of pyrene-coupled coumarin derivatives obtained via CA-aldehyde | Inhibitory for the proliferation of PC3 prostate cancer cells Material for cell imaging | [23] |
| Supramolecular hydrogel from CA-derived coumarin-tris-based amphiphiles | Stimuli-responsive (pH or Fe3+ ion) drug delivery system for in vivo formulations | [24] |
| Nanovesicles from the biobased surfactant N-cardanyl tauramide | Temperature stimuli-responsive drug delivery system | [29] |
| CA-based nanovesicles via a solvent-free batch method hosting a CA–porphyrin hybrid, chlorogenic acids, phthalazine derivatives, and cannabidiol | Nanosystems for photodynamic therapy, antioxidant and cytotoxic activities against different cancer cell lines, and preserving bioactive embedded molecules | [30,31,32,33] |
| Fluorescent CA-based nanovesicles via a solvent-free batch method embedded with RhB | Biocompatible green nanotools for bioimaging Active uptake in human macrophages and HeLa cells | [34] |
| CA-based amphiphilic nanostructures by a microfluidic route (micelles, vesicles) | Bioactive nanosystems for drug delivery | [35] |
| Nanovesicles via a solvent-free batch method based on CA-benzoxazines synthesized via a green route | Bioactive green nanosystems | [36] |
| Nanoformulation | Characterization Data | Bioactivity | Ref. |
|---|---|---|---|
| Nanoliposomes based on hydrogenated AA conjugated to a CD133 monoclonal antibody | Size = 100.90 ± 5.24 nm PDI = 0.28 ± 0.02 ZP = −40.7 ± 3.2 mV EE = 100% | Cytotoxic against NTERA-2 cancer stem cells Apoptosis | [38] |
| Antineoplastic drug: mitoxantrone loaded into liposomal carriers enriched with encapsulated AA in the liposomal bilayer using a vitamin C gradient | Size = 112 ± 3 nm PDI = 0.041 ± 0.003 ZP = −4.31 ± 0.49 mV EE = 99.5 ± 3.5% | Epigenetic agent anticancer | [39] |
| Liposomes containing AA, mitoxantrone, and ammonium ascorbate | Size = 119 ± 1.5 nm PDI = 0.05 ZP = −3.71 ± 0.5 mV EE = 89.9% | Apoptosis via reactive oxygen species production through the killing of cancer cells in a monolayer culture towards melanoma cells | [40] |
| AA-loaded zein nanoparticles | Size = 381.6 nm PDI = 0.067 ZP = −15.9 mV AA (%w/v) = 0.00093 | Bacteriostatic for Staphylococcus aureus and Pseudomonas aeruginosa Bactericide activity for S. aureus Fungistatic/fungicide for Candida rugosa, Candida albicans, Candida parapsilosis, Candida tropicalis, Candida jardinii, Candida glabratta, and Candida auris Inhibitory/bactericidal for Planktonic S. mutans Larvicidal for A. aegypti larvae | [41,42,43] |
| AA-loaded solid lipid nanoparticles, coated with chitosan and DNase | Size = 212.8 ± 4.21 nm PDI = 0.285 ± 0.04 ZP = +13.5 ± 1.92 mV EE = 73.8 ± 1.23% | Antimicrobial efficacy against Staphylococcus aureus | [44] |
| AA, gemcitabine used for the development of docetaxel nanoparticles | Size = 163 ± 8 nm PDI = 0.13 ± 0.09 ZP = −27 ± 1 mV EE = 9.1 ± 0.6% | Tumor targeting through VEGF receptors overexpressed in tumors Combination of gemcitabine and docetaxel provides synergistic activity by targeting multiple pathways | [45] |
| Docetaxel-loaded AA functionalized liposomes | Size = 126.4 ± 6.2 nm PDI = 0.239 ± 0.03 EE = 72.35 ± 3.46% | Reduction in tumor volume and toxicity in comparison with marketed formulation (Taxotere®) | [46] |
| AA, CD, chitosan, alginate, and gum arabic matrices | Size = 70/250 nm ZP = −18.8/−9.8 mV | Inhibitory capacity for all strains of dermatophytes and antimicrobial control | [47] |
| AA nanocapsules obtained via interfacial polymerization using the inverse miniemulsion technique with TDI | 1 equivalent TDI: Size = 310 ± 17 nm ZP = −34 mV 2 equivalent TDI: Size = 582 ± 153 nm ZP = −43 mV | Active in vitro against Bacillus subtilis colonies in the bacterial tests | [48] |
| Self-aggregated nanoparticles from chitosan modified with AA | Size = 214 nm ZP = −20.3 mV EE = 27 ± 3% | Release and stabilization of insulin in the intestinal environment | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, E.; Lazzoi, M.R.; Mergola, L.; Del Sole, R.; Mele, G. Advances in Nanomaterials Based on Cashew Nut Shell Liquid. Nanomaterials 2023, 13, 2486. https://doi.org/10.3390/nano13172486
Bloise E, Lazzoi MR, Mergola L, Del Sole R, Mele G. Advances in Nanomaterials Based on Cashew Nut Shell Liquid. Nanomaterials. 2023; 13(17):2486. https://doi.org/10.3390/nano13172486
Chicago/Turabian StyleBloise, Ermelinda, Maria Rosaria Lazzoi, Lucia Mergola, Roberta Del Sole, and Giuseppe Mele. 2023. "Advances in Nanomaterials Based on Cashew Nut Shell Liquid" Nanomaterials 13, no. 17: 2486. https://doi.org/10.3390/nano13172486






