Silver–Gold Alloy Nanoparticles (AgAu NPs): Photochemical Synthesis of Novel Biocompatible, Bimetallic Alloy Nanoparticles and Study of Their In Vitro Peroxidase Nanozyme Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
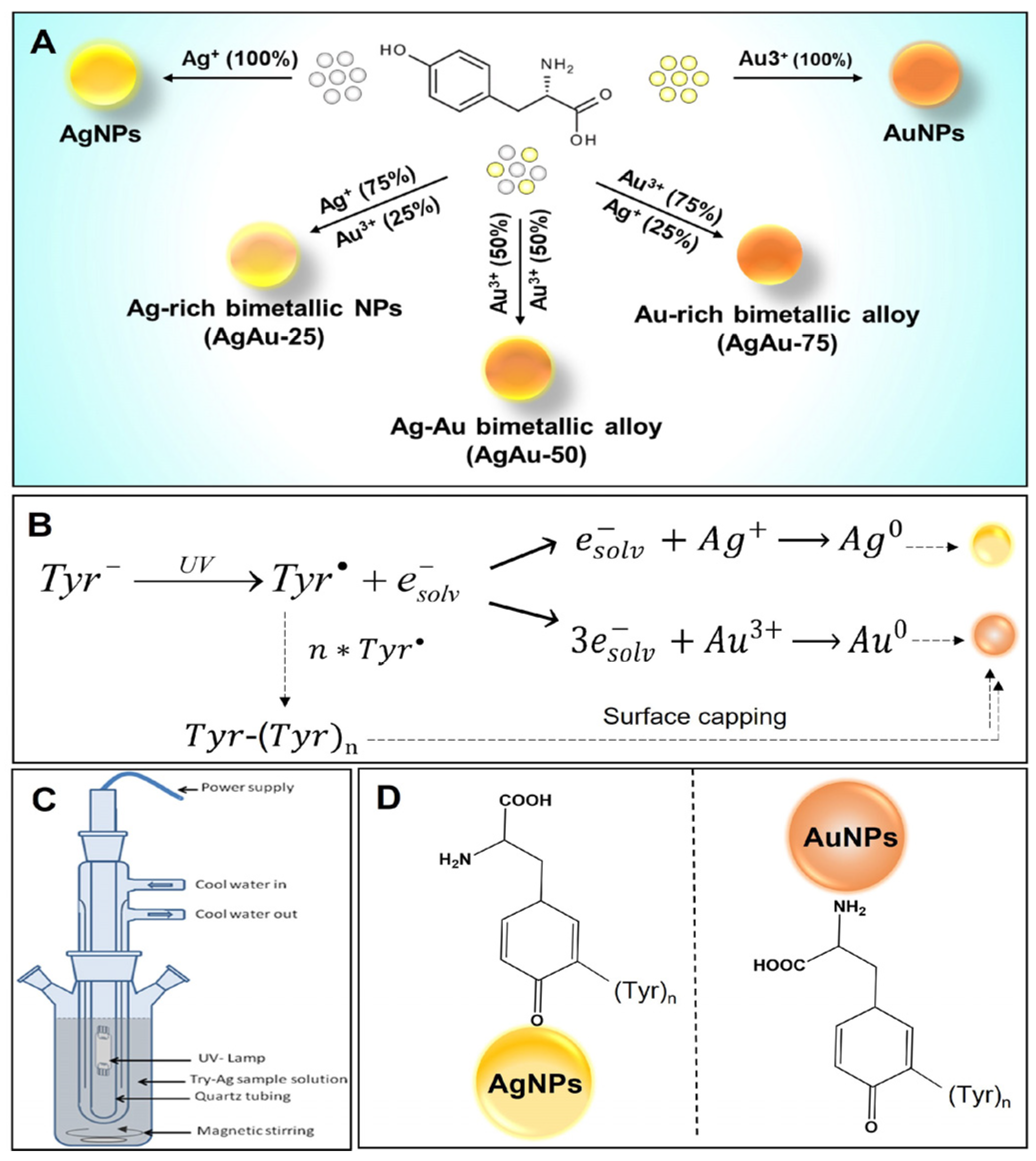
2.2. Photochemical Synthesis of Monometallic AgNPs and AuNPs
2.3. Photochemical Synthesis of Bimetallic AgAu NPs
2.4. Column Chromatography-Based Purification of the AgNPs, AuNPs, and AgAu NPs
2.5. Physicochemical Characterization of Monometallic and Bimetallic AgAu NPs
2.5.1. X-ray Powder Diffraction
2.5.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.3. X-ray Fluorescence Spectroscopy (XRF)
2.5.4. Transmission Electron Microscopy (TEM)
2.5.5. Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AE
2.6. Cell Culture
2.7. Cell Cytotoxicity Assays
2.8. Quantitation of the Intracellular Reactive Oxygen Species (ROS)
2.9. In Vitro Peroxidase Nanozyme Activity of NPs
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Anuj, K.S.; Gupta, B.D. Fibre-optic sensor based on surface plasmon resonance with Ag–Au alloy nanoparticle films. Nanotechnology 2006, 17, 124. [Google Scholar] [CrossRef]
- Yen, C.-W.; Lin, M.-L.; Wang, A.; Chen, S.-A.; Chen, J.-M.; Mou, C.-Y. CO Oxidation Catalyzed by Au−Ag Bimetallic Nanoparticles Supported in Mesoporous Silica. J. Phys. Chem. C 2009, 113, 17831–17839. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Synergistic Catalysis over Bimetallic Alloy Nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]
- Freeman, R.G.; Hommer, M.B.; Grabar, K.C.; Jackson, M.A.; Natan, M.J. Ag-Clad Au nanoparticles: Novel aggregation, optical, and ourface-Enhanced raman scattering properties. J. Phys. Chem. 1996, 100, 718–724. [Google Scholar] [CrossRef]
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; Wang, Z.L.; El-Sayed, M.A. Alloy Formation of Gold−Silver Nanoparticles and the Dependence of the Plasmon Absorption on Their Composition. J. Phys. Chem. B 1999, 103, 3529–3533. [Google Scholar] [CrossRef]
- Tokonami, S.; Morita, N.; Takasaki, K.; Toshima, N. Novel Synthesis, Structure, and Oxidation Catalysis of Ag/Au Bimetallic Nanoparticles. J. Phys. Chem. C 2010, 114, 10336–10341. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, H.T.; Abbasi, E.; Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 376–380. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J. Mol. Liq. 2016, 221, 179–189. [Google Scholar] [CrossRef]
- Wang, A.-Q.; Chang, C.-M.; Mou, C.-Y. Evolution of Catalytic Activity of Au−Ag Bimetallic Nanoparticles on Mesoporous Support for CO Oxidation. J. Phys. Chem. B 2005, 109, 18860–18867. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Xu, L.; Luo, Z.; Fan, Z.; Zhang, X.; Tan, C.; Li, H.; Zhang, H.; Xue, C. Triangular Ag–Pd alloy nanoprisms: Rational synthesis with high-efficiency for electrocatalytic oxygen reduction. Nanoscale 2014, 6, 11738–11743. [Google Scholar] [CrossRef] [PubMed]
- Abhijith, K.S.; Sharma, R.; Ranjan, R.; Thakur, M.S. Facile synthesis of gold–silver alloy nanoparticles for application in metal enhanced bioluminescence. Photochem. Photobiol. Sci. 2014, 13, 986–991. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Fu, Z.-W.; Qin, D. Transformation of Ag Nanocubes into Ag–Au Hollow Nanostructures with Enriched Ag Contents to Improve SERS Activity and Chemical Stability. ACS Appl. Mater. Interfaces 2014, 6, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.; Vinogradova, E.; Ruiz-Zepeda, F.; Velazquez-Salazar, J.J.; Jose-Yacaman, M. Biocompatible gold/silver nanostars for surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 651–655. [Google Scholar] [CrossRef]
- Bond, G.C. Gold: A relatively new catalyst. Catal. Today 2002, 72, 5–9. [Google Scholar] [CrossRef]
- Fernández, J.L.; Raghuveer, V.; Manthiram, A.; Bard, A.J. Pd−Ti and Pd−Co−Au Electrocatalysts as a Replacement for Platinum for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2005, 127, 13100–13101. [Google Scholar] [CrossRef]
- Atwan, M.H.; Northwood, D.O.; Gyenge, E.L. Evaluation of colloidal Ag and Ag-alloys as anode electrocatalysts for direct borohydride fuel cells. Int. J. Hydrogen Energy 2007, 32, 3116–3125. [Google Scholar] [CrossRef]
- Foglia, S.; Ledda, M.; Fioretti, D.; Iucci, G.; Papi, M.; Capellini, G.; Lolli, M.G.; Grimaldi, S.; Rinaldi, M.; Lisi, A. In vitro biocompatibility study of sub-5 nm silica-coated magnetic iron oxide fluorescent nanoparticles for potential biomedical application. Sci. Rep. 2017, 7, 46513. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; Di Gioia, C.; Carletti, R.; Ciasca, G.; Foglia, S.; Palmieri, V.; Marchese, R.; et al. Biocompatibility assessment of sub-5 nm silica-coated superparamagnetic iron oxide nanoparticles in human stem cells and in mice for potential application in nanomedicine. Nanoscale 2020, 12, 1759–1778. [Google Scholar] [CrossRef]
- Xie, M.; Xu, Y.; Huang, J.; Li, Y.; Wang, L.; Yang, L.; Mao, H. Going even smaller: Engineering sub-5 nm nanoparticles for improved delivery, biocompatibility, and functionality. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2020, 12, e1644. [Google Scholar] [CrossRef]
- Matur, M.; Madhyastha, H.; Shruthi, T.S.; Madhyastha, R.; Srinivas, S.P.; Navya, P.N.; Daima, H.K. Engineering bioactive surfaces on nanoparticles and their biological interactions. Sci. Rep. 2020, 10, 19713. [Google Scholar] [CrossRef]
- Shreyash, N.; Bajpai, S.; Khan, M.A.; Vijay, Y.; Tiwary, S.K.; Sonker, M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 11428–11457. [Google Scholar] [CrossRef]
- Degliangeli, F.; Kshirsagar, P.; Brunetti, V.; Pompa, P.P.; Fiammengo, R. Absolute and direct microRNA quantification using DNA–gold nanoparticle probes. J. Am. Chem. Soc. 2014, 136, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Perinot, A.; Kshirsagar, P.; Malvindi, M.A.; Pompa, P.P.; Fiammengo, R.; Caironi, M.J.S.R. Direct-written polymer field-effect transistors operating at 20 MHz. Sci. Rep. 2016, 6, 38941. [Google Scholar] [CrossRef]
- Carmicheal, J.; Hayashi, C.; Huang, X.; Liu, L.; Lu, Y.; Krasnoslobodtsev, A.; Lushnikov, A.; Kshirsagar, P.G.; Patel, A.; Jain, M.; et al. Label-free characterization of exosome via surface enhanced Raman spectroscopy for the early detection of pancreatic cancer. J. Nanomed. Nanotechnol. Biol. 2019, 16, 88–96. [Google Scholar] [CrossRef]
- Kshirsagar, P.; Rachagani, S.; Seshacharyulu, P.; Muniyan, S.; Mahato, R.; Batra, S.K.; Jain, M. Abstract LB-331: Advanced DNA-gold nanoprobe-based direct and sensitive quantification of novel microRNAs in prostate cancer. Cancer Res. 2017, 77, LB-331. [Google Scholar] [CrossRef]
- Kshirsagar, P.; Rachagani, S.; Seshacharyulu, P.; Muniyan, S.; Sharma, S.; Mahato, R.; Batra, S.K.; Jain, M. Abstract 2583: DNA-linked gold nanoprobe assay: An efficient technology for direct and high-sensitive quantification of serum microRNAs in prostate cancer. Cancer Res. 2019, 79, 2583. [Google Scholar] [CrossRef]
- Navya, P.N.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, M.S.; Atarod, M.; Sajjadi, M.; Isaabadi, Z. An Introduction to Green Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Daima, H.K.; Selvakannan, P.; Homan, Z.; Bhargava, S.K.; Bansal, V. Tyrosine Mediated Gold, Silver and Their Alloy Nanoparticles Synthesis: Antibacterial Activity Toward Gram Positive and Gram Negative Bacterial Strains. In Proceedings of the 2011 International Conference on Nanoscience, Technology and Societal Implications, Bhubaneswar, India, 8–10 December 2011; pp. 1–6. [Google Scholar]
- Kshirsagar, P.; Sangaru, S.S.; Brunetti, V.; Malvindi, M.A.; Pompa, P.P. Synthesis of fluorescent metal nanoparticles in aqueous solution by photochemical reduction. Nanotechnology 2014, 25, 045601. [Google Scholar] [CrossRef]
- Kshirsagar, P.G.; Sangaru, S.S. Amine and Acid-Functionalized Gold Nanoparticles via Thiol-Ene Click Chemistry: A Bio-Orthogonal Route for Surface Bioconjugation. Biomed. J. Sci. 2021, 36, 28821–28826. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Kshirsagar, P.; Sangaru, S.S.; Malvindi, M.A.; Martiradonna, L.; Cingolani, R.; Pompa, P.P. Synthesis of highly stable silver nanoparticles by photoreduction and their size fractionation by phase transfer method. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 264–270. [Google Scholar] [CrossRef]
- Kshirsagar, P.G.; Gulati, M.; Junker, W.M.; Aithal, A.; Spagnol, G.; Das, S.; Mallya, K.; Gautam, S.K.; Kumar, S.; Sorgen, P.; et al. Characterization of recombinant β subunit of human MUC4 mucin (rMUC4β). Sci. Rep. 2021, 11, 23730. [Google Scholar] [CrossRef] [PubMed]
- Hadžija, O.; Špoljar, B. Quantitative determination of carboxylate by infrared spectroscopy: Application to humic acids. Fresenius J. Anal. Chem. 1995, 351, 692–693. [Google Scholar] [CrossRef]
- Lehrer, S.S.; Fasman, G.D. Ultraviolet Irradiation Effects in Poly-L-tyrosine and Model Compounds. Identification of Bityrosine as a Photoproduct*. Biochemistry 1967, 6, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Bent, D.V.; Hayon, E. Excited state chemistry of aromatic amino acids and related peptides. III. Tyrosine. J. Am. Chem. Soc. 1975, 97, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Li, W.; Francis, G.A.; Goldstein, J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Investig. 1993, 91, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Skaff, O.; Jolliffe, K.A.; Hutton, C.A. Synthesis of the Side Chain Cross-Linked Tyrosine Oligomers Dityrosine, Trityrosine, and Pulcherosine. J. Org. Chem. 2005, 70, 7353–7363. [Google Scholar] [CrossRef]
- Rothschild, K.J.; He, Y.W.; Gray, D.; Roepe, P.D.; Pelletier, S.L.; Brown, R.S.; Herzfeld, J. Fourier transform infrared evidence for proline structural changes during the bacteriorhodopsin photocycle. Proc. Natl. Acad. Sci. USA 1989, 86, 9832–9835. [Google Scholar] [CrossRef] [PubMed]
- Daima, H.K.; Selvakannan, P.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. J. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Selvakannan, P.; Pasricha, R.; Mandale, A.; Sastry, M. Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef]
- Noguez, C. Surface Plasmons on Metal Nanoparticles: The Influence of Shape and Physical Environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Gómez, L.A.; de Araújo, C.B.; Brito-Silva, A.M.; Galembeck, A. Solvent effects on the linear and nonlinear optical response of silver nanoparticles. Appl. Phys. B 2008, 92, 61–66. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Experimental results and discussion. In Optical Properties of Metal Clusters; Springer: Berlin/Heidelberg, Germany, 1995; pp. 275–436. [Google Scholar]
- Major, K.; De, C.; Obare, S. Recent Advances in the Synthesis of Plasmonic Bimetallic Nanoparticles. Plasmonics 2009, 4, 61–78. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Mallik, K.; Mandal, M.; Pradhan, N.; Pal, T. Seed Mediated Formation of Bimetallic Nanoparticles by UV Irradiation: A Photochemical Approach for the Preparation of “Core−Shell” Type Structures. Nano Lett. 2001, 1, 319–322. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mallin, M.P.; Murphy, C.J. Solution-Phase Synthesis of Sub-10 nm Au−Ag Alloy Nanoparticles. Nano Lett. 2002, 2, 1235–1237. [Google Scholar] [CrossRef]
- Mari, A.; Imperatori, P.; Marchegiani, G.; Pilloni, L.; Mezzi, A.; Kaciulis, S.; Cannas, C.; Meneghini, C.; Mobilio, S.; Suber, L. High Yield Synthesis of Pure Alkanethiolate-Capped Silver Nanoparticles. Langmuir 2010, 26, 15561–15566. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yuan, J.; Chen, Y.; Palmer, R.E.; Wilcoxon, J.P. Direct imaging of core-shell structure in silver-gold bimetallic nanoparticles. Appl. Phys. Lett. 2005, 87, 243103. [Google Scholar] [CrossRef]
- Okamoto, H.; Massalski, T. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Naha, P.C.; Lau, K.C.; Hsu, J.C.; Hajfathalian, M.; Mian, S.; Chhour, P.; Uppuluri, L.; McDonald, E.S.; Maidment, A.D.; Cormode, D.P. Gold silver alloy nanoparticles (GSAN): An imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale 2016, 8, 13740–13754. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.; Shukla, R.; Bhargava, S.K.; Bansal, V. Fine-tuning the antimicrobial profile of biocompatible gold nanoparticles by sequential surface functionalization using polyoxometalates and lysine. PLoS ONE 2013, 8, e79676. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, P.; Seshacharyulu, P.; Muniyan, S.; Rachagani, S.; Smith, L.M.; Thompson, C.; Shah, A.; Mallya, K.; Kumar, S.; Jain; et al. DNA-gold nanoprobe-based integrated biosensing technology for non-invasive liquid biopsy of serum miRNA: A new frontier in prostate cancer diagnosis. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102566. [Google Scholar] [CrossRef] [PubMed]
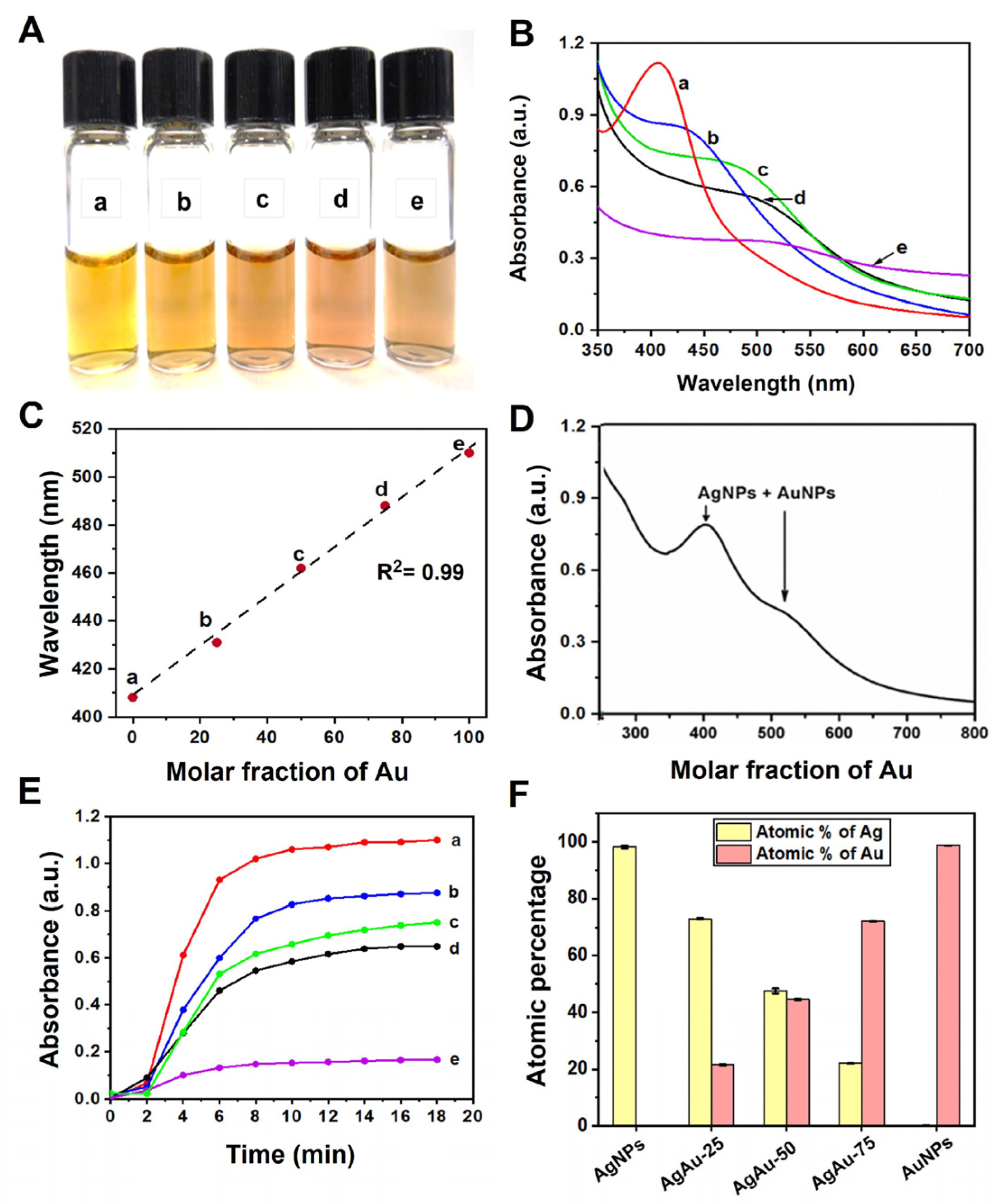
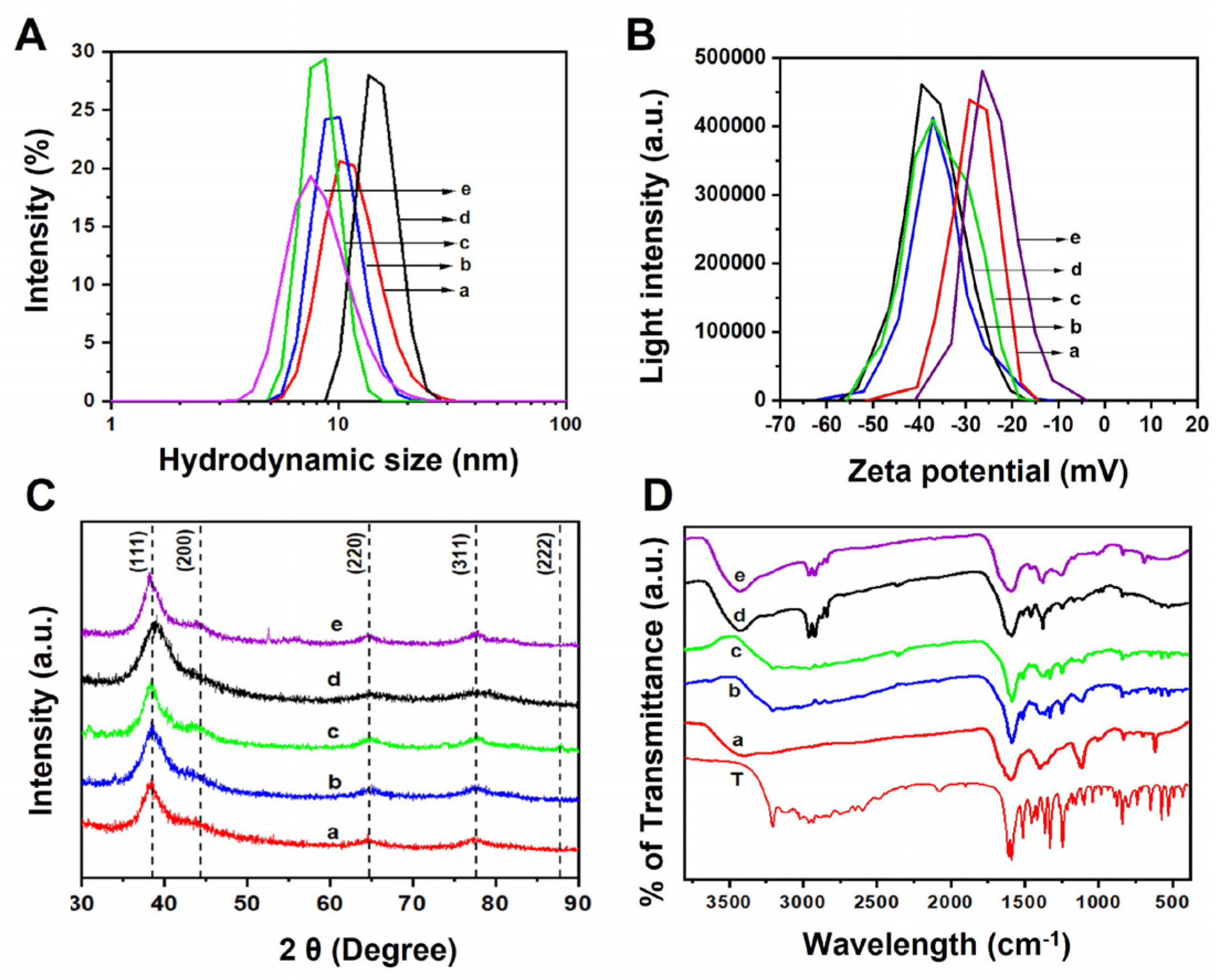
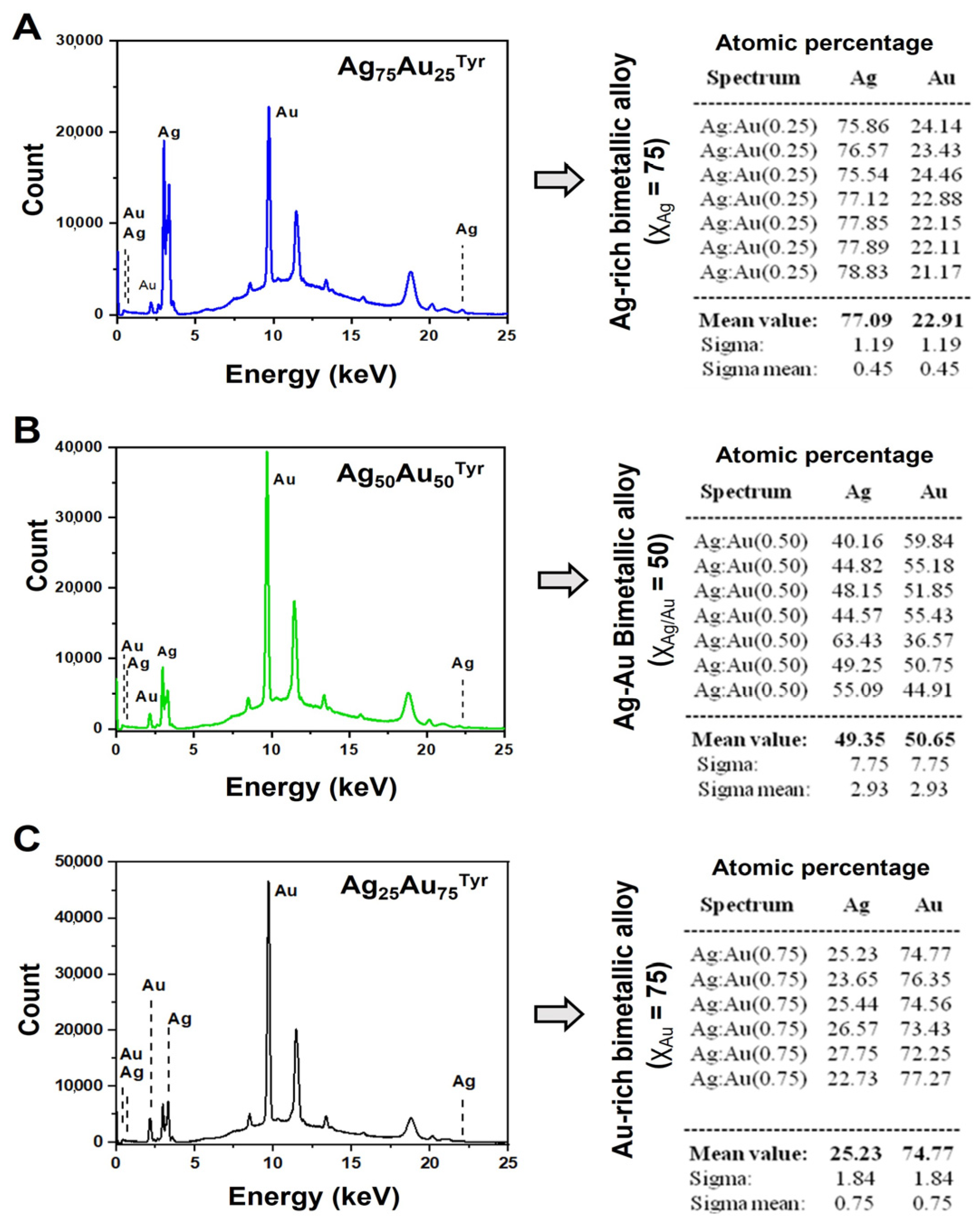


| NPs | χAg | χAu | UV, SPR a (λmax, nm) | Zeta [ζ] b Potential (mV) | Diameter (XRD, nm) c | Atomic % by XRF d | HD (ϕ) e (DLS, nm) | Diameter f (TEM, nm) |
|---|---|---|---|---|---|---|---|---|
| AgNPs | 1.00 | 0.00 | 408 | −26.0 | 3.5 | - | 10 ± 2 | 4.0 ± 2.2 |
| AgAu-25 | 0.75 | 0.25 | 430 | −37.0 | 3.1 | 77:23 | 8 ± 1 | 4.7 ± 1.8 |
| AgAu-50 | 0.50 | 0.50 | 462 | −37.2 | 3.8 | 49:51 | 9 ± 1 | 3.0 ± 1.5 |
| AgAu-75 | 0.25 | 0.75 | 488 | −39.3 | 3.0 | 25:75 | 8 ± 1 | 4.7 ± 2.1 |
| AuNPs | 0.00 | 1.00 | 510 | −29.2 | 3.8 | - | 7 ± 1 | 3.3 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kshirsagar, P.G.; De Matteis, V.; Pal, S.; Sangaru, S.S. Silver–Gold Alloy Nanoparticles (AgAu NPs): Photochemical Synthesis of Novel Biocompatible, Bimetallic Alloy Nanoparticles and Study of Their In Vitro Peroxidase Nanozyme Activity. Nanomaterials 2023, 13, 2471. https://doi.org/10.3390/nano13172471
Kshirsagar PG, De Matteis V, Pal S, Sangaru SS. Silver–Gold Alloy Nanoparticles (AgAu NPs): Photochemical Synthesis of Novel Biocompatible, Bimetallic Alloy Nanoparticles and Study of Their In Vitro Peroxidase Nanozyme Activity. Nanomaterials. 2023; 13(17):2471. https://doi.org/10.3390/nano13172471
Chicago/Turabian StyleKshirsagar, Prakash G., Valeria De Matteis, Sudipto Pal, and Shiv Shankar Sangaru. 2023. "Silver–Gold Alloy Nanoparticles (AgAu NPs): Photochemical Synthesis of Novel Biocompatible, Bimetallic Alloy Nanoparticles and Study of Their In Vitro Peroxidase Nanozyme Activity" Nanomaterials 13, no. 17: 2471. https://doi.org/10.3390/nano13172471







