Comparative Analysis of Interfacial Adaptation and Depth Penetration of Recent HiFlow versus Regular Bioceramic Sealers in Conjunction with BC Gutta-Percha Points Using Two Different Obturation Techniques—A Preliminary Report of an Ex Vivo Study
Abstract
:1. Introduction
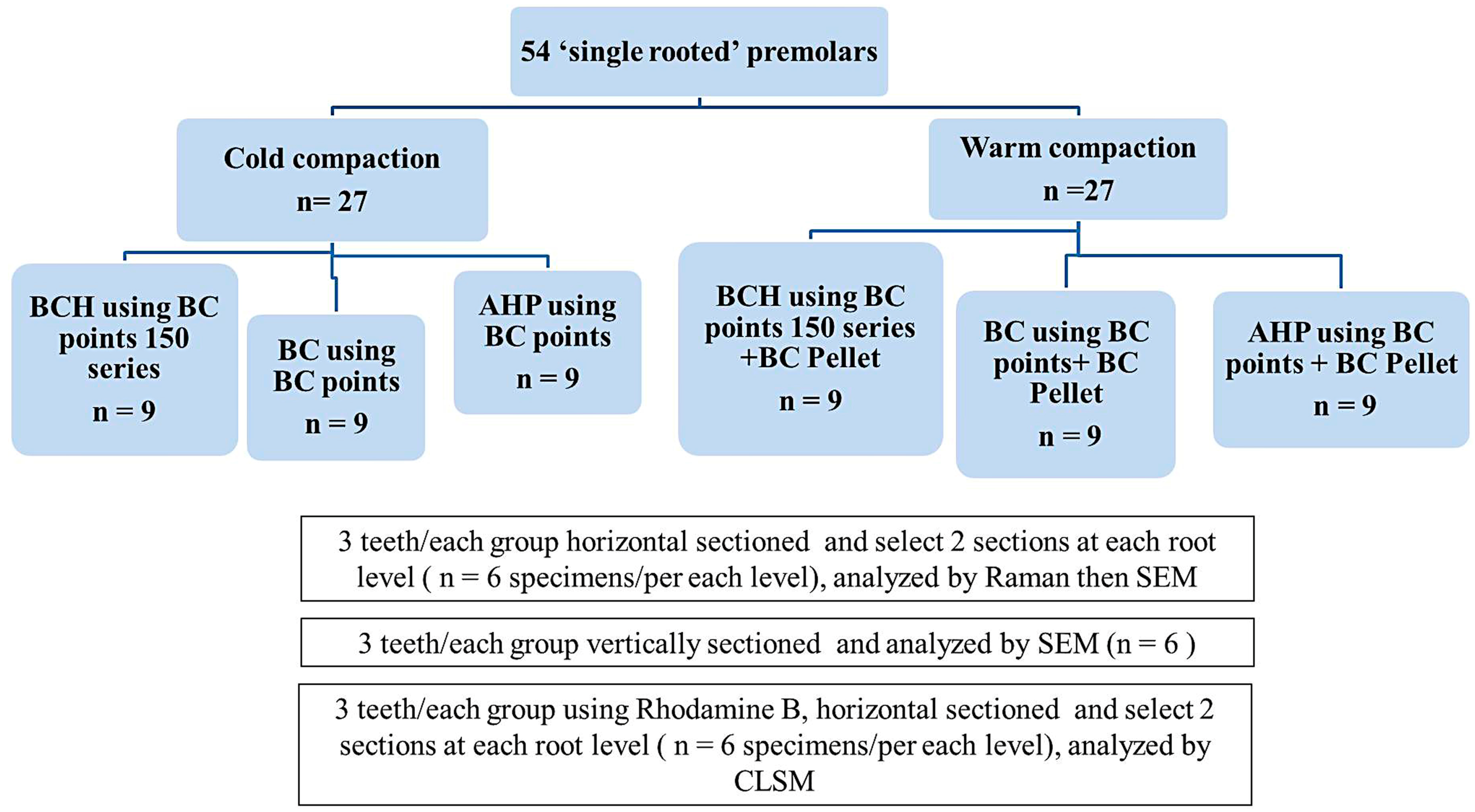
2. Materials and Methods
2.1. Specimen Preparation
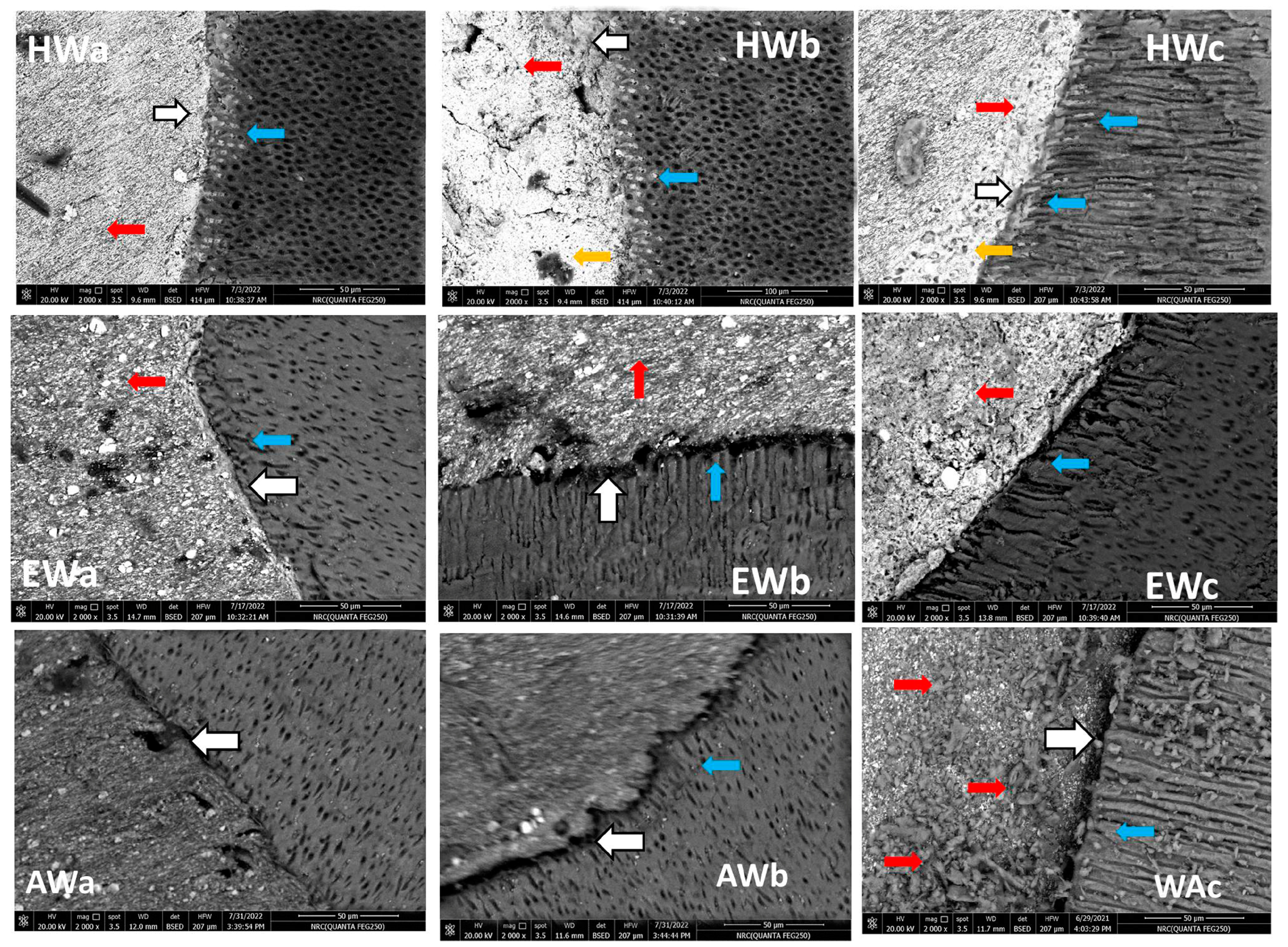
2.2. Dentinal Tubules Adaptation and Penetration Test of Sealers Using Scanning Electron Microscopy (SEM)
2.3. Raman Spectroscopy Analysis
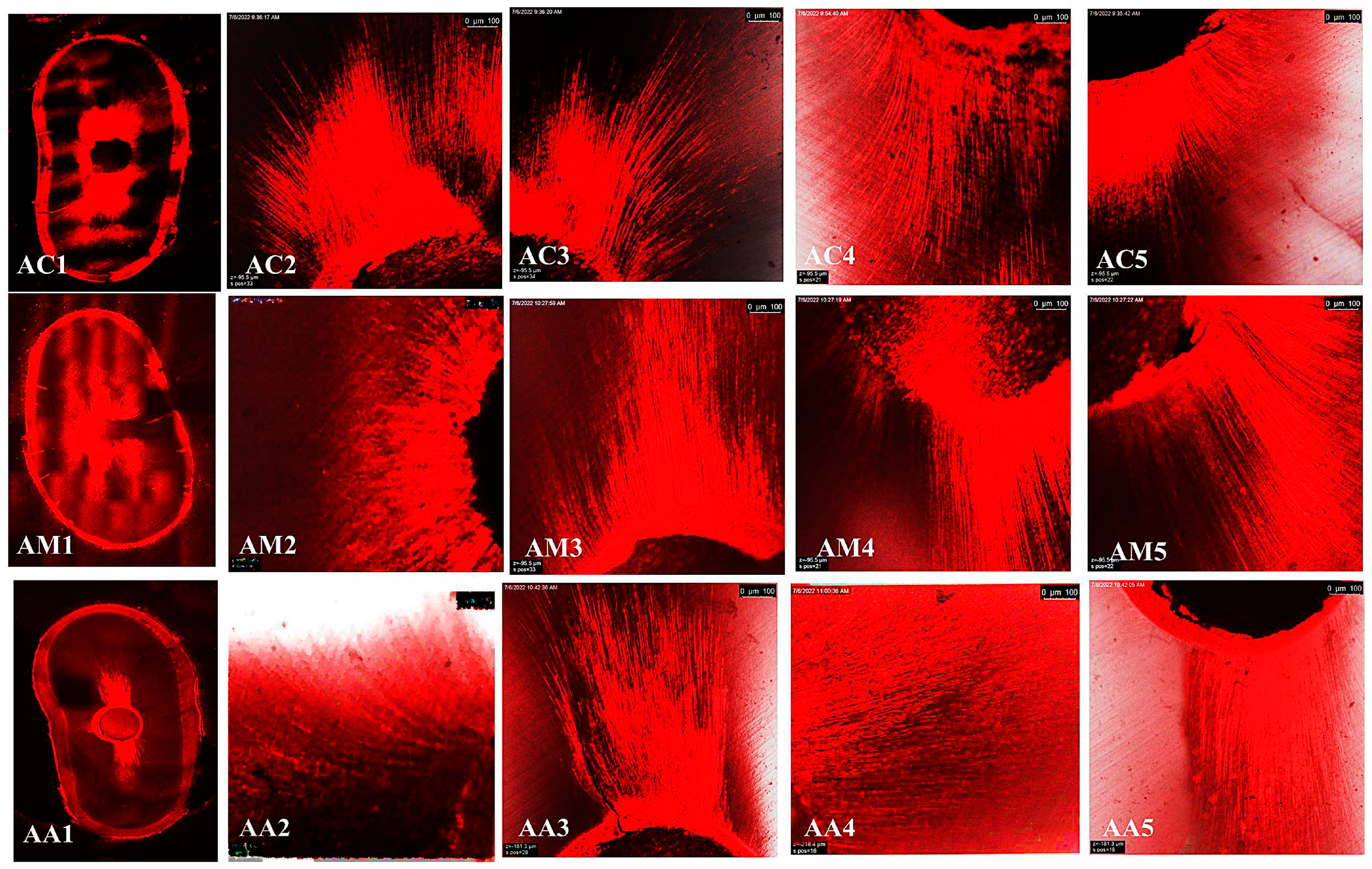
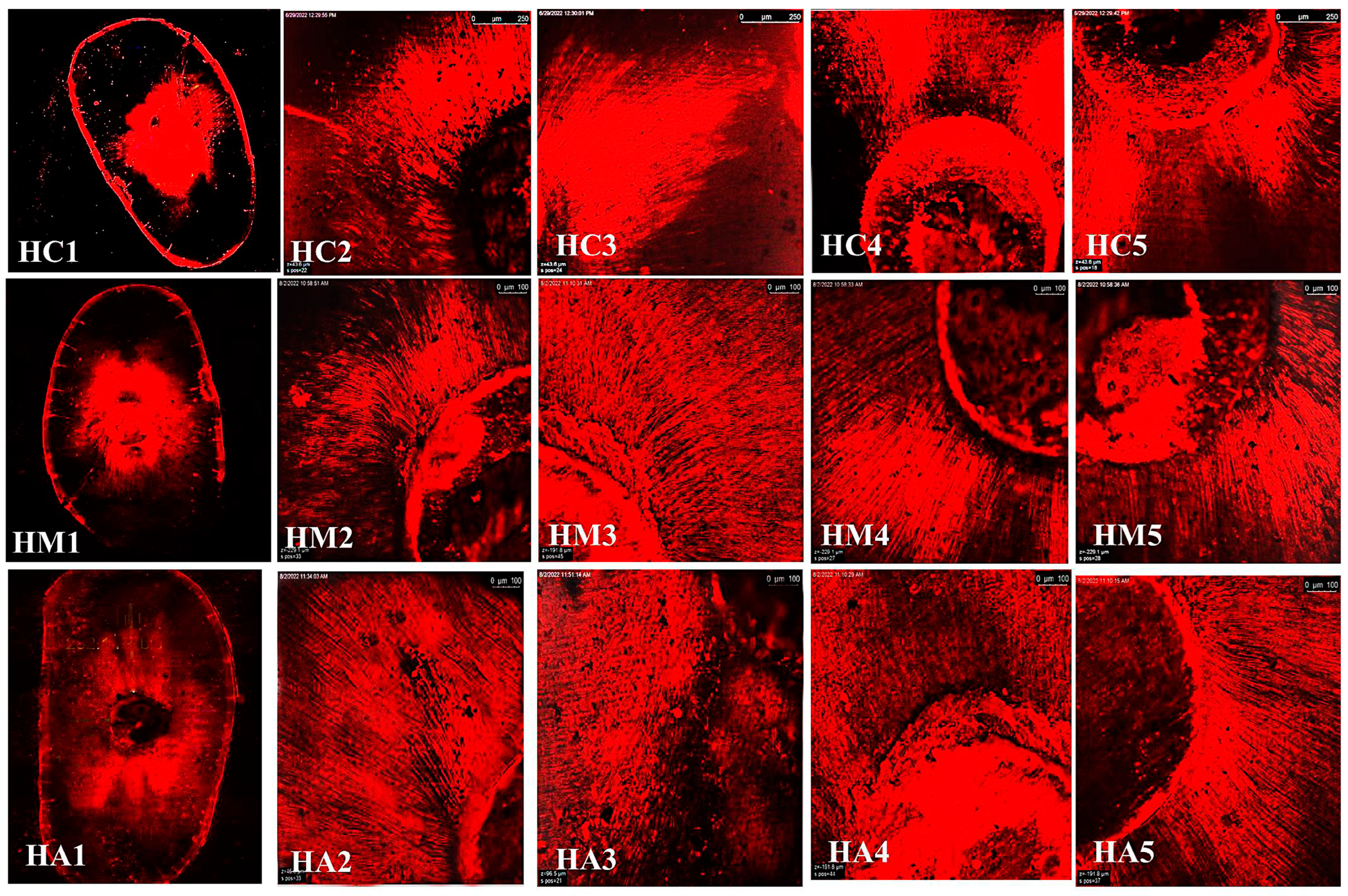
2.4. Depth Penetration Analysis Using Confocal Laser Scanning Microscopy (CLSM):
2.5. Flow/Film Thickness Test
2.6. Statistical Analysis
3. Results
3.1. SEM
3.2. Raman Spectroscopy
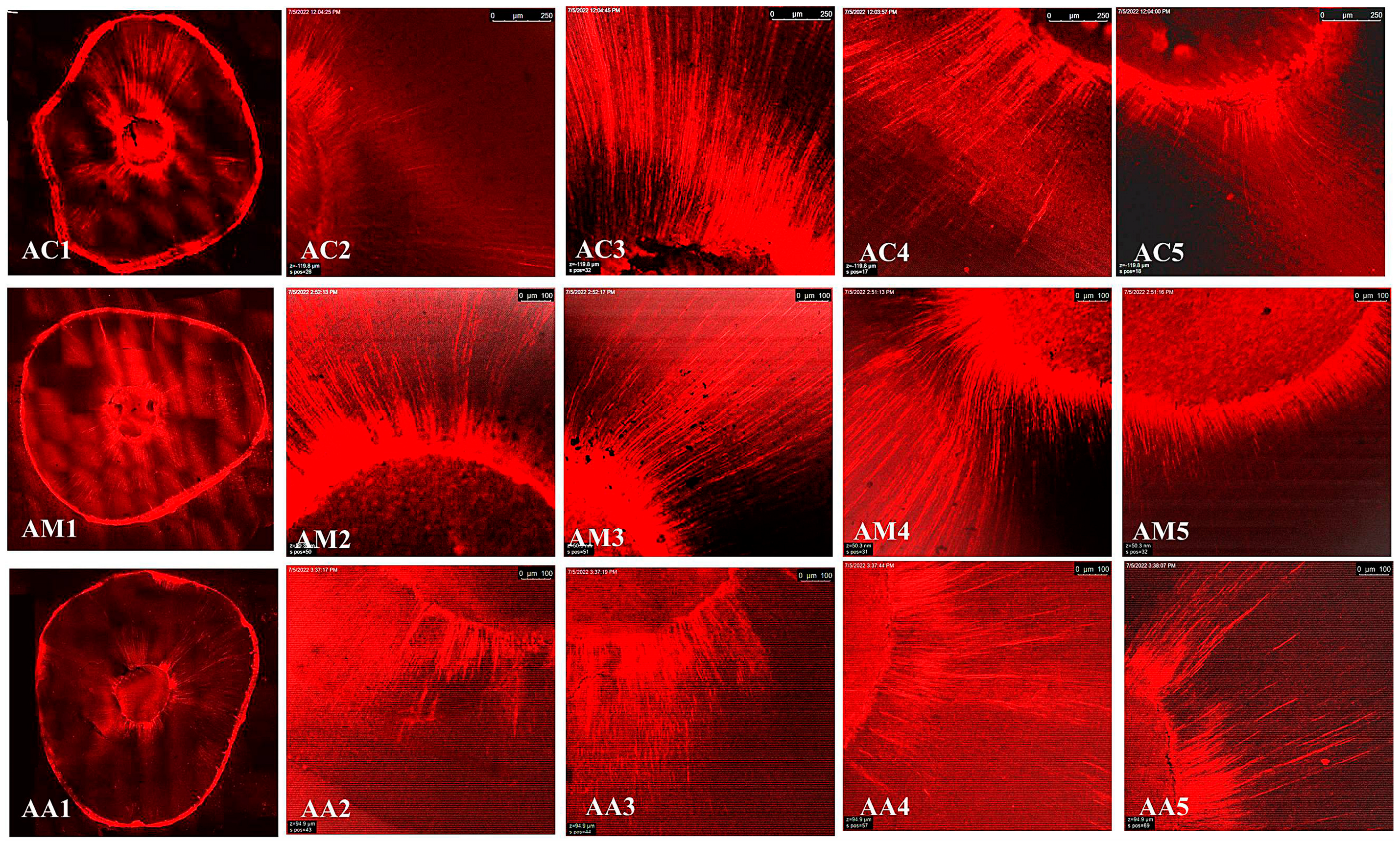
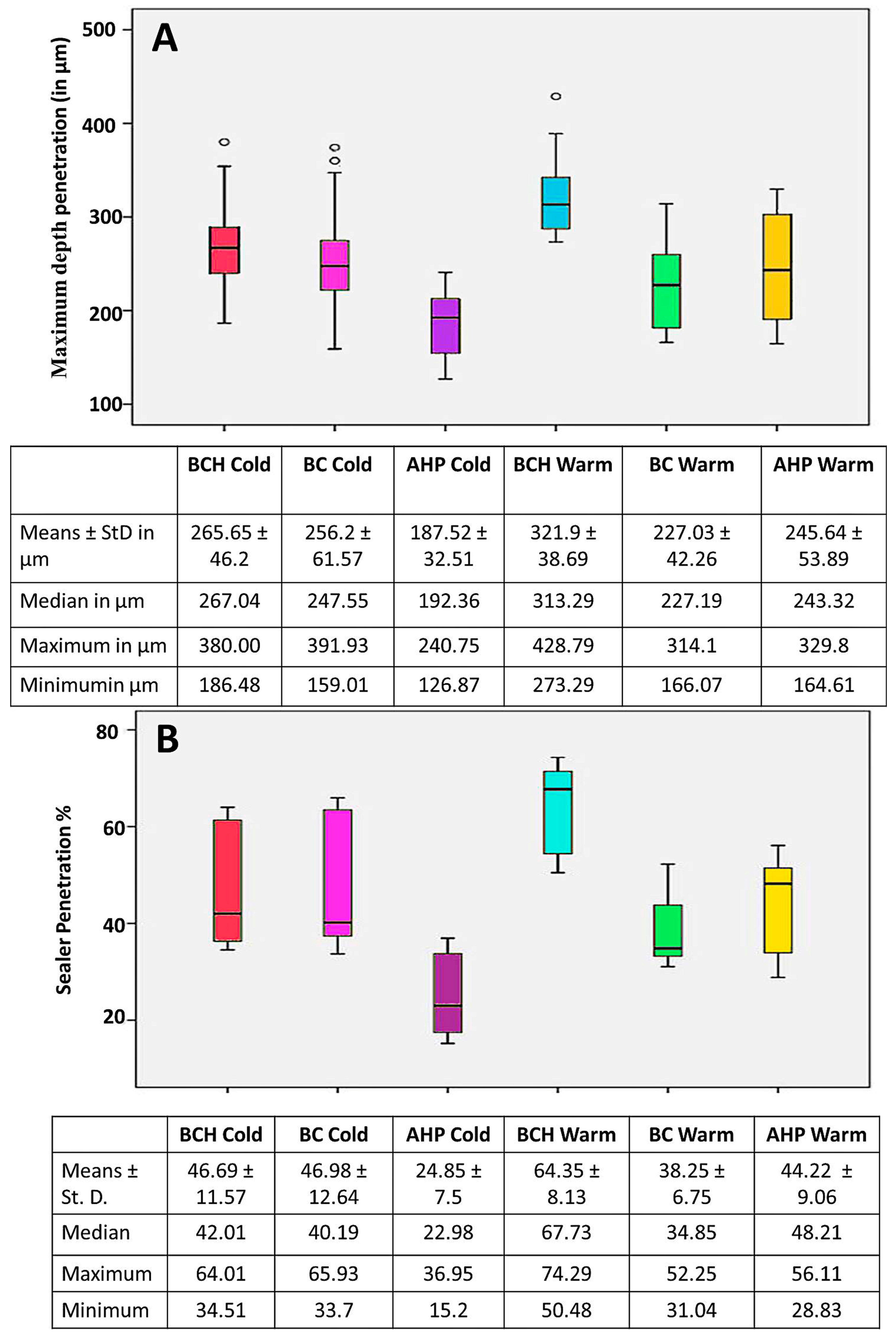
3.3. Confocal Laser Scanning Microscopy (CLSM)
Maximum Depth Penetration and Penetration Percent (%)
3.4. Flow and Film Thickness
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schilder, H. Filling root canals in three dimensions. Dent. Clin. N. Am. 1967, 16, 723. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schmidt, S.; Rohrbach, A.; Berlandi, J.; Bürklein, S.; Schäfer, E. Debunking the Concept of Dentinal Tubule Penetration of Endodontic Sealers: Sealer Staining with Rhodamine B Fluorescent Dye Is an Inadequate Method. Materials 2021, 14, 3211. [Google Scholar] [CrossRef]
- Wu, M.-K.; Van Der Sluis, L.W.; Wesselink, P.R. Fluid transport along gutta-percha backfills with and without sealer. J. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, M.A.; White, R.R.; Walton, R.E. Determining the optimal obturation length: A meta-analysis of literature. J. Endod. 2005, 31, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.Y.; Kutty, M.G.; Che Ab Aziz, Z.A. Push-out bond strength of experimental apatite calcium phosphate based coated gutta-percha. Int. J. Biomater. 2018, 2018, 1731857. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Davis, G.; Baysan, A. Physico-Chemical Properties of Calcium-Silicate vs. Resin Based Sealers—A Systematic Review and Meta-Analysis of Laboratory-Based Studies. Materials 2022, 15, 229. [Google Scholar] [CrossRef]
- AbuZeid, S.T.; Alnoury, A. Characterisation of the Bioactivity and the Solubility of a New Root Canal Sealer. Int. Dent. J. 2023, 73, 760–769. [Google Scholar] [CrossRef]
- Candeiro, G.T.d.M.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of Radiopacity, pH, Release of Calcium Ions, and Flow of a Bioceramic Root Canal Sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- Estivalet, M.S.; de Araújo, L.P.; Immich, F.; da Silva, A.F.; Ferreira, N.d.S.; da Rosa, W.L.d.O.; Piva, E. Bioactivity Potential of Bioceramic-Based Root Canal Sealers: A Scoping Review. Life 2022, 12, 1853. [Google Scholar] [CrossRef]
- Abu Zeid, S.T.; Mokeem Saleh, A.A.; Khafagi, M.G.E.-D.; Abou Neel, E.A. Setting reaction of new bioceramic root canal sealers. Spectrosc. Lett. 2018, 51, 426–430. [Google Scholar] [CrossRef]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Šimundić Munitić, M.; Poklepović Peričić, T.; Utrobičić, A.; Bago, I.; Puljak, L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: A systematic review. PLoS ONE 2019, 14, e0223575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Ungor, M.; Onay, E.; Orucoglu, H. Push-out bond strengths: The Epiphany–Resilon endodontic obturation system compared with different pairings of Epiphany, Resilon, AH Plus and gutta-percha. Int. Endod. J. 2006, 39, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.-f.; Haapasalo, M. Physical Properties of 5 Root Canal Sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Bai, W.; Liang, Y.; Gao, X.J. Real temperature of the continuous-wave pluggers. Beijing Da Xue Xue Bao Yi Xue Ban 2015, 47, 834–837. [Google Scholar]
- Brasseler USA. TotalFill® BC Sealer™ & BC Sealer HiFlow. Available online: http://media.brasselerusa.com/userfiles/IFU%2CManuals%2CBrochures/B_5019_ENG_HiFlow%20NPR.pdf (accessed on 1 November 2022).
- Donnermeyer, D.; Ibing, M.; Bürklein, S.; Weber, I.; Reitze, M.P.; Schäfer, E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: Hydraulic calcium silicate-based sealers. Materials 2021, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Brasseler USA. BUSA. Redefining Endodontics, Bioceramic Technology. Available online: https://brasselerusadental.com/wp-content/uploads/sites/9/2017/11/B_3644_Bioceramic-Guide.pdf (accessed on 1 January 2023).
- Cimpean, S.I.; Burtea, A.L.C.; Chiorean, R.S.; Dudescu, M.C.; Antoniac, A.; Robu, A.; Campian, R.S.; Timis, L.I. Evaluation of Bond Strength of Four Different Root Canal Sealers. Materials 2022, 15, 4966. [Google Scholar] [CrossRef]
- Silva, E.J.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T.K.d.S. Solubility of bioceramic-and epoxy resin-based root canal sealers: A systematic review and meta-analysis. Aust. Endod. J. 2021, 47, 690–702. [Google Scholar] [CrossRef]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.A.; Berzins, D.W.; Bahcall, J.K. An in vitro comparison of bond strength of various obturation materials to root canal dentin using a push-out test design. J. Endod. 2007, 33, 856–858. [Google Scholar] [CrossRef]
- De-Deus, G.; Reis, C.; Di Giorgi, K.; Brandão, M.C.; Audi, C.; Fidel, R.A.S. Interfacial adaptation of the Epiphany self-adhesive sealer to root dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 381–386. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, R.; Khalil, I.; Le Brun, G.; Pellen, F.; Le Jeune, B.; Daou, M.; El Osta, N.; Naaman, A.; Abboud, M. Dentinal tubule penetration of AH Plus, BC Sealer and a novel tricalcium silicate sealer: A confocal laser scanning microscopy study. Clin. Oral Investig. 2019, 23, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- ISO 6876; Dental Root Canal Sealing Materials. International Standardization Organization: Geneva, Swizerland. Available online: https://www.iso.org/standard/45117.html (accessed on 5 February 2023).
- Yamauchi, S.; Watanabe, S.; Okiji, T. Effects of heating on the physical properties of premixed calcium silicate-based root canal sealers. J. Oral Sci. 2021, 63, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, S.; Edrees, H.Y.; Mokeem Saleh, A.A.; Alothmani, O.S. Physicochemical properties of two generations of MTA-based root canal sealers. Materials 2021, 14, 5911. [Google Scholar] [CrossRef] [PubMed]
- Retana-Lobo, C.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Benavides-García, M.; Hernández-Meza, E.; Reyes-Carmona, J. Push-out bond strength, characterization, and ion release of premixed and powder-liquid bioceramic sealers with or without gutta-percha. Scanning 2021, 2021, 6617930. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.; Rao, H.M. Gutta-percha in endodontics-A comprehensive review of material science. J. Conserv. Dent. Endod. 2019, 22, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, K.; Jin, Q.; Zhao, F.; Huang, Z. Presence of voids after three obturation techniques in band-shaped isthmuses: A micro-computed tomography study. BMC Oral Health 2021, 21, 227. [Google Scholar] [CrossRef]
- Bouillaguet, S.; Shaw, L.; Barthelemy, J.; Krejci, I.; Wataha, J. Long-term sealing ability of pulp canal sealer, AH-Plus, GuttaFlow and epiphany. Int. Endod. J. 2008, 41, 219–226. [Google Scholar] [CrossRef]
- Patri, G.; Agrawal, P.; Anushree, N.; Arora, S.; Kunjappu, J.J.; Shamsuddin, S.V. A scanning electron microscope analysis of sealing potential and marginal adaptation of different root canal sealers to dentin: An in vitro study. J. Contemp. Dent. Pract. 2020, 21, 73–77. [Google Scholar] [PubMed]
- Al-Haddad, A.; Kasim, N.H.A.; Ab Aziz, Z.A.C. Interfacial adaptation and thickness of bioceramic-based root canal sealers. Dent. Mater. J. 2015, 34, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-based root canal sealers: A review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Guimarães, B.M.; Ordinola-Zapata, R.; Bramante, C.M.; Cavenago, B.C.; Garcia, R.B.; Bernardineli, N.; Andrade, F.B.; Moraes, I.G.; Duarte, M.A. Physical properties and interfacial adaptation of three epoxy resin–based sealers. J. Endod. 2011, 37, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, S.T.; Alamoudi, R.A.; Mokeem Saleh, A.A. Impact of Water Solubility on Chemical Composition and Surface Structure of Two Generations of Bioceramic Root Canal Sealers. Appl. Sci. 2022, 12, 873. [Google Scholar] [CrossRef]
- Weis, M.V.; Parashos, P.; Messer, H. Effect of obturation technique on sealer cement thickness and dentinal tubule penetration. Int. Endod. J. 2004, 37, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kwak, S.W.; Ha, J.-H.; Lee, W.; Kim, H.-C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017, 2017, 2582849. [Google Scholar] [CrossRef]
- Chen, B.; Haapasalo, M.; Mobuchon, C.; Li, X.; Ma, J.; Shen, Y. Cytotoxicity and the effect of temperature on physical properties and chemical composition of a new calcium silicate–based root canal sealer. J. Endod. 2020, 46, 531–538. [Google Scholar] [CrossRef]
- Bernardes, R.A.; de Amorim Campelo, A.; Junior, D.S.S.; Pereira, L.O.; Duarte, M.A.H.; Moraes, I.G.; Bramante, C.M. Evaluation of the flow rate of 3 endodontic sealers: Sealer 26, AH Plus, and MTA Obtura. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e47–e49. [Google Scholar] [CrossRef]
- Yadav, S.; Nawal, R.R.; Chaudhry, S.; Talwar, S. Assessment of quality of root canal filling with C Point, Guttacore and Lateral Compaction Technique: A confocal laser scanning microscopy study. Eur. Endod. J. 2020, 5, 236–241. [Google Scholar]
- Lacey, S.; Pitt Ford, T.; Yuan, X.F.; Sherriff, M.; Watson, T. The effect of temperature on viscosity of root canal sealers. Int. Endod. J. 2006, 39, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Camilleri, J. Investigation of the effect of sealer use on the heat generated at the external root surface during root canal obturation using warm vertical compaction technique with System B heat source. J. Endod. 2014, 40, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Belli, S.; Eraslan, O.; Eskitascioglu, G.; Karbhari, V. Monoblocks in root canals: A finite elemental stress analysis study. Int. Endod. J. 2011, 44, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Monoblocks in root canals: A hypothetical or a tangible goal. J. Endod. 2007, 33, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Trope, M. Gutta-percha: The end of an era. Alpha Omegan 2004, 97, 16–22. [Google Scholar]
- Meneses, C.B.; Gambini, A.F.; Olivi, L.T.; Dos Santos, M.; Sipert, C.R. Effect of CPoint, EndoSequence BC, and gutta-percha points on viability and gene expression of periodontal ligament fibroblasts. Eur. Endod. J. 2019, 4, 57. [Google Scholar] [PubMed]
- Ørstavik, D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Alegre, A.C.; Verdú, S.A.; López, J.I.Z.; Alcina, E.P.; Climent, J.R.; Sabater, A.P. Intratubular penetration capacity of HiFlow bioceramic sealer used with warm obturation techniques and single cone: A confocal laser scanning microscopic study. Heliyon 2022, 8, e10388. [Google Scholar] [CrossRef]





| Technique | Cold Compaction | Warm Compaction | |||||
|---|---|---|---|---|---|---|---|
| Sealers | BCH | BC | AHP | BCH | BC | AHP | |
| Means ± SD (in µm) of Film thickness detected by SEM images. | at cervical | 25.98 ± 5.58 | 40.98 ± 6.29 | 65.39 ± 6.37 | 22.93 ± 4.27 | 24.01 ± 6.22 * | 22.55 ± 2.09 |
| at Middle | 21.57 ± 7.14 | 33.66 ± 5.29 | 64.89 ± 9.53 * | 15.66 ± 5.4 | 21.15 ± 3.4 * | 18.53 ± 2.77 | |
| at apical | 21.56 ± 4.46 | 26.34 ± 3.81 | 57.84 ± 9.75 * | 11.3 ± 1.72 | 16.7 ± 1.54 * | 10.67 ± 0.85 | |
| Flow (mm) on glass plate Means ± SD (in mm) Median | 19.45 ± 1.09 (20) | 23.35 ± 0.47 * (23) | 18.35 ± 0.47 (18) | 25.4 ± 1.26 (25.00) | 24.35 ± 0.47 * (24.00) | 19.35 ± 0.47 (19) | |
| Film thickness on glass plate (µm) | 0.4 | 0.55 * | 0.5 | 0.4 | 0.8 * | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zeid, S.T.; Alamoudi, R.A. Comparative Analysis of Interfacial Adaptation and Depth Penetration of Recent HiFlow versus Regular Bioceramic Sealers in Conjunction with BC Gutta-Percha Points Using Two Different Obturation Techniques—A Preliminary Report of an Ex Vivo Study. J. Funct. Biomater. 2024, 15, 134. https://doi.org/10.3390/jfb15050134
Abu-Zeid ST, Alamoudi RA. Comparative Analysis of Interfacial Adaptation and Depth Penetration of Recent HiFlow versus Regular Bioceramic Sealers in Conjunction with BC Gutta-Percha Points Using Two Different Obturation Techniques—A Preliminary Report of an Ex Vivo Study. Journal of Functional Biomaterials. 2024; 15(5):134. https://doi.org/10.3390/jfb15050134
Chicago/Turabian StyleAbu-Zeid, Sawsan T., and Ruaa A. Alamoudi. 2024. "Comparative Analysis of Interfacial Adaptation and Depth Penetration of Recent HiFlow versus Regular Bioceramic Sealers in Conjunction with BC Gutta-Percha Points Using Two Different Obturation Techniques—A Preliminary Report of an Ex Vivo Study" Journal of Functional Biomaterials 15, no. 5: 134. https://doi.org/10.3390/jfb15050134





