Genome-Wide Identification and Expression Analysis of the Broad-Complex, Tramtrack, and Bric-à-Brac Domain-Containing Protein Gene Family in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Sequence Analysis of the StBTB Gene Family
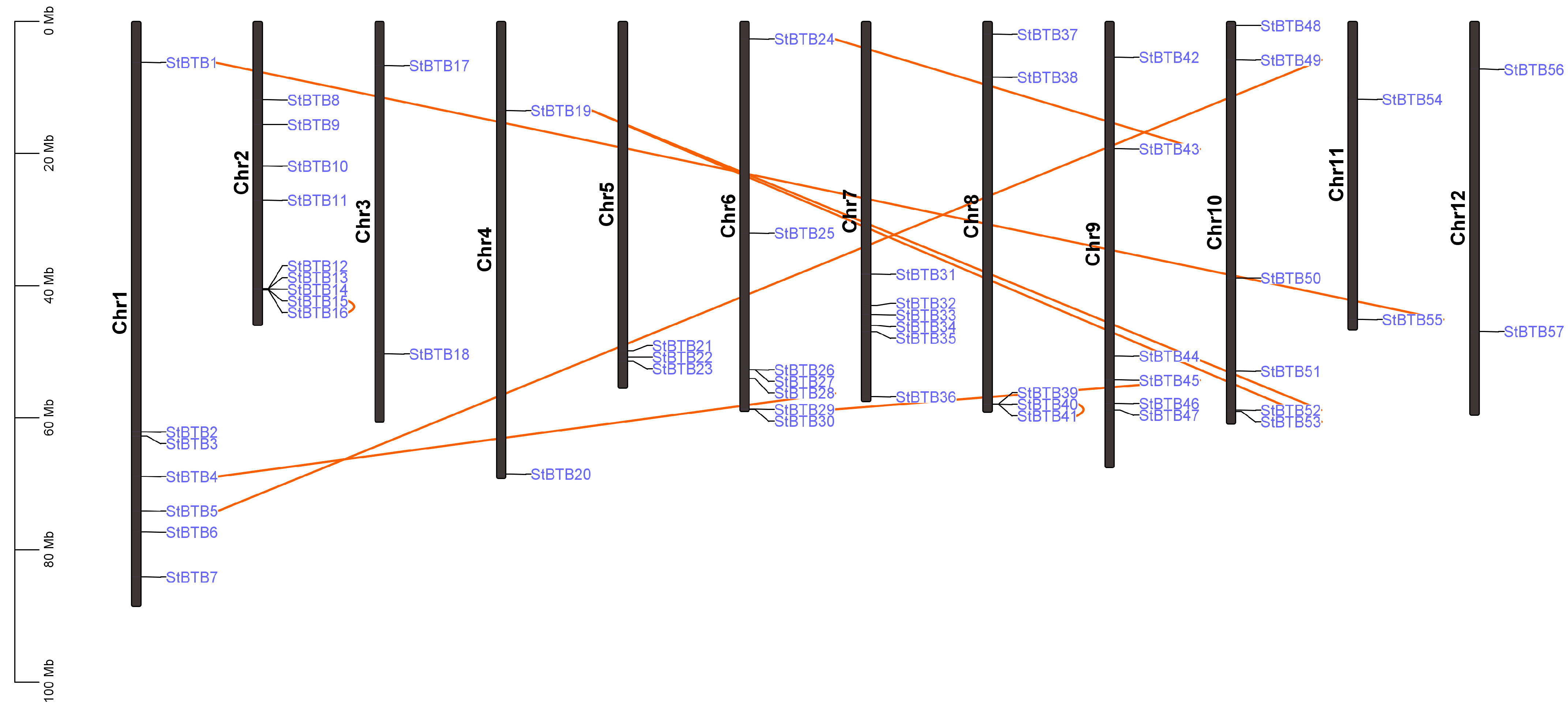
2.2. Chromosomal Distribution and Gene Duplication Event
2.3. Gene Structure and Conserved Motif Analyses of the StBTBs
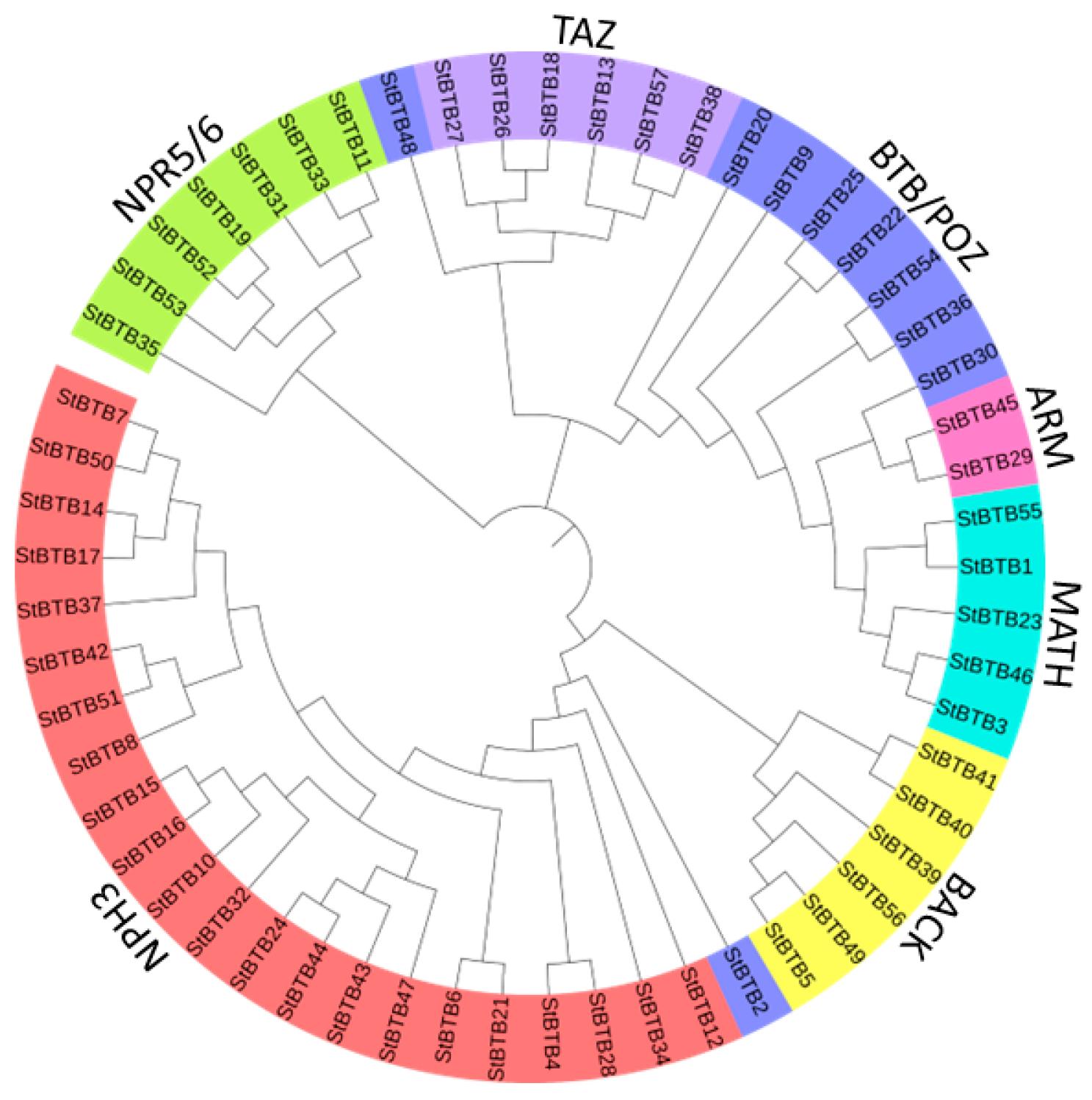
2.4. Multiple Sequence Alignment and Phylogenetic Tree Construction
2.5. Tissue-Specific Expression Analysis of StBTBs
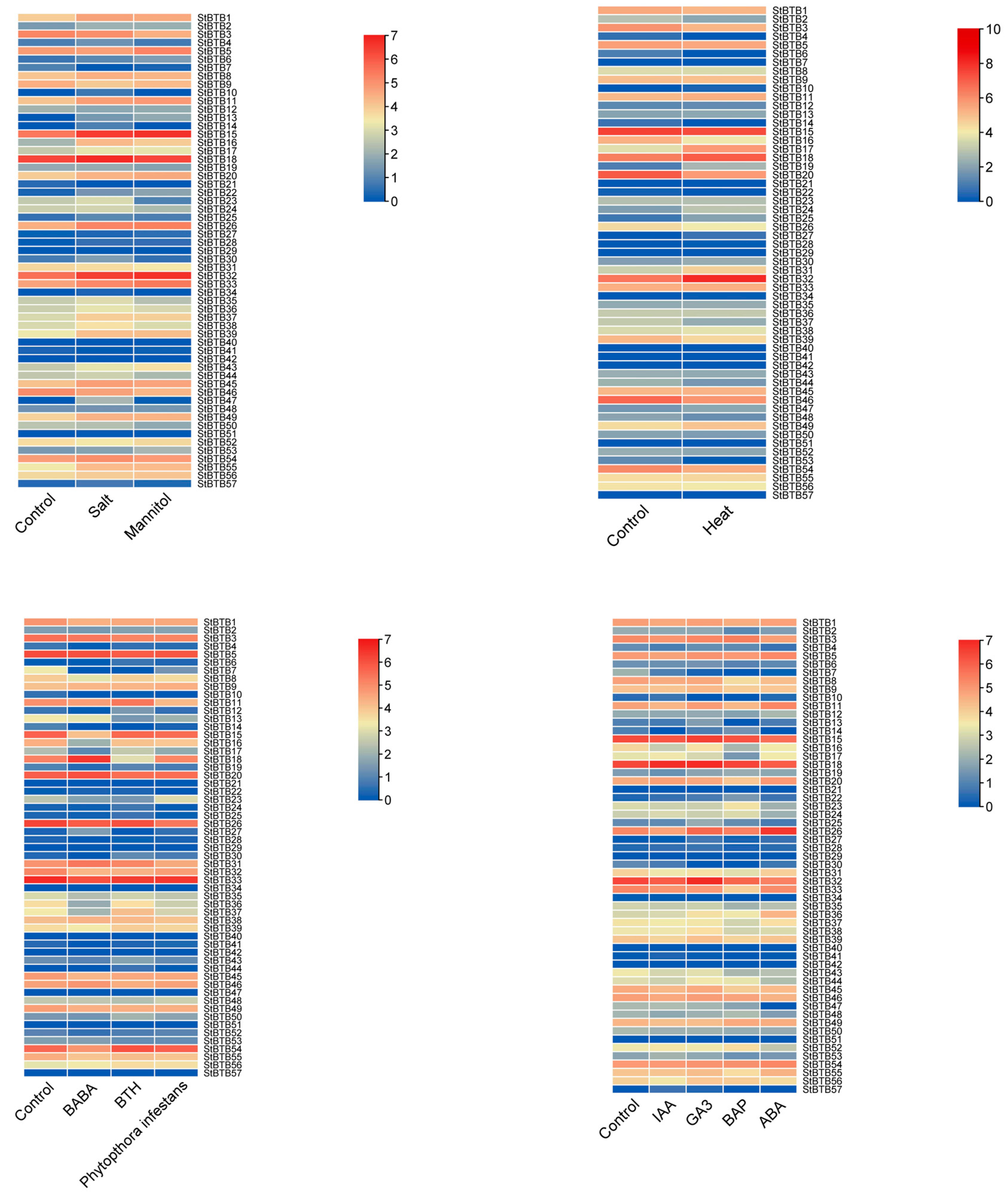
2.6. Expression Analysis of StBTBs under Various Hormonal and Stress Treatments
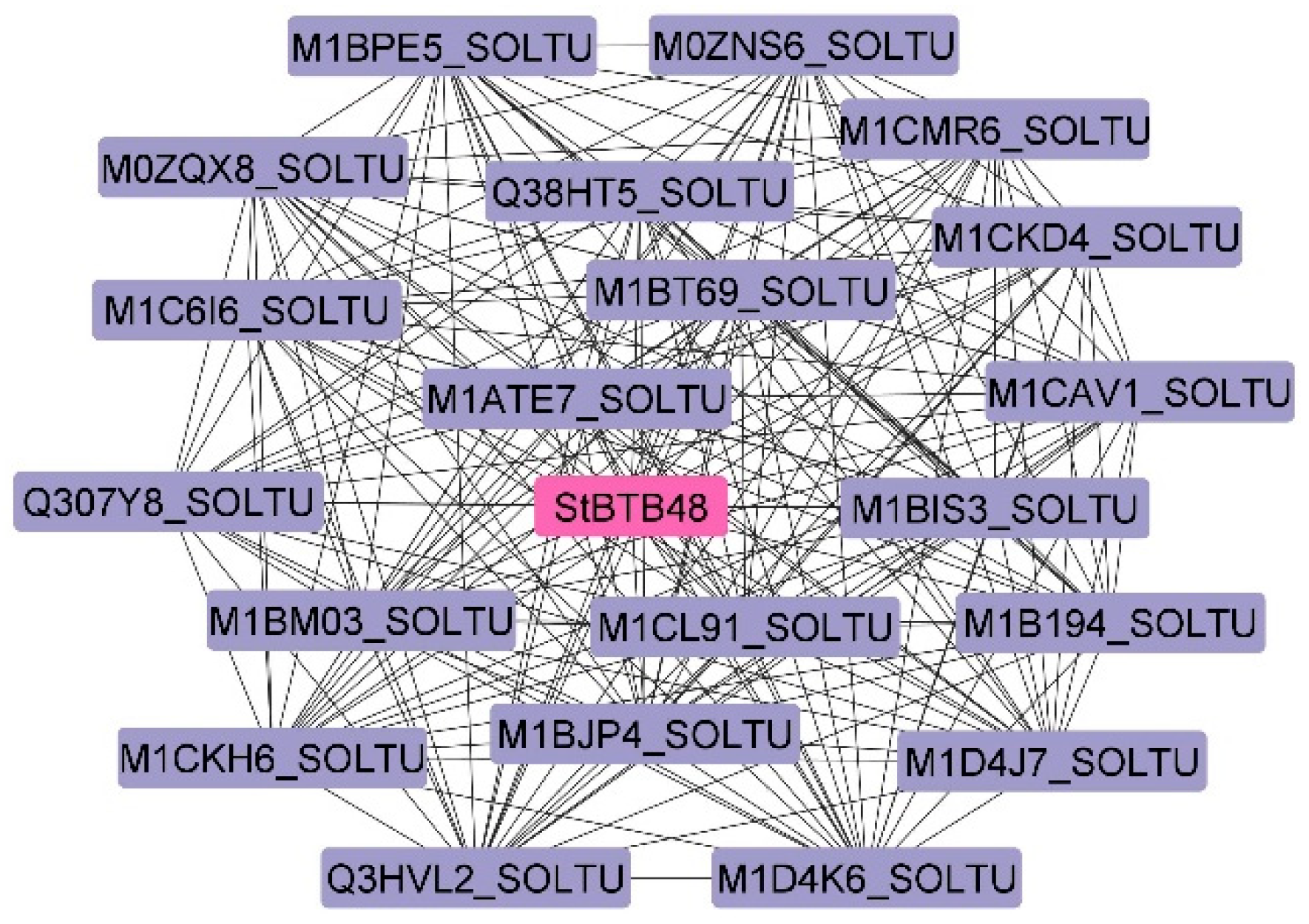
2.7. Protein–Protein Interaction and GO Analysis
2.8. microRNA Target Site Analysis
3. Results
3.1. Identification of BTB Genes in Potato and Their Sequence Analysis
3.2. Chromosomal Mapping and Gene Duplication Analysis
3.3. Gene Structure and Conserved Motif Analysis
3.4. Phylogenetic Analysis
3.5. Tissue-Specific Gene Expression Analysis of StBTBs
3.6. Expression Profile Analysis under Hormonal and Stress Treatments
3.7. Protein–Protein Interaction and GO Analysis
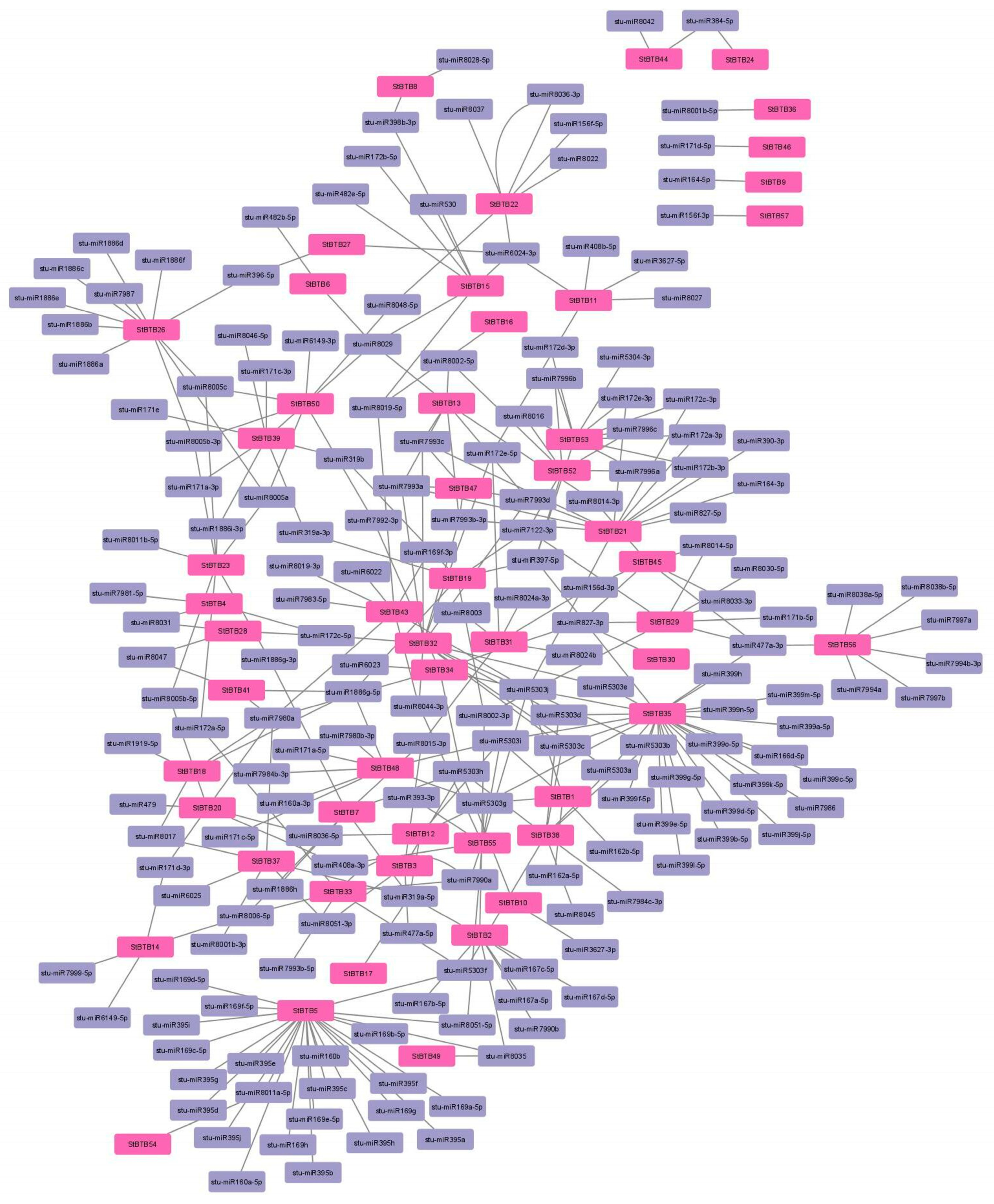
3.8. microRNA Target Site Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bardwell, V.J.; Treisman, R. The POZ domain: A conserved protein-protein interaction motif. Genes Dev. 1994, 8, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Zollman, S.; Godt, D.; Privé, G.G.; Couderc, J.L.; Laski, F.A. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc. Natl. Acad. Sci. USA 1994, 91, 10717–10721. [Google Scholar] [CrossRef]
- Perez-Torrado, R.; Yamada, D.; Defossez, P.A. Born to bind: The BTB protein–protein interaction domain. Bioessays 2006, 28, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.; Nandra, S.K.; Privé, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.F.; Engel, C.K.; Privé, G.G. Crystal structure of the BTB domain from PLZF. Proc. Natl. Acad. Sci. USA 1998, 95, 12123–12128. [Google Scholar] [CrossRef] [PubMed]
- Bonchuk, A.; Balagurov, K.; Georgiev, P. BTB domains: A structural view of evolution, multimerization, and protein–protein interactions. Bioessays 2023, 45, 2200179. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Wang, W.; Xiong, Y. Cullin-RING E3 ubiquitin ligases: Bridges to destruction. Macromol. Protein Complexes Struct. Funct. 2017, 83, 323–347. [Google Scholar]
- Chaharbakhshi, E.; Jemc, J.C. Broad-complex, tramtrack, and bric-à-brac (BTB) proteins: Critical regulators of development. Genesis 2016, 54, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Shalmani, A.; Ullah, U.; Muhammad, I.; Zhang, D.; Sharif, R.; Jia, P.; Saleem, N.; Gul, N.; Rakhmanova, A.; Tahir, M.M.; et al. The TAZ domain-containing proteins play important role in the heavy metals stress biology in plants. Environ. Res. 2021, 197, 111030. [Google Scholar] [CrossRef] [PubMed]
- Araus, V.; Vidal, E.A.; Puelma, T.; Alamos, S.; Mieulet, D.; Guiderdoni, E.; Gutiérrez, R.A. Members of BTB gene family of scaffold proteins suppress nitrate uptake and nitrogen use efficiency. Plant Physiol. 2016, 171, 1523–1532. [Google Scholar]
- Ahmad, K.F.; Melnick, A.; Lax, S.; Bouchard, D.; Liu, J.; Kiang, C.L.; Mayer, S.; Takahashi, S.; Licht, J.D.; Privé, G.G. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol. Cell 2003, 12, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.; Beutler, B. The BTB-ZF transcription factors. Cell Cycle 2012, 11, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sato, K.; Yamamoto, D. Sex-switching of the Drosophila brain by two antagonistic chromatin factors. Fly 2013, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H. Zinc finger and BTB domain-containing protein 3 is essential for the growth of cancer cells. BMB Rep. 2014, 47, 405. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Wee, S.; Anderson, S.; Yates, J.; Wolf, D.A. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell 2003, 12, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Quint, A.; Brand, D.; Vivian-Smith, A.; Offringa, R. BTB and TAZ domain scaffold proteins perform a crucial function in Arabidopsis development. Plant J. 2009, 58, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, K.K.; Misra, A.; Ren, S.; McKnight, T.D. BT2, a BTB protein, mediates multiple responses to nutrients, stresses, and hormones in Arabidopsis. Plant Physiol. 2009, 150, 1930–1939. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, H.; He, S.; Zhu, H.; Gao, S.; Xing, S.; Wei, Z.; Zhao, N.; Liu, Q. The sweetpotato BTB-TAZ protein gene, IbBT4, enhances drought tolerance in transgenic Arabidopsis. Front. Plant Sci. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Peng, L.; Xiong, J.; Li, X.; Wang, J.; Li, X.; Yang, Y. AtSIBP1, a novel BTB domain-containing protein, positively regulates salt signaling in Arabidopsis thaliana. Plants 2019, 8, 573. [Google Scholar] [CrossRef]
- Cao, H.; Li, X.; Dong, X. Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 6531–6536. [Google Scholar] [CrossRef]
- Zhao, M.; Ge, Y.; Xu, Z.; Ouyang, X.; Jia, Y.; Liu, J.; Zhang, M.; An, Y. A BTB/POZ domain-containing protein negatively regulates plant immunity in Nicotiana benthamiana. Biochem. Biophys. Res. Commun. 2022, 600, 54–59. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Shumayla, S.S.; Kumar, R.; Mendu, V.; Singh, K.; Upadhyay, S.K. Genomic dissection and expression profiling revealed functional divergence in Triticum aestivum leucine rich repeat receptor like kinases (TaLRRKs). Front. Plant Sci. 2016, 7, 1374. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Su, X.; Wang, Y.; Yang, W.; Pan, Y.; Su, C.; Zhang, X. Genome-wide identification and expression analysis of the BTB domain-containing protein gene family in tomato. Genes Genom. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Bhuria, M.; Verma, D.; Garewal, N.; Singh, K. Genome-Wide Identification of BTB Domain-Containing Gene Family in Grapevine (Vitis vinifera L.). Agriculture 2023, 13, 252. [Google Scholar] [CrossRef]
- Motchoulski, A.; Liscum, E. Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 1999, 286, 961–964. [Google Scholar] [CrossRef]
- Jun, J.H.; Ha, C.M.; Fletcher, J.C. BLADE-ON-PETIOLE1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating ASYMMETRIC LEAVES2. Plant Cell 2010, 22, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, H.R.; Joshi, R.; Pareek, A.; Singla-Pareek, S.L. MATH-domain family shows response toward abiotic stress in Arabidopsis and rice. Front. Plant Sci. 2016, 7, 923. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Bernhardt, A.; Dieterle, M.; Hano, P.; Mutlu, A.; Estelle, M.; Genschik, P.; Hellmann, H. Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 2005, 137, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.J.; Hanada, K.; Shiu, S.H.; Vierstra, R.D. Large-scale, lineage-specific expansion of a bric-à-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell 2007, 19, 2329–2348. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.M.; Martínez-García, V.; Lefebvre, S. Phylogeny of the TRAF/MATH domain. In TNF Receptor Associated Factors (TRAFs); Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–24. [Google Scholar]
- Qi, P.; Yan, H.; PeiFen, D.; QingXuan, X.; Ying, R.; ChunLin, L. Construction of SSH library with different stages of seeds development in Brassica napus L. Acta Agron. Sin. 2009, 35, 1576–1583. [Google Scholar]
- Zhao, L.; Huang, Y.; Hu, Y.; He, X.; Shen, W.; Liu, C.; Ruan, Y. Phylogenetic Analysis of Brassica rapa MATH-Domain Proteins. Curr. Genom. 2013, 14, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, H.I.; Korea Kumho Petrochemical Co., Ltd. Nucleic Acid Molecule Encoding an Armadillo Repeat Protein, Aria and a Method Utilizing Aria to Generate Salt Tolerant Plants. U.S. Patent 7,049,482, 3 May 2006. [Google Scholar]
- Juranić, M.; Srilunchang, K.O.; Krohn, N.G.; Leljak-Levanić, D.; Sprunck, S.; Dresselhaus, T. Germline-specific MATH-BTB substrate adaptor MAB1 regulates spindle length and nuclei identity in maize. Plant Cell 2012, 24, 4974–4991. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.J.; Gagne, J.M.; Salter, D.W.; Hellmann, H.; Estelle, M.; Ma, L.; Vierstra, R.D. Cullins 3a and 3b assemble with members of the broad complex/ tramtrack/ bric-à-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 2005, 280, 18810–18821. [Google Scholar] [CrossRef] [PubMed]
- Shalmani, A.; Huang, Y.B.; Chen, Y.B.; Muhammad, I.; Li, B.B.; Ullah, U.; Jing, X.Q.; Bhanbhro, N.; Liu, W.T.; Li, W.Q.; et al. The highly interactive BTB domain targeting other functional domains to diversify the function of BTB proteins in rice growth and development. Int. J. Biol. Macromol. 2021, 192, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Juranić, M.; Dresselhaus, T. Phylogenetic analysis of the expansion of the MATH-BTB gene family in the grasses. Plant Signal Behav. 2014, 9, e28242. [Google Scholar] [CrossRef]
- Ren, S.; Mandadi, K.K.; Boedeker, A.L.; Rathore, K.S.; McKnight, T.D. Regulation of telomerase in Arabidopsis by BT2, an apparent target of TELOMERASE ACTIVATOR1. Plant Cell 2007, 19, 23–31. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Liu, K.K.; Zhang, H.X.; Cheng, G.X.; Ali, M.; Ul Haq, S.; Wei, A.M.; Gong, Z.H. Contribution of CaBPM4, a BTB domain–containing gene, to the response of pepper to Phytophthora capsici infection and abiotic stresses. Agronomy 2019, 9, 417. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, H.; Li, R.; Han, D.; Wang, L.; Wu, J.; Xu, P.; Zhang, S. GmBTB/POZ, a novel BTB/POZ domain-containing nuclear protein, positively regulates the response of soybean to Phytophthora sojae infection. Mol. Plant Pathol. 2019, 20, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Li, H.L.; Qiao, Z.W.; Zhang, J.C.; Sun, W.J.; Wang, C.K.; Yang, K.; You, C.X.; Hao, Y.J. The BTB-TAZ protein MdBT2 negatively regulates the drought stress response by interacting with the transcription factor MdNAC143 in apple. Plant Sci. 2020, 301, 110689. [Google Scholar] [CrossRef] [PubMed]
- Woodger, F.J.; Jacobsen, J.V.; Gubler, F. GMPOZ, a BTB/POZ domain nuclear protein, is a regulator of hormone responsive gene expression in barley aleurone. Plant Cell Physiol. 2004, 45, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Liu, W.; Meng, Q.; Zhang, W.; Chen, A.; Sun, S.; Xu, G. Identification of microRNAs in six solanaceous plants and their potential link with phosphate and mycorrhizal signaling. J. Integr. Plant Biol. 2014, 56, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Adam, H.; Díaz-Mendoza, M.; Zurczak, M.; Gonzalez-Schain, N.D.; Suarez-Lopez, P. Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 2009, 136, 2873–2881. [Google Scholar] [CrossRef]
- Pei, L.L.; Zhang, L.L.; Liu, X.; Jiang, J. Role of microRNA miR171 in plant development. PeerJ 2023, 11, e15632. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in abiotic stress response and characteristics regulation of plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef]



| Gene Name | SpudDB Accession Number | Chromosomal Location | CDS Length | AA Length | Subcellular Localization |
|---|---|---|---|---|---|
| StBTB1 | Soltu.DM.01G005950.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G005950.1, (accessed on 12 October 2023) | 1:6212257–6219717 | 1218 | 405 | Cytoplasm |
| StBTB2 | Soltu.DM.01G023290.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G023290.1, (accessed on 12 October 2023) | 1:62115639–62125961 | 2598 | 865 | Nucleus |
| StBTB3 | Soltu.DM.01G023810.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G023290.1, (accessed on 12 October 2023) | 1:62789505–62798820 | 1224 | 407 | Chloroplast |
| StBTB4 | Soltu.DM.01G029170.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G029170.1, (accessed on 12 October 2023) | 1:68879361–68877284 | 1725 | 574 | Chloroplast |
| StBTB5 | Soltu.DM.01G034510.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G034510.1, (accessed on 12 October 2023) | 1:74078735–74083692 | 1659 | 552 | Nucleus |
| StBTB6 | Soltu.DM.01G038390.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G038390.1, (accessed on 12 October 2023) | 1:77301342–77303414 | 1665 | 554 | Nucleus |
| StBTB7 | Soltu.DM.01G046570.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.01G046570.1, (accessed on 12 October 2023) | 1:84079985–84086687 | 1881 | 626 | Nucleus |
| StBTB8 | Soltu.DM.02G002620.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu (accessed on 12 October 2023) | 2:11879293–11874980 | 1890 | 629 | Nucleus |
| StBTB9 | Soltu.DM.02G003900.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G003900.1, (accessed on 12 October 2023) | 2:15605796–15599933 | 840 | 279 | Peroxisome |
| StBTB10 | Soltu.DM.02G007630.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G007630.1, (accessed on 12 October 2023) | 2:21886913–21882888 | 1275 | 424 | Cytoplasm |
| StBTB11 | Soltu.DM.02G012330.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G012330.1, (accessed on 12 October 2023) | 2:27057682–27063283 | 1746 | 581 | Nucleus |
| StBTB12 | Soltu.DM.02G027710.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G027710.1, (accessed on 12 October 2023) | 2:40452119–40448666 | 1467 | 488 | Chloroplast |
| StBTB13 | Soltu.DM.02G027750.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G027750.1, (accessed on 12 October 2023) | 2:40471945–40474925 | 1137 | 378 | Nucleus |
| StBTB14 | Soltu.DM.02G027770.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G027770.1, (accessed on 12 October 2023) | 2:40517486–40522346 | 1842 | 613 | Chloroplast |
| StBTB15 | Soltu.DM.02G027830.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G027830.1, (accessed on 12 October 2023) | 2:40600737–40596954 | 1791 | 596 | Nucleus |
| StBTB16 | Soltu.DM.02G027880.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.02G027880.1, (accessed on 12 October 2023) | 2:40646109–40645050 | 501 | 166 | Nucleus |
| StBTB17 | Soltu.DM.03G005510.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.03G005510.1, (accessed on 12 October 2023) | 3:6670844–6676484 | 1884 | 627 | Cytoplasm |
| StBTB18 | Soltu.DM.03G025100.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.03G025100.1, (accessed on 12 October 2023) | 3:50328857–50331675 | 1038 | 345 | Nucleus |
| StBTB19 | Soltu.DM.04G012100.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.04G012100.1, (accessed on 12 October 2023) | 4:13482468–13485467 | 1386 | 461 | Cytoplasm |
| StBTB20 | Soltu.DM.04G037630.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.04G037630.1, (accessed on 12 October 2023) | 4:68525044–68518203 | 2415 | 804 | Chloroplast |
| StBTB21 | Soltu.DM.05G021440.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.05G021440.1, (accessed on 12 October 2023) | 5:49859963–49865413 | 1446 | 481 | Nucleus |
| StBTB22 | Soltu.DM.05G022360.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.05G022360.1, (accessed on 12 October 2023) | 5:50791378–50794505 | 801 | 266 | Cytoplasm |
| StBTB23 | Soltu.DM.05G022990.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.05G022990.1, (accessed on 12 October 2023) | 5:51463053–51457832 | 1023 | 340 | Nucleus |
| StBTB24 | Soltu.DM.06G002220.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G002220.1, (accessed on 12 October 2023) | 6:2651681–2655195 | 1800 | 599 | Chloroplast |
| StBTB25 | Soltu.DM.06G010640.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G010640.1, (accessed on 12 October 2023) | 6:32043750–32040570 | 840 | 279 | Chloroplast |
| StBTB26 | Soltu.DM.06G026950.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G026950.1, (accessed on 12 October 2023) | 6:52716023–52712688 | 1050 | 349 | Nucleus |
| StBTB27 | Soltu.DM.06G026960.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G026960.1, (accessed on 12 October 2023) | 6:52723186–52718338 | 1050 | 349 | Nucleus |
| StBTB28 | Soltu.DM.06G028810.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G028810.1, (accessed on 12 October 2023) | 6:54046853–54048811 | 1722 | 573 | Chloroplast |
| StBTB29 | Soltu.DM.06G034720.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G034720.1, (accessed on 12 October 2023) | 6:58701505–58709231 | 1617 | 538 | Chloroplast |
| StBTB30 | Soltu.DM.06G034740.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.06G034740.1, (accessed on 12 October 2023) | 6:58720995–58723523 | 441 | 146 | Chloroplast |
| StBTB31 | Soltu.DM.07G011890.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.07G011890.1, (accessed on 12 October 2023) | 7:38290258–38273905 | 1884 | 627 | Chloroplast |
| StBTB32 | Soltu.DM.07G013880.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.07G013880.1, (accessed on 12 October 2023) | 7:42990889–42988509 | 1731 | 576 | Plastid |
| StBTB33 | Soltu.DM.07G014680.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.07G014680.1, (accessed on 12 October 2023) | 7:44408833–44404274 | 1728 | 575 | Cytoplasm/Nucleus |
| StBTB34 | Soltu.DM.07G016030.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.07G016030.1, (accessed on 12 October 2023) | 7:46031384–46034171 | 2064 | 687 | Chloroplast |
| StBTB35 | Soltu.DM.07G016870.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=SoltuDM.07G016870.1, (accessed on 12 October 2023) | 7:46995457–47002655 | 1734 | 577 | Cytoplasm |
| StBTB36 | Soltu.DM.07G027790.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.07G027790.1, (accessed on 12 October 2023) | 7:56826287–56828986 | 987 | 328 | Cytoplasm |
| StBTB37 | Soltu.DM.08G001470.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.08G001470.1, (accessed on 12 October 2023) | 8:1948150–1941686 | 1848 | 615 | Cytoplasm |
| StBTB38 | Soltu.DM.08G006060.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.08G006060.1, (accessed on 12 October 2023) | 8:8434101–8428805 | 1212 | 403 | Cytoplasm |
| StBTB39 | Soltu.DM.08G028580.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.08G028580.1, (accessed on 12 October 2023) | 8:57912747–57917717 | 1560 | 519 | Nucleus |
| StBTB40 | Soltu.DM.08G028600.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.08G028600.1, (accessed on 12 October 2023) | 8:57927168–57928872 | 1380 | 459 | Chloroplast |
| StBTB41 | Soltu.DM.08G028610.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.08G028610.1, (accessed on 12 October 2023) | 8:57933232–57934873 | 1332 | 443 | Chloroplast |
| StBTB42 | Soltu.DM.09G005820.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G005820.1 (accessed on 12 October 2023) | 9:5410787–5407618 | 1785 | 594 | Cytoplasm |
| StBTB43 | Soltu.DM.09G011290.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G011290.1, (accessed on 12 October 2023) | 9:19305560–19308657 | 1737 | 578 | Vacuole |
| StBTB44 | Soltu.DM.09G017950.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G017950.1, (accessed on 12 October 2023) | 9:50663650–50660954 | 1824 | 607 | Nucleus |
| StBTB45 | Soltu.DM.09G019780.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G019780.1, (accessed on 12 October 2023) | 9:54297721–54289424 | 2127 | 708 | Cytoplasm |
| StBTB46 | Soltu.DM.09G022320.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G022320.1, (accessed on 12 October 2023) | 9:57831226–57825083 | 1236 | 411 | Nucleus |
| StBTB47 | Soltu.DM.09G023040.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.09G023040.1, (accessed on 12 October 2023) | 9:58865131–58869419 | 1890 | 629 | Chloroplast |
| StBTB48 | Soltu.DM.10G000680.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G000680.1, (accessed on 12 October 2023) | 10:619135–609397 | 2250 | 749 | Plastid |
| StBTB49 | Soltu.DM.10G005800.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G005800.1, (accessed on 12 October 2023) | 10:5842039–5836297 | 1656 | 551 | Nucleus |
| StBTB50 | Soltu.DM.10G013480.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G013480.1, (accessed on 12 October 2023) | 10:38866471–38857805 | 2349 | 782 | Cytoplasm |
| StBTB51 | Soltu.DM.10G021040.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G021040.1, (accessed on 12 October 2023) | 10:52920912–52916854 | 1833 | 610 | Nucleus |
| StBTB52 | Soltu.DM.10G027630.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G027630.1, (accessed on 12 October 2023) | 10:58822974–58825292 | 1464 | 487 | Cytoplasm |
| StBTB53 | Soltu.DM.10G027950.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.10G027950.1, (accessed on 12 October 2023) | 10:59080668–59078099 | 1473 | 490 | Nucleus |
| StBTB54 | Soltu.DM.11G011280.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.11G011280.1, (accessed on 12 October 2023) | 11:11798335–11794985 | 987 | 328 | Cytoplasm |
| StBTB55 | Soltu.DM.11G025060.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.11G025060.1, (accessed on 12 October 2023) | 11:45125219–45116416 | 1227 | 408 | Nucleus |
| StBTB56 | Soltu.DM.12G008220.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.12G008220.1, (accessed on 12 October 2023) | 12:7252303–7257056 | 1668 | 555 | Nucleus |
| StBTB57 | Soltu.DM.12G019670.1 http://spuddb.uga.edu/cgi-bin/annotation_report.cgi?orf=Soltu.DM.12G019670.1, (accessed on 12 October 2023) | 12:46941436–46943083 | 795 | 264 | Cytoplasm |
| Motif No. | Motif Name | Sequence of Motifs |
|---|---|---|
| Motif 1 | NPH3 | IPGGAEAFELAAKFCYGVNFEJTAYNVAALRCAAEYLEMTEEYSKGNLISKTEVFLNSVV |
| Motif 2 | NPH3 | VPDYARPVHDGLYRAIDIYLKAHPGLSDSERKRLCKLMDCQKLSQEACTHAAQNERLPLRVIVQVLFFEQL |
| Motif 3 | N.A. | YVERAYKYRPVKVLEFELPHQQCVVYLDLKRDECANLFPAGRVYSQAFHLGGQGFFLSAHCNMDQQSAFHCFGLFLGMQEKGSVSFAVDYEFAVRTKPGEEYVSKYKGNYTFTGGKAVGYRNLFGIPWTPFLAEDSLYFINGILHLRAELTI |
| Motif 4 | BTB | SELASDVTINVGGRSFHLHKFILAARSGVFRKLFSNGNE |
| Motif 5 | BACK | IHASEEAALMDLLKFMYSNTLSTKTPPGLLDVLMAADKFEVASCMRYCSRELQNLPMTSESALLYLDLPSSVLMADAVRPLADAAKQFLAARFKDITKFQEEVLSLPLAGIEAVLSSDDLQIASEDAVYDFVLKWARTHYPKJEERREILTSRLCRLIRFPFMTCRKLRKVLTCNDFDHELASKFVLEALFYKAEAPHRQ |
| Motif 6 | MATH | TSSRSVTETVNGSHRFVINGYSLAKGMGIGKYITSDTFTVGGHQWAIYFYPDGKNPEDNSTYVSLFIALASEGTDVRALFELTLIDQSGKGKHKVHSHFDRALESGPYTLKYRGSMWGYKRFFRRALLETSDYLKDDCLKIHCTVGVVRST |
| Motif 7 | NPH3 | SVPCSFLLKLLRSAIMLDASPSCRSELERRIGLQLDQATLBDLLIPA |
| Motif 8 | NPH3 | DWWVEDLSVLSIDLYKRVIVAMKARGVK |
| Motif 9 | NPH3 | SWKDSIIVLQTCENLLPLAEELKJVSRCIDSIASKACVD |
| Motif 10 | NPH3 | PGSPSLSSLVKVAKLVDGYLAEIAPDPNLKLSKFISLAE |
| Motif 11 | N.A. | HALDLLAAADKYGVEQLKRLCEKALAEDLSIENVLDVLQ |
| Motif 12 | BTB | IKINDVPYEAFKAMLRFLYSGKLKEEPM |
| Motif 13 | TAZ | PCSKFDTCQGLQLLIRHFATCKRRVPGGCJRCKRMWQILRLHSSICDQPDDCKVPLCRQFKZK |
| Motif 14 | TAZ | NFKKIEETEGWKFLQHHDPVLELEILQFMDEADJRKKRRRRHKREQNLYLQLSEAMDCLEHICREGCTSVGPHDKEP |
| Motif 15 | NPR5/6 | SHHHHEHDLSSADELEDKKIRRIRRALDSDDVELVKLLLMGEGLNLDEAIALHYAVEYCSREVVKELLELG |
| Motif 16 | NPR5/6 | VVNPAGPRGKTPLHIAAERKEPDIIAVLLDKGADPNVRTLDGITPLDILRTLTR |
| Motif 17 | ARM | HYEAVGVIGNLVHSSPNIKKEVLLAGALQPVIGLLSSSCPESQREAALLLGQFAATDSDCKIHIVQRGAVPPLIEMLQSPDAQLREMSAFALGRLAQDTHNQAGIAHCGGIIPLLKLLDSKNGSLQHNAAFALYGLADNEDNVADLIKVGGVQKLQDGEFIVQPTRDCVAKTLKRLEEKIHGRILGHLLYLMRIGEKVIQ |
| Motif 18 | NPH3 | YAGETLYDVDTVQRILENFL |
| Motif 19 | N.A. | ASLRRENRELKLELEKMRMRVADLEKEHVSMKQEIQK |
| Motif 20 | NPH3 | SEKKQRVILETIVSLLPKEKG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiana; Katwal, A.; Chauhan, H.; Upadhyay, S.K.; Singh, K. Genome-Wide Identification and Expression Analysis of the Broad-Complex, Tramtrack, and Bric-à-Brac Domain-Containing Protein Gene Family in Potato. Agriculture 2024, 14, 771. https://doi.org/10.3390/agriculture14050771
Aiana, Katwal A, Chauhan H, Upadhyay SK, Singh K. Genome-Wide Identification and Expression Analysis of the Broad-Complex, Tramtrack, and Bric-à-Brac Domain-Containing Protein Gene Family in Potato. Agriculture. 2024; 14(5):771. https://doi.org/10.3390/agriculture14050771
Chicago/Turabian StyleAiana, Anita Katwal, Hanny Chauhan, Santosh Kumar Upadhyay, and Kashmir Singh. 2024. "Genome-Wide Identification and Expression Analysis of the Broad-Complex, Tramtrack, and Bric-à-Brac Domain-Containing Protein Gene Family in Potato" Agriculture 14, no. 5: 771. https://doi.org/10.3390/agriculture14050771








