Surgical Aortic Valve Replacement and Renal Dysfunction: From Acute Kidney Injury to Chronic Disease
Abstract
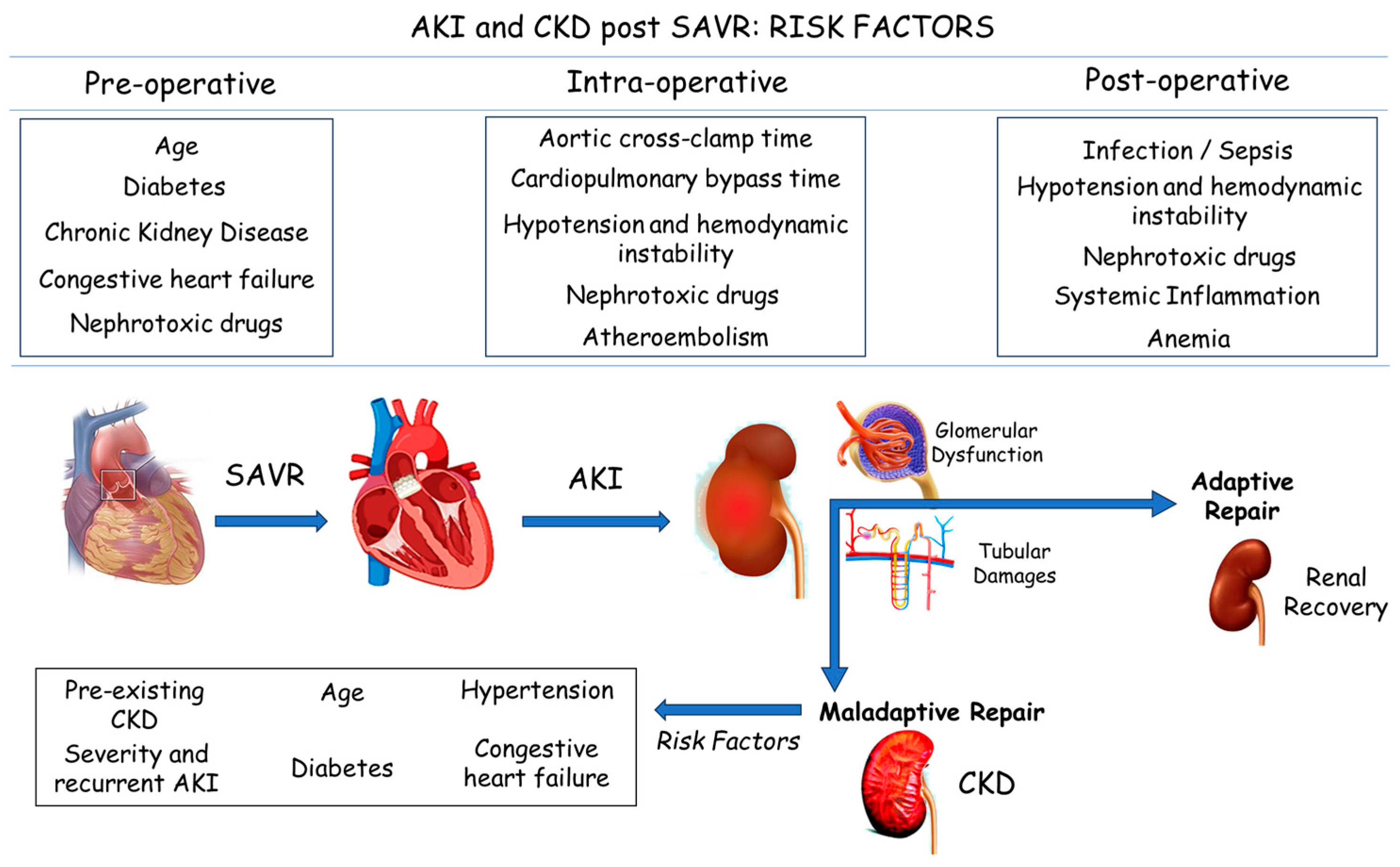
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables and Outcomes
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. AKI Prevalence and Dialysis Treatment
3.3. RRT, Kidney Recovery, and All-Cause Death
3.4. Endocarditis and AKI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Akhoundi, A.; Ahmed, A.H.; Kashani, K.B. Actual versus ideal body weight for acute kidney injury diagnosis and classification in critically ill patients. BMC Nephrol. 2014, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Delgado, J.C.; Esteve, F.; Torrado, H.; Rodríguez-Castro, D.; Carrio, M.L.; Farrero, E.; Javierre, C.; Ventura, J.L.; Manez, R. Influence of acute kidney injury on short- and long-term outcomes in patients undergoing cardiac surgery: Risk factors and prognostic value of a modified RIFLE classification. Crit. Care 2013, 17, R293. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.B.; Lebovitz, E.E.; Gelzinis, T.A.; Hilmi, I.A. Preoperative risk factors for unexpected postoperative intensive care unit admission: A retrospective case analysis. Anaesth. Crit. Care Pain. Med. 2018, 37, 571–575. [Google Scholar] [CrossRef]
- Prowle, J.R.; Forni, L.G.; Bell, M.; Chew, M.S.; Edwards, M.; Grams, M.E.; Grocott, M.P.W.; Liu, K.D.; McIlroy, D.; Murray, P.T.; et al. Postoperative acute kidney injury in adult non-cardiac surgery: Joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat. Rev. Nephrol. 2021, 17, 605–618. [Google Scholar] [CrossRef]
- Lassnigg, A.; Schmidlin, D.; Mouhieddine, M.; Bachmann, L.M.; Druml, W.; Bauer, P.; Hiesmayr, M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. JASN 2004, 15, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Keeling, W.B.; Sarin, E.L.; Guyton, R.A.; Kilgo, P.D.; Dara, A.B.; Puskas, J.D.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann. Thorac. Surg. 2011, 91, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic kidney disease and valvular heart disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 96, 836–849. [Google Scholar] [CrossRef]
- Azarbal, A.; Leadholm, K.L.; Ashikaga, T.; Solomon, R.J.; Dauerman, H.L. Frequency and prognostic significance of acute kidney recovery in patients who underwent transcatheter aortic valve implantation. Am. J. Cardiol. 2018, 121, 634–641. [Google Scholar] [CrossRef]
- Lahoud, R.; Butzel, D.W.; Parsee, A.; Huang, Y.-L.; Solomon, R.J.; DeVries, J.T.; Flynn, J.M.; Iribarne, A.; Lee, P.V.; Ross, C.S.; et al. Acute kidney recovery in patients who underwent transcatheter versus surgical aortic valve replacement (from the northern new england cardiovascular disease study group). Am. J. Cardiol. 2020, 125, 788–794. [Google Scholar] [CrossRef]
- Vives, M.; Hernandez, A.; Parramon, F.; Estanyol, N.; Pardina, B.; Muñoz, A.; Alvarez, P.; Hernandez, C. Acute kidney injury after cardiac surgery: Prevalence, impact and management challenges. Int. J. Nephrol. Renov. Dis. 2019, 12, 153–166. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patanè, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The Roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. J. Am. Soc. Nephrol. 2021, 32, 2994–3015. [Google Scholar] [CrossRef] [PubMed]
- Howitt, S.H.; Grant, S.W.; Caiado, C.; Carlson, E.; Kwon, D.; Dimarakis, I.; Malagon, I.; McCollum, C. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: A validation study. BMC Nephrol. 2018, 19, 149. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Bohbot, Y.; Candellier, A.; Diouf, M.; Rusinaru, D.; Altes, A.; Pasquet, A.; Maréchaux, S.; Vanoverschelde, J.; Tribouilloy, C. Severe aortic stenosis and chronic kidney disease: Outcomes and impact of aortic valve replacement. J. Am. Heart Assoc. 2020, 9, e017190. [Google Scholar] [CrossRef]
- Ferro, C.J.; Chue, C.D.; de Belder, M.A.; Moat, N.; Wendler, O.; Trivedi, U. UK TAVI Steering Group, National Institute for Cardiovascular Outcomes Research. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): An analysis of the UK TAVI registry. Heart 2015, 101, 546–552. [Google Scholar] [CrossRef]
- Mylotte, D.; Osnabrugge, R.L.; Windecker, S.; Lefèvre, T.; de Jaegere, P.; Jeger, R.; Wenaweser, P.; Maisano, F.; Moat, N.; Søndergaard, L.; et al. Transcatheter aortic valve replacement in Europe: Adoption trends and factors influencing device utilization. J. Am. Coll. Cardiol. 2013, 62, 210–219. [Google Scholar] [CrossRef]
- Mas-Peiro, S.; Faerber, G.; Bon, D.; Herrmann, E.; Bauer, T.; Bleiziffer, S.; Bekeredjian, R.; Böning, A.; Frerker, C.; Beckmann, A.; et al. Impact of chronic kidney disease in 29 893 patients undergoing transcatheter or surgical aortic valve replacement from the German Aortic Valve Registry. Eur. J. Cardio-Thorac. Surg. 2021, 59, 532–544. [Google Scholar] [CrossRef]
- Rivera, F.B.; Al-Abcha, A.; Ansay, M.F.M.; Magalong, J.V.U.; Tang, V.A.S.; Ona, H.M.; Miralles, K.A.; Sausa, R.; Uy, R.A.F.; Lerma, E.V.; et al. Transcatheter Aortic Valve Replacement-Associated Acute Kidney Injury: An Update. Cardiorenal Med. 2023, 13, 143–157. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Liu, S.; Yu, X.; Zou, J.; Ding, X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 82–89. [Google Scholar] [CrossRef]
- Mylotte, D.; Osnabrugge, R.L.; Windecker, S.; Lefèvre, T.; de Jaegere, P.; Jeger, R.; Wenaweser, P.; Maisano, F.; Moat, N.; Søndergaard, L.; et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 814–822. [Google Scholar]
- Karim, H.M.; Yunus, M.; Saikia, M.K.; Kalita, J.P.; Mandal, M. Incidence and progression of cardiac surgery-associated acute kidney injury and its relationship with bypass and cross clamp time. Ann. Card. Anaesth. 2017, 20, 22–27. [Google Scholar]
- Al-Sarraf, N.; Thalib, L.; Hughes, A.; Houlihan, M.; Tolan, M.; Young, V.; McGovern, E. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int. J. Surg. 2011, 9, 104–109. [Google Scholar] [CrossRef]
- Swinkels, B.M.; Ten Berg, J.M.; Kelder, J.C.; Vermeulen, F.E.; Van Boven, W.J.; de Mol, B.A. Effect of aortic cross-clamp time on late survival after isolated aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 222–228. [Google Scholar] [CrossRef]
- Liakopoulos, O.J.; Kuhn, E.W.; Choi, Y.H.; Chang, W.; Wittwer, T.; Madershahian, N.; Wassmer, G.; Wahlers, T. Myocardial protection in cardiac surgery patients requiring prolonged aortic cross-clamp times: A single-center evaluation of clinical outcomes comparing two blood cardioplegic strategies. J. Cardiovasc. Surg. 2010, 51, 895–905. [Google Scholar]
- Bolignano, D.; Coppolino, G.; Romeo, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology 2010, 15, 23–26. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, J.; Chen, Z.; Feng, J.; Yang, X.; Liang, W.; Ding, G. Transition of acute kidney injury to chronic kidney disease: Role of metabolic reprogramming. Metab. Clin. Exp. 2022, 131, 155194. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Duan, J.; Pan, S.; Cheng, F.; Qiao, Y.; Feng, Q.; Liu, D.; Liu, Z. The Road from AKI to CKD: Molecular Mechanisms and Therapeutic Targets of Ferroptosis. Cell Death Dis. 2023, 14, 426. [Google Scholar] [CrossRef]
- Menez, S.; Ju, W.; Menon, R.; Moledina, D.G.; Thiessen Philbrook, H.; McArthur, E.; Jia, Y.; Obeid, W.; Mansour, S.G.; Koyner, J.L.; et al. Urinary EGF and MCP-1 and risk of CKD after cardiac surgery. JCI Insight 2021, 6, e147464. [Google Scholar] [CrossRef]
- Samad, Z.; Sivak, J.A.; Phelan, M.; Schulte, P.J.; Patel, U.; Velazquez, E.J. Prevalence and Outcomes of Left-Sided Valvular Heart Disease Associated With Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e006044. [Google Scholar] [CrossRef]
- Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; Kato, T.; et al. Sudden Death in Patients With Severe Aortic Stenosis: Observations From the CURRENT AS Registry. J. Am. Heart Assoc. 2018, 7, e008397. [Google Scholar] [CrossRef]
- Savica, V.; Bellinghieri, G.; Monardo, P.; Muraca, U.; Santoro, D. An update on calcium metabolism alterations and cardiovascular risk in patients with chronic kidney disease: Questions, myths and facts. J. Nephrol. 2013, 26, 456–464. [Google Scholar] [CrossRef]
- Savica, V.; Santoro, D.; Monardo, P.; Mallamace, A.; Bellinghieri, G. Sevelamer carbonate in the treatment of hyperphosphatemia in patients with chronic kidney disease on hemodialysis. Ther. Clin. Risk Manag. 2008, 4, 821–826. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Smeriglio, A.; Campo, S.; La Camera, E.; Lanteri, G.; Giunta, E.; Monardo, P.; Trombetta, D. In Vitro Simulated Hemoperfusion on Seraph®-100 as a Promising Strategy to Counteract Sepsis. Biomedicines 2024, 12, 575. [Google Scholar] [CrossRef]
- Wang, G.; He, Y.; Guo, Q.; Zhao, Y.; He, J.; Chen, Y.; Chen, W.; Zhou, Y.; Peng, Z.; Deng, K.; et al. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with the lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit. Care 2023, 27, 275. [Google Scholar] [CrossRef]
| All Patients (n: 462) | AKI Group (n: 76) | No-AKI Group (n: 386) | p | |
|---|---|---|---|---|
| Age, mean ± SD | 66.2 ±11.4 | 69.4 ± 7.5 | 54.6 ± 4.9 | <0.05 |
| Male, n (%) | 244 (53) | 41 (54) | 203 (52) | >0.05 |
| Female, n (%) | 218 (47) | 35 (46) | 183 (47) | >0.05 |
| Hypertension, n (%) | 405 (88) | 73 (96) | 332 (86) | >0.05 |
| Hyperlipidemia, n (%) | 195 (42) | 60 (78) | 135 (35) | <0.05 |
| Smoking, n (%) | 131 (28) | 62 (81) | 69 (18) | <0.05 |
| Diabetes, n (%) | 132 (29) | 59 (77) | 73 (19) | <0.05 |
| CKD, n (%) | 78 (17) | 51 (67) | 27 (4) | <0.05 |
| Stage I–II | 33 | 8 | 25 | <0.05 |
| Stage III | 29 | 27 | 2 | <0.05 |
| Stage IV | 16 | 16 | - | <0.05 |
| Aortic cross-clamp time, min | 101 ± 49 | 127 ± 31 | 91 ± 27 | <0.05 |
| Cardiopulmonary bypass time, min | 126 ± 43 | 151 ±19 | 101 ± 29 | <0.05 |
| RRT, n (%) | 32 (7) | 32 (42) | - | - |
| LOS in the ICU days, n (%) | 11.4 ± 7.9 | 13.6 ± 4.9 | 3.8 ± 1.4 | <0.05 |
| Death, n (%) | 23 (5) | 21 (28) | 2 (0.5) | <0.05 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Hyperlipidemia | 1.07 | 0.94–1.12 | 0.27 | |||
| Age | 1.06 | 1.02–1.07 | 0.04 | 1.03 | 0.97–1.04 | 0.08 |
| Diabetes | 1.05 | 1.02–1.09 | 0.03 | 1.02 | 0.99–1.04 | 0.06 |
| Smoking | 1.06 | 0.97–1.18 | 0.37 | |||
| CKD | 1.13 | 1.04–1.22 | 0.002 | 1.10 | 1.04–1.25 | 0.0004 |
| CKD I–II | 1.03 | 1.01–1.05 | 0.04 | 1.02 | 0.97–1.03 | 0.07 |
| CKD III | 1.07 | 1.02–1.11 | 0.02 | 1.06 | 1.04–1.09 | 0.03 |
| CKD IV | 1.10 | 1.02–1.14 | 0.01 | 1.09 | 1.01–1.11 | 0.01 |
| Aortic cross-clamp time | 1.09 | 1.05–1.16 | 0.001 | 1.04 | 1.02–1.09 | 0.03 |
| cardiopulmonary bypass time | 1.08 | 1.08–1.22 | 0.004 | 1.08 | 1.03–1.12 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacquaniti, A.; Ceresa, F.; Campo, S.; Smeriglio, A.; Trombetta, D.; Patanè, F.; Monardo, P. Surgical Aortic Valve Replacement and Renal Dysfunction: From Acute Kidney Injury to Chronic Disease. J. Clin. Med. 2024, 13, 2933. https://doi.org/10.3390/jcm13102933
Lacquaniti A, Ceresa F, Campo S, Smeriglio A, Trombetta D, Patanè F, Monardo P. Surgical Aortic Valve Replacement and Renal Dysfunction: From Acute Kidney Injury to Chronic Disease. Journal of Clinical Medicine. 2024; 13(10):2933. https://doi.org/10.3390/jcm13102933
Chicago/Turabian StyleLacquaniti, Antonio, Fabrizio Ceresa, Susanna Campo, Antonella Smeriglio, Domenico Trombetta, Francesco Patanè, and Paolo Monardo. 2024. "Surgical Aortic Valve Replacement and Renal Dysfunction: From Acute Kidney Injury to Chronic Disease" Journal of Clinical Medicine 13, no. 10: 2933. https://doi.org/10.3390/jcm13102933






