Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis
Abstract
1. Introduction
2. Methods
3. Results
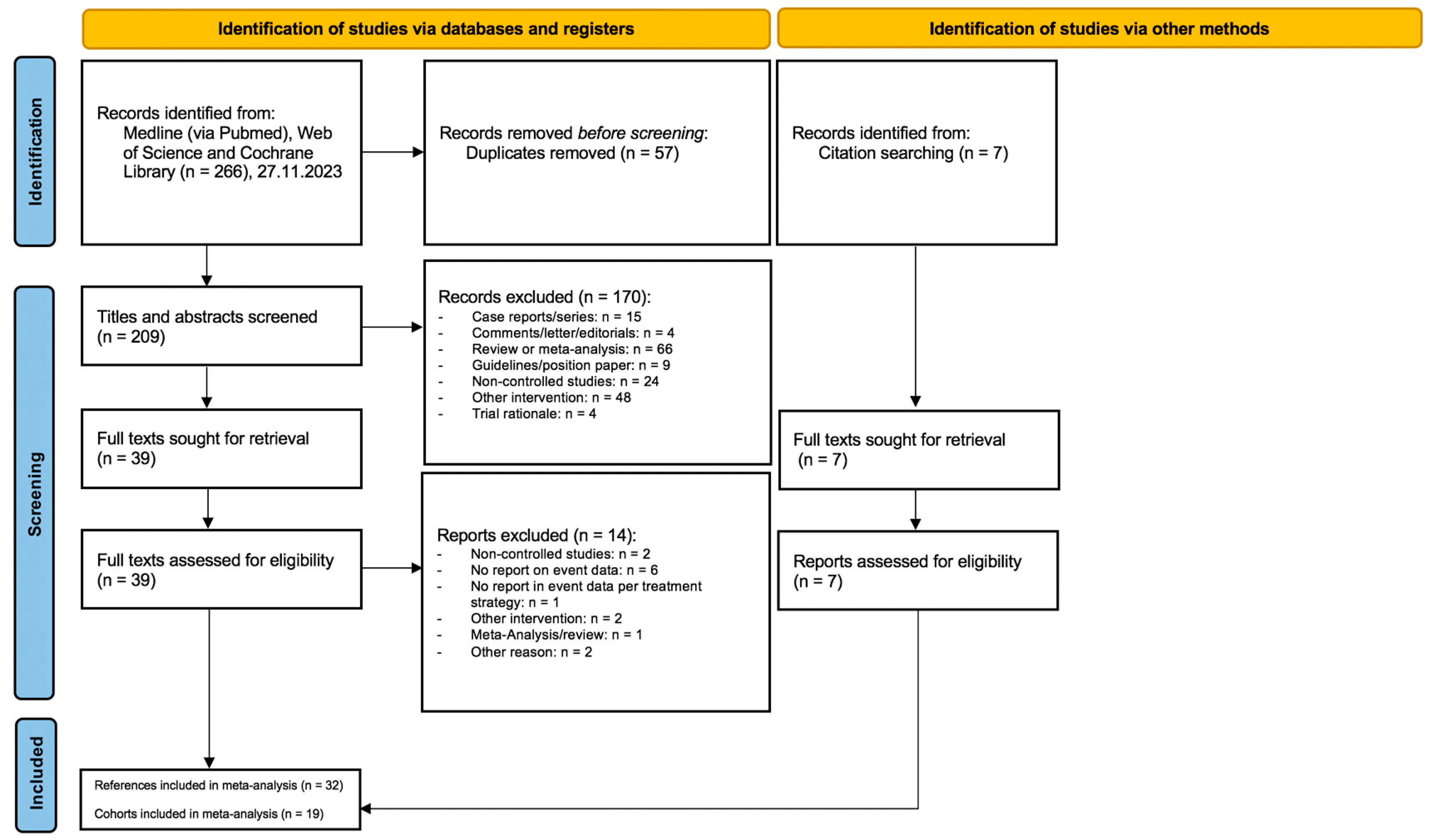
3.1. Study Selection
3.2. Studies and Cohorts
3.3. Risk of Bias Assessment
3.4. Patient Baseline Characteristics and Procedural Data
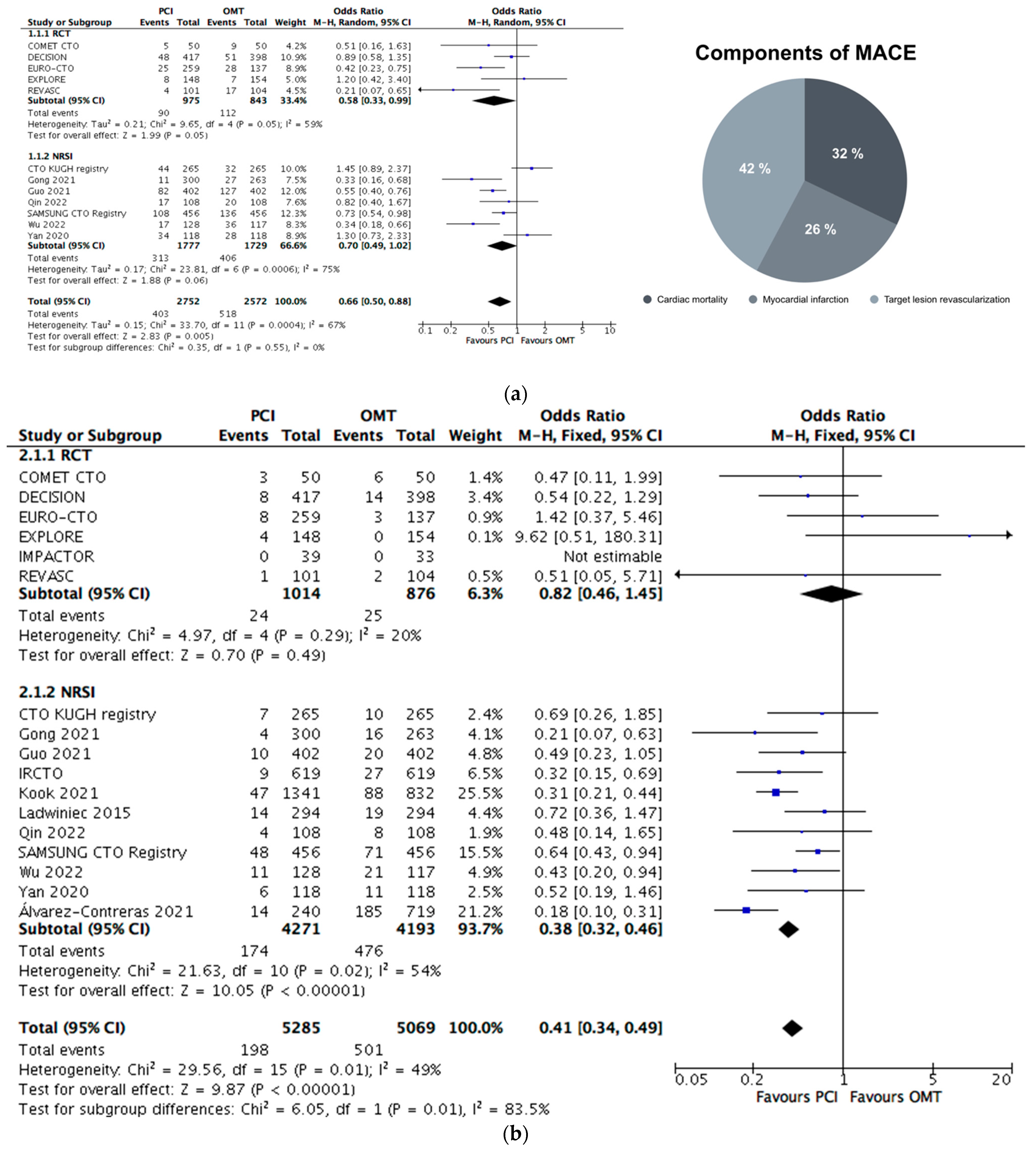
4. Primary Outcome Analysis
4.1. MACE Equivalent
4.2. Mortality&MI
4.3. Sensitivity Analysis of Primary Outcome
4.4. Subgroup Analyses of Primary Outcome
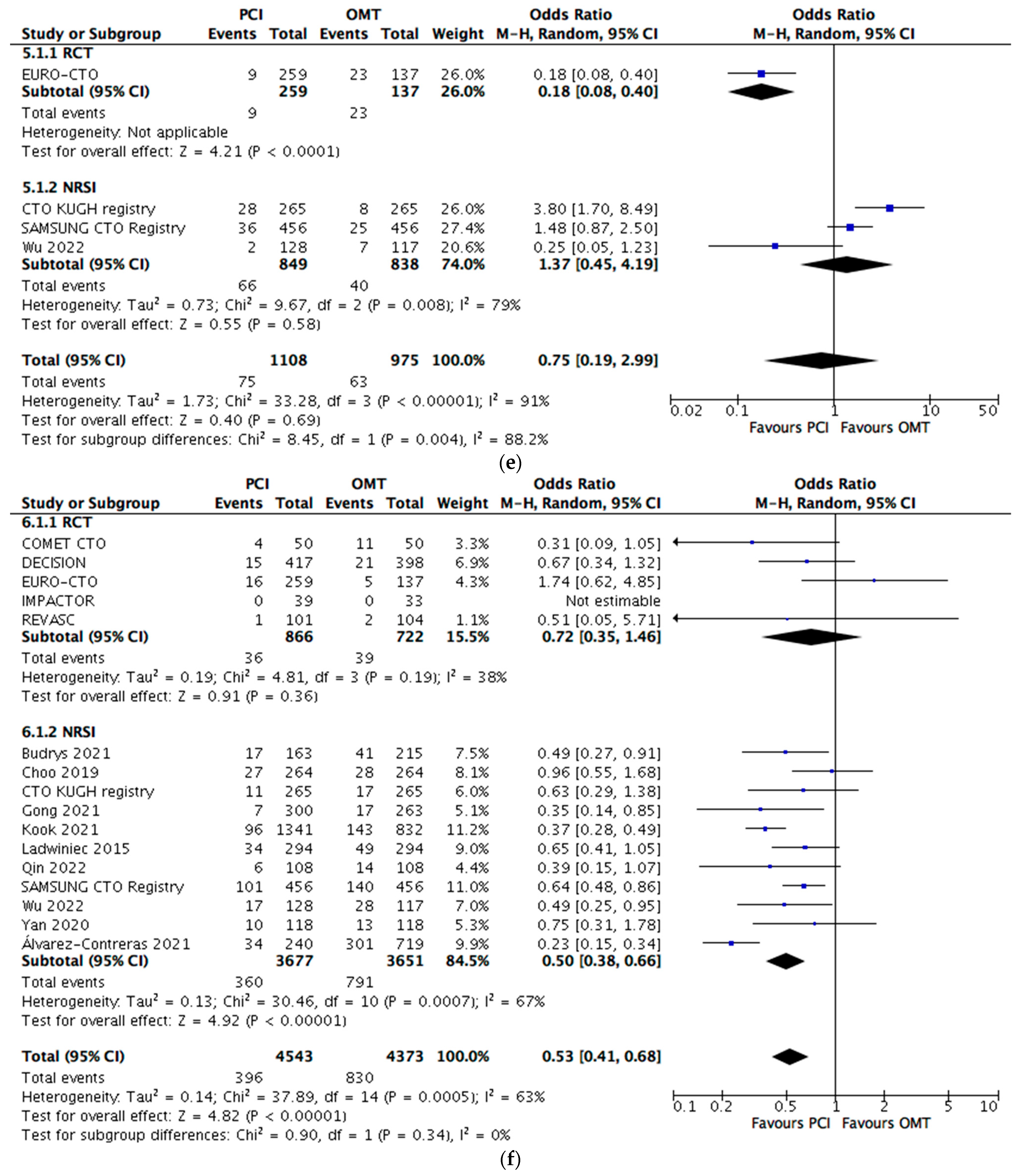
5. Secondary Outcome Analyses
5.1. Cardiac Mortality
5.2. Myocardial Infarction
5.3. TVR
5.4. TLR
5.5. All-Cause Mortality
5.6. Stroke
5.7. MACCE equivalent
6. Discussion
- ▪
- PCI was associated with a 34% lower likelihood of a constructed primary outcome MACE equivalent; this was consistent in RCTs and replicable after an adjustment for confounding factors in the ITT subgroup analysis and after the exclusion of studies considering patients with concomitant ACS.
- ▪
- Primary outcome reduction was mainly related to TVR or TLR, but Mortality&MI outcome analysis PCI was associated with a decreased event rate again.
- ▪
- PCIs were associated with a 47% decrease in all-cause mortality.
- ▪
- PCIs were associated with a 46% lower likelihood of stroke and a subsequent 38% decrease in the MACCE equivalent.
- ▪
- There was little to no detectable difference in TLR and TVR rates between the interventions.
- -
- All cause death, MI, clinically driven repeat revascularization (REVASC, IMPACTOR);
- -
- All cause death, MI, stroke, any revascularization (DECISION);
- -
- Cardiac death, MI, CABG (EXPLORE);
- -
- Cardiac death, non-fatal MI, ischemic driven TLR (EURO CTO);
- -
- Cardiovascular death, non-fatal MI, any revascularization (COMET CTO);
Limitations and Strengths
7. Conclusions
8. Impact on Daily Practice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass graft |
| CTO | Chronic total occlusion |
| CI | Confidence interval |
| ITT | Intention-to-treat |
| LAD | Left anterior artery descending |
| LCX | Left circumflex artery |
| LVEF | Left ventricular ejection fraction |
| MI | Myocardial infarction |
| MVD | Multi-vessel disease |
| NRSI | Non-randomized controlled study of intervention |
| OMT | Optimal medical treatment |
| OR | Odds ratio |
| PCI | Percutaneous coronary intervention |
| PSM | Propensity-score matching |
| RCA | Right coronary artery |
| RCT | Randomized controlled trial |
| RoB | Risk of bias |
| TIMI | Thrombolysis in myocardial infarction |
| TLR | Target lesion revascularization |
| TVR | Target vessel revascularization |
References
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef]
- Azzalini, L.; Jolicoeur, E.M.; Pighi, M.; Millán, X.; Picard, F.; Tadros, V.X.; Fortier, A.; L’Allier, P.L.; Ly, H.Q. Epidemiology, Management Strategies, and Outcomes of Patients with Chronic Total Coronary Occlusion. Am. J. Cardiol. 2016, 118, 1128–1135. [Google Scholar] [CrossRef]
- Råmunddal, T.; Hoebers, L.P.; Henriques, J.P.; Dworeck, C.; Angerås, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Chronic total occlusions in Sweden—A report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS ONE 2014, 9, e103850. [Google Scholar] [CrossRef]
- Tomasello, S.D.; Boukhris, M.; Giubilato, S.; Marzà, F.; Garbo, R.; Contegiacomo, G.; Marzocchi, A.; Niccoli, G.; Gagnor, A.; Varbella, F.; et al. Management strategies in patients affected by chronic total occlusions: Results from the Italian Registry of Chronic Total Occlusions. Eur. Heart J. 2015, 36, 3189–3198. [Google Scholar] [CrossRef]
- Werner, G.S.; Gitt, A.K.; Zeymer, U.; Juenger, C.; Towae, F.; Wienbergen, H.; Senges, J. Chronic total coronary occlusions in patients with stable angina pectoris: Impact on therapy and outcome in present day clinical practice. Clin. Res. Cardiol. 2009, 98, 435–441. [Google Scholar] [CrossRef]
- Råmunddal, T.; Hoebers, L.P.; Henriques, J.P.S.; Dworeck, C.; Angerås, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Prognostic Impact of Chronic Total Occlusions A Report From SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC-Cardiovasc. Interv. 2016, 9, 1535–1544. [Google Scholar] [CrossRef]
- Claessen, B.E.; Dangas, G.D.; Weisz, G.; Witzenbichler, B.; Guagliumi, G.; Mockel, M.; Brener, S.J.; Xu, K.; Henriques, J.P.; Mehran, R.; et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur. Heart J. 2012, 33, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.R.; Werner, G.S.; Boukhris, M.; Azzalini, L.; Mashayekhi, K.; Carlino, M.; Avran, A.; Konstantinidis, N.V.; Grancini, L.; Bryniarski, L.; et al. Percutaneous recanalisation of chronic total occlusions: 2019 consensus document from the EuroCTO Club. EuroIntervention 2019, 15, 198–208. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Gkargkoulas, F.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Patel, M.; Mahmud, E.; Choi, J.W.; Burke, M.N.; et al. Temporal Trends in Chronic Total Occlusion Percutaneous Coronary Interventions: Insights From the PROGRESS-CTO Registry. J. Invasive Cardiol. 2020, 32, 153–160. [Google Scholar] [CrossRef]
- Megaly, M.; Buda, K.; Mashayekhi, K.; Werner, G.S.; Grantham, J.A.; Rinfret, S.; McEntegart, M.; Brilakis, E.S.; Alaswad, K. Comparative Analysis of Patient Characteristics in Chronic Total Occlusion Revascularization Studies: Trials vs Real-World Registries. JACC Cardiovasc. Interv. 2022, 15, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nan, N.; Xue, Y.; Zhang, M.; Tian, J.; Chen, C.; Zhang, M.; Song, X. Comparison of successful versus failed percutaneous coronary intervention in patients with chronic total occlusion: A systematic review and meta-analysis. Cardiol. J. 2022, 31, 15–23. [Google Scholar] [CrossRef]
- Rha, S.W.; Choi, B.G.; Baek, M.J.; Ryu, Y.G.; Li, H.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; Jang, W.Y.; et al. Five-Year Outcomes of Successful Percutaneous Coronary Intervention with Drug-Eluting Stents versus Medical Therapy for Chronic Total Occlusions. Yonsei Med. J. 2018, 59, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Kucukseymen, S.; Iannaccone, M.; Grantham, J.A.; Sapontis, J.; Juricic, S.; Ciardetti, N.; Mattesini, A.; Stojkovic, S.; Strauss, B.H.; Wijeysundera, H.C.; et al. Association of Successful Percutaneous Revascularization of Chronic Total Occlusions with Quality of Life: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2324522. [Google Scholar] [CrossRef]
- Roth, C.; Goliasch, G.; Aschauer, S.; Gangl, C.; Ayoub, M.; Distelmaier, K.; Frey, B.; Lang, I.M.; Berger, R.; Mashayekhi, K.; et al. Impact of treatment strategies on long-term outcome of CTO patients. Eur. J. Intern. Med. 2020, 77, 97–104. [Google Scholar] [CrossRef]
- Christakopoulos, G.E.; Christopoulos, G.; Carlino, M.; Jeroudi, O.M.; Roesle, M.; Rangan, B.V.; Abdullah, S.; Grodin, J.; Kumbhani, D.J.; Vo, M.; et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am. J. Cardiol. 2015, 115, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Hoebers, L.P.; Claessen, B.E.; Elias, J.; Dangas, G.D.; Mehran, R.; Henriques, J.P. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int. J. Cardiol. 2015, 187, 90–96. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Bufe, A.; Werner, G.S.; Werner, N.; Meyer-Gessner, M.; Liebetrau, C.; Zahn, R.; Levenson, B.; Möllmann, H.; Nef, H.; et al. Behandlung von chronischen Koronarverschlüssen (CTO)–Positionspapier der Deutschen Gesellschaft für Kardiologie. Der Kardiol. 2021, 15, 320–340. [Google Scholar] [CrossRef]
- Werner, G.S.; Hildick-Smith, D.; Martin Yuste, V.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. Three-year outcomes of A Randomized Multicentre Trial Comparing Revascularization and Optimal Medical Therapy for Chronic Total Coronary Occlusions (EuroCTO). EuroIntervention 2023, 19, 571–579. [Google Scholar] [CrossRef]
- Juricic, S.A.; Stojkovic, S.M.; Galassi, A.R.; Stankovic, G.R.; Orlic, D.N.; Vukcevic, V.D.; Milasinovic, D.G.; Aleksandric, S.B.; Tomasevic, M.V.; Dobric, M.R.; et al. Long-term follow-up of patients with chronic total coronary artery occlusion previously randomized to treatment with optimal drug therapy or percutaneous revascularization of chronic total occlusion (COMET-CTO). Front. Cardiovasc. Med. 2023, 9, 1014664. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Spitzer, E.; McFadden, E.; Vranckx, P.; Garcia-Garcia, H.M.; Seltzer, J.H.; Held, C.; de Vries, T.; Menon, V.; Brown, K.J.; Soliman, O.I.I.; et al. Critical Appraisal of Contemporary Clinical Endpoint Definitions in Coronary Intervention Trials: A Guidance Document. JACC Cardiovasc. Interv. 2019, 12, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, B.R.; Alexander, K.P.; Cyr, D.D.; Berger, J.S.; Reynolds, H.R.; Bangalore, S.; Boden, W.E.; Lopes, R.D.; Demkow, M.; Piero Perna, G.; et al. Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons. Circulation 2021, 143, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2, a revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Ladwiniec, A.; Cunnington, M.S.; Rossington, J.; Mather, A.N.; Alahmar, A.; Oliver, R.M.; Nijjer, S.S.; Davies, J.E.; Thackray, S.; Alamgir, F.; et al. Collateral donor artery physiology and the influence of a chronic total occlusion on fractional flow reserve. Circ. Cardiovasc. Interv. 2015, 8, e002219. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Yun, S.C.; Han, S.; Kang, H.; Kang, S.J.; Kim, Y.H.; Lee, C.W.; et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation 2019, 139, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Obedinskiy, A.A.; Kretov, E.I.; Boukhris, M.; Kurbatov, V.P.; Osiev, A.G.; Ibn Elhadj, Z.; Obedinskaya, N.R.; Kasbaoui, S.; Grazhdankin, I.O.; Prokhorikhin, A.A.; et al. The IMPACTOR-CTO Trial. JACC Cardiovasc. Interv. 2018, 11, 1309–1311. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Nuhrenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Loffelhardt, N.; et al. A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion: The REVASC Trial. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Ge, L.; Qian, J.; Ma, J.; Ge, J. A comparison of long-term clinical outcomes between percutaneous coronary intervention (PCI) and medical therapy in patients with chronic total occlusion in noninfarct-related artery after PCI of acute myocardial infarction. Clin. Cardiol. 2022, 45, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Contreras, L.; Flores-Umanzor, E.; Cepas-Guillen, P.; Ferreira-Gonzalez, I.; Freixa, X.; Regueiro, A.; Brugaletta, S.; Sabate, M.; Martin-Yuste, V. Clinical Impact of Medical Therapy Versus Revascularization in Patients with Chronic Coronary Total Occlusions. J. Invasive Cardiol. 2021, 33, E2–E8. [Google Scholar] [PubMed]
- Henriques, J.P.; Hoebers, L.P.; Råmunddal, T.; Laanmets, P.; Eriksen, E.; Bax, M.; Ioanes, D.; Suttorp, M.J.; Strauss, B.H.; Barbato, E.; et al. Percutaneous Intervention for Concurrent Chronic Total Occlusions in Patients with STEMI: The EXPLORE Trial. J. Am. Coll. Cardiol. 2016, 68, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, I.M.; Elias, J.; García-García, H.M.; Hoebers, L.P.; Ouweneel, D.M.; Scheunhage, E.M.; Delewi, R.; Råmunddal, T.; Eriksen, E.; Claessen, B.; et al. Value of the SYNTAX Score in ST-Elevation Myocardial Infarction Patients with a Concomitant Chronic Total Coronary Occlusion (from the EXPLORE Trial). Am. J. Cardiol. 2019, 123, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Juricic, S.A.; Tesic, M.B.; Galassi, A.R.; Petrovic, O.N.; Dobric, M.R.; Orlic, D.N.; Vukcevic, V.D.; Stankovic, G.R.; Aleksandric, S.B.; Tomasevic, M.V.; et al. Randomized Controlled Comparison of Optimal Medical Therapy with Percutaneous Recanalization of Chronic Total Occlusion (COMET-CTO). Int. Heart J. 2021, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Budrys, P.; Bajoras, V.; Rees, M.; Saule, I.M.; Davidavicius, G.; Berukstis, A.; Baranauskas, A. Treatment of chronic total occlusion with percutaneous coronary intervention is associated with improved survival as compared to medical treatment alone: Insights from a single-centre, registry. Rev. Cardiovasc. Med. 2021, 22, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Rha, S.W.; Choi, B.G.; Choi, S.Y.; Byun, J.K.; Jang, W.Y.; Kim, W.; Na, J.O.; Choi, C.U.; Kim, E.J.; et al. Percutaneous Coronary Intervention for Chronic Total Occlusion in Single Coronary Arteries. Tex. Heart Inst. J. 2021, 48, e197023. [Google Scholar] [CrossRef]
- Choi, S.Y.; Choi, B.G.; Rha, S.W.; Baek, M.J.; Ryu, Y.G.; Park, Y.; Byun, J.K.; Shim, M.; Li, H.; Mashaly, A.; et al. Percutaneous Coronary Intervention Versus Optimal Medical Therapy for Chronic Total Coronary Occlusion with Well-Developed Collaterals. J. Am. Heart Assoc. 2017, 6, e006357. [Google Scholar] [CrossRef]
- Choo, E.H.; Koh, Y.S.; Seo, S.M.; Lee, J.M.; Kim, H.Y.; Park, H.J.; Kim, P.J.; Chang, K.; Jeon, D.S.; Kim, D.B.; et al. Comparison of successful percutaneous coronary intervention versus optimal medical therapy in patients with coronary chronic total occlusion. J. Cardiol. 2019, 73, 156–162. [Google Scholar] [CrossRef]
- Gong, X.; Zhou, L.; Ding, X.; Chen, H.; Li, H. The impact of successful chronic total occlusion percutaneous coronary intervention on long-term clinical outcomes in real world. BMC Cardiovasc. Disord. 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Meng, S.; Lv, H.; Zhong, L.; Wu, J.; Ding, H.; Xu, J.; Zhang, X.; Huang, R. Long-Term Outcomes of Successful Recanalization Compared with Optimal Medical Therapy for Coronary Chronic Total Occlusions in Patients with and without Left Ventricular Systolic Dysfunction. Front. Cardiovasc. Med. 2021, 8, 654730. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhong, L.; Chen, K.; Wu, J.; Huang, R.C. Long-term clinical outcomes of optimal medical therapy vs. successful percutaneous coronary intervention for patients with coronary chronic total occlusions. Hell. J. Cardiol. 2018, 59, 281–287. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Guo, L.; Zhong, L.; Xiao, J.; Meng, S.; Wang, Y.; Ding, H.; Zhang, B.; Zhu, H.; et al. Effect of complete percutaneous revascularization on improving long-term outcomes of patients with chronic total occlusion and multi-vessel disease. Chin. Med. J. 2023, 136, 959–966. [Google Scholar] [CrossRef]
- Kook, H.; Yang, J.H.; Cho, J.Y.; Jang, D.H.; Kim, M.S.; Lee, J.; Lee, S.H.; Joo, H.J.; Park, J.H.; Hong, S.J.; et al. Differential clinical impact of chronic total occlusion revascularization based on left ventricular systolic function. Clin. Res. Cardiol. 2021, 110, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Ladwiniec, A.; Allgar, V.; Thackray, S.; Alamgir, F.; Hoye, A. Medical therapy, percutaneous coronary intervention and prognosis in patients with chronic total occlusions. Heart 2015, 101, 1907–1914. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yang, J.H.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Lee, S.H.; Gwon, H.C.; Choi, S.H. Impact of Chronic Total Coronary Occlusion Location on Long-term Survival After Percutaneous Coronary Intervention. Rev. Esp. De Cardiol. 2019, 72, 717–723. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yang, J.H.; Choi, S.H.; Hwang, J.K.; Jang, W.J.; Hahn, J.Y.; Song, Y.B.; Choi, J.H.; Lee, S.H.; Gwon, H.C. Optimal medical therapy may be a better initial strategy in patients with chronic total occlusion of a single coronary artery. Int. J. Cardiol. 2016, 210, 56–62. [Google Scholar] [CrossRef]
- Jang, W.J.; Yang, J.H.; Choi, S.H.; Bin Song, Y.; Hahn, J.Y.; Choi, J.H.; Kim, W.S.; Lee, Y.T.; Gwon, H.C. Long-Term Survival Benefit of Revascularization Compared with Medical Therapy in Patients with Coronary Chronic Total Occlusion and Well-Developed Collateral Circulation. JACC-Cardiovasc. Interv. 2015, 8, 271–279. [Google Scholar] [CrossRef]
- Park, T.K.; Lee, S.H.; Choi, K.H.; Lee, J.M.; Yang, J.H.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Gwon, H.C.; Lee, S.H.; et al. Late Survival Benefit of Percutaneous Coronary Intervention Compared with Medical Therapy in Patients with Coronary Chronic Total Occlusion: A 10-Year Follow-Up Study. J. Am. Heart Assoc. 2021, 10, e019022. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, B.S.; Jang, W.J.; Ahn, J.; Park, T.K.; Bin Song, Y.; Hahn, J.Y.; Choi, J.H.; Lee, S.H.; Gwon, H.C.; et al. Optimal Medical Therapy vs. Percutaneous Coronary Intervention for Patients with Coronary Chronic Total Occlusion. Circ. J. 2015, 80, 211–217. [Google Scholar] [CrossRef]
- Wu, X.; Cai, J.; Zhang, Q.; Huang, H. Assessing the Clinical Influence of Chronic Total Occlusions (CTOs) Revascularization and the Impact of Vascularization Completeness on Patients with Left Ventricular (LV) Systolic Dysfunction. Comput. Intell. Neurosci. 2022, 2022, 9128206. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.F.; Yuan, F.; Liu, H.; Xu, F.; Zhang, M.; Wang, W.; Zhang, M.D.; Tian, J.F.; Cui, K.Y.; Zhou, K.; et al. Percutaneous Coronary Intervention Offers Survival Benefit to Stable Patients with One Single Chronic Total Occlusion and Diabetes: A Propensity Score-Matched Analysis. Angiology 2020, 71, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Alaswad, K.; Megaly, M.; Karmpaliotis, D.; Masoumi, A.; Jaber, W.A.; Nicholson, W.; Rinfret, S.; et al. A Systematic Review and Meta-Analysis of Clinical Outcomes of Patients Undergoing Chronic Total Occlusion Percutaneous Coronary Intervention. J. Invasive Cardiol. 2022, 34, E763–E775. [Google Scholar]
- Abo-Aly, M.; Misumida, N.; Backer, N.; ElKholey, K.; Kim, S.M.; Ogunbayo, G.O.; Abdel-Latif, A.; Ziada, K.M. Percutaneous Coronary Intervention with Drug-Eluting Stent Versus Optimal Medical Therapy for Chronic Total Occlusion: Systematic Review and Meta-Analysis. Angiology 2019, 70, 908–915. [Google Scholar] [CrossRef]
- Iannaccone, M.; D’Ascenzo, F.; Piazza, F.; De Benedictis, M.; Doronzo, B.; Behnes, M.; Garbo, R.; Mashayekhi, K. Optimal medical therapy vs. coronary revascularization for patients presenting with chronic total occlusion: A meta-analysis of randomized controlled trials and propensity score adjusted studies. Catheter. Cardiovasc. Interv. 2019, 93, E320–E325. [Google Scholar] [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.A.; van Es, G.A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2018, 39, 2192–2207. [Google Scholar] [CrossRef]
- Di Marco, A.; Paglino, G.; Oloriz, T.; Maccabelli, G.; Baratto, F.; Vergara, P.; Bisceglia, C.; Anguera, I.; Sala, S.; Sora, N.; et al. Impact of a chronic total occlusion in an infarct-related artery on the long-term outcome of ventricular tachycardia ablation. J. Cardiovasc. Electrophysiol. 2015, 26, 532–539. [Google Scholar] [CrossRef]
- Allahwala, U.K.; Jolly, S.S.; Dzavik, V.; Cairns, J.A.; Kedev, S.; Balasubramanian, K.; Stankovic, G.; Moreno, R.; Valettas, N.; Bertrand, O.; et al. The Presence of a CTO in a Non-Infarct-Related Artery During a STEMI Treated with Contemporary Primary PCI Is Associated with Increased Rates of Early and Late Cardiovascular Morbidity and Mortality: The CTO-TOTAL Substudy. JACC Cardiovasc. Interv. 2018, 11, 709–711. [Google Scholar] [CrossRef]
- Muraca, I.; Carrabba, N.; Virgili, G.; Bruscoli, F.; Migliorini, A.; Pennesi, M.; Pontecorboli, G.; Marchionni, N.; Valenti, R. Chronic total occlusion revascularization: A complex piece to “complete” the puzzle. World J. Cardiol. 2022, 14, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Simsek, B.; Rempakos, A.; Kostantinis, S.; Alexandrou, M.; Karacsonyi, J.; Rangan, B.V.; Mastrodemos, O.C.; Mutlu, D.; Abi Rafeh, N.; Alaswad, K.; et al. International survey of chronic total occlusion percutaneous coronary intervention operators. Catheter. Cardiovasc. Interv. 2024, 103, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, G.; Karmpaliotis, D.; Alaswad, K.; Yeh, R.W.; Jaffer, F.A.; Wyman, R.M.; Lombardi, W.L.; Menon, R.V.; Grantham, J.A.; Kandzari, D.E.; et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int. J. Cardiol. 2015, 198, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Brilakis, E.S.; Azzalini, L. Chronic total occlusion percutaneous coronary intervention: Managing perforation complications. Expert Rev. Cardiovasc. Ther. 2021, 19, 71–87. [Google Scholar] [CrossRef]
- Råmunddal, T.; Holck, E.N.; Karim, S.; Eftekhari, A.; Escaned, J.; Ioanes, D.; Walsh, S.; Spratt, J.; Veien, K.; Jensen, L.O.; et al. International randomized trial on the effect of revascularization or optimal medical therapy of chronic total coronary occlusions with myocardial ischemia-ISCHEMIA-CTO trial-rationale and design. Am. Heart J. 2023, 257, 41–50. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Rafeh, N.A.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention A Global Expert Consensus Document. Circulation 2019, 140, 420–433. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial I. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart. 2018, 13, 305–338. [Google Scholar] [CrossRef]

| (a) Randomized-controlled trials | ||||||||||||||||
| Trials | EXPLORE | REVASC | IMPACTOR | DECISION | EURO-CTO | COMET-CTO | ||||||||||
| Study characteristics | ||||||||||||||||
| Study period | 11/2007–04/2015 | 08/2007–09/2015 | 10/2010–04/2014 | 03/2010–09/2016 | 03/2012–05/2015 | 10/2015–05/2017 | ||||||||||
| Study design | RCT, multicentric, Europe and North America, 1:1 randomization | RCT, monocentric, Germany, 1:1 randomization | RCT, monocentric, Russia, 1:1 randomization | RCT, multicentric, Asia, 1:1 randomization | RCT, multicentric, Europe. 2:1 randomization | RCT, monocentric, Serbia, 1:1 randomization | ||||||||||
| Randomized patients | 304, 302 analyzed | 205 | 94, 72 analyzed | 834, 815 analyzed | 448, 396 analyzed | 100, 99 analyzed | ||||||||||
| CTO definition | TIMI 0, vessel diameter ≥2.5 mm | TIMI 0 ≥3 months, vessel diameter 2.5 to 4 mm | - | TIMI 0 ≥3 months, vessel diameter ≥2.5 mm | TIMI 0 ≥3 months, vessel diameter ≥2.5 mm | TIMI 0, vessel diameter ≥2.5 mm | ||||||||||
| Indication for treatment | CTO as bystander in ACS | Stable angina, proof of myocardial ischemia and myocardial viability | Stable angina, proof of myocardial ischemia | - | Symptomatic lesion, proof of myocardial viability | Stable angina, proof of myocardial ischemia and myocardial viability | ||||||||||
| Exclusion of prior CABG | No | No | No | No | No | No | ||||||||||
| Exclusion of patients with concomitant ACS | No | Yes | Yes | Yes (ST segment myocardial infarction) | Yes | Yes | ||||||||||
| Inclusion of failed revascularization in PCI group Intention-to-treat principle | Yes | Yes | No | Yes | Yes | Yes | ||||||||||
| Comparator treatment | OMT | OMT | OMT | OMT | OMT | OMT | ||||||||||
| Follow-up period, median | 4 months | 12 months | 12 months | 48 months | 37.4 months ‡ | 56 months ‡ | ||||||||||
| Baseline characteristics of patients included | ||||||||||||||||
| PCI | Patients, n | 148 | 101 | 39 | 417 | 259 | 50 | |||||||||
| OMT | Patients, n | 154 | 104 | 33 | 398 | 137 | 50 | |||||||||
| PCI | Age, median | 60 [10] | 65 | - | 62.2 [10.2] ‡ | 65.2 [9.7] | 61 (7) ‡ | |||||||||
| OMT | Age, median | 60 [10] | 68 | - | 62.9 [9.9] ‡ | 64.7 [9.9] | 63 (5) ‡ | |||||||||
| PCI | Male patients, n | 131 (88.5) | 91 (90.1) | - | 344 (83.3) # | 215 (83.0) | 38 (76) | |||||||||
| OMT | Male patients, n | 126 (81.8) | 90 (86.5) | - | 319 (81.6) # | 118 (86.1) | 44 (88) | |||||||||
| PCI | Hypertension, n | 59 (39.9) | 81 (80.2) | - | 262 (63.4) # | 189 (73.0) | 43 (86) | |||||||||
| OMT | Hypertension, n | 69 (44.8) | 93 (89.4) | - | 238 (60.9) # | 98 (71.5) | 43 (86) | |||||||||
| PCI | Diabetes, n | 22 (14.9) | 32 (31.6) | - | 132 (32.0) # | 85 (32.8) | 14 (28) | |||||||||
| OMT | Diabetes, n | 25 (16.2) | 31 (29.8) | - | 134 (34.3) # | 40 (29.2) | 18 (36) | |||||||||
| PCI | Smoking, n | 77 (52.0) | 23 (22.8) | - | 125 (30.3) # | 190 (73.4) | 30 (60) | |||||||||
| OMT | Smoking, n | 76 (49.4) | 21 (20.2) | - | 102 (26.1) # | 92 (67.2) | 37 (74) | |||||||||
| PCI | Dyslipidemia, n | 51 (34.5) | - | - | 249 (60.3) # | 210 (81.1) | 36 (72) | |||||||||
| OMT | Dyslipidemia, n | 52 (33.8) | - | - | 217 (55.5) # | 111 (81.0) | 35 (70) | |||||||||
| PCI | LVEF, mean | 41 [11] † | - | - | 57.3 [9.8] # | 54.5 [10.8] | 54.9 [9.4] | |||||||||
| OMT | LVEF, mean | 42 [12] † | - | - | 57.6 [9.1] # | 55.7 [10.8] | 51.3 [11.3] | |||||||||
| PCI | MVD, n | - | 87 (86.1) | - | 302 (73.1) | 129 (49.8) | - | |||||||||
| OMT | MVD, n | - | 94 (90.4) | - | 288 (73.7) | 75 (54.7) | - | |||||||||
| PCI | CTO location RCA, n | 64 (43.2) | 58 (57.4) | 39/39 (100) | 186 (45.0) | 165/259 (63.7) | 28 (56) | |||||||||
| OMT | CTO location RCA, n | 78 (50.6) | 71 (68.3) | 33/33 (100) | 186 (47.6) | 81/141 (57.4) | 39 (78) | |||||||||
| PCI | CTO location LAD, n | 36 (24.3) | 23 (22.8) | 0 (0) | 185 (44.8) | 66/259 (25.5) | 12 (24) | |||||||||
| OMT | CTO location LAD, n | 39 (25.3) | 17 (16.3) | 0 (0) | 163 (41.7) | 38/141 (27.0) | 5 (10) | |||||||||
| PCI | CTO location LCX, n | 48 (32.4) | 20 (19.8) | 0 (0) | 42 (10.2) | 28/259 (10.8) | 10 (20) | |||||||||
| OMT | CTO location LCX, n | 37 (24.0) | 16 (15.4) | 0 (0) | 42 (10.7) | 22/141 (15.6) | 6 (12) | |||||||||
| PCI | CTO location other, n | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||||||
| OMT | CTO location other, n | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||||||
| PCI | J-CTO score, median | 2 [1] | 2 | - | 2.1 [1.2] ‡ | 1.82 [1.07] ‡ | 1.48 [1.27] ‡ | |||||||||
| OMT | J-CTO score, median | 2 [1] | 2 | - | 2.2 [1.2] ‡ | 1.67 [0.91] ‡ | 1.72 [1.09] ‡ | |||||||||
| Procedural aspects in PCI group | ||||||||||||||||
| Number of PCI procedures/attempts | 147 | - | 384 | 274 | 50 | |||||||||||
| Successful CTO-PCI, n | 106 (72.1) | 89/101 (88.1) | 39/39 (100) | 348/384 (90.6) | 220/254 (86.6) | 47/50 (94) | ||||||||||
| Antegrade approach, n | 124 (84.3) | 61/101 (60.4) | - | - | 176/274 (64.2) | 44(50 (88) | ||||||||||
| Retrograde approach, n | 23 (15.7) | 40/101 (39.6) | - | - | 98/274 (35.8) | 3/50 (6) | ||||||||||
| Fluoroscopy time, (min), mean | - | 37 † | - | 42 [34.0] | 49.6 [34.9] | 29.4 [21] | ||||||||||
| Contrast volume (mL), mean | - | 280 † | - | 340.7 [156.9] | 285 [198] | 289.6 [105.6] | ||||||||||
| (b) Non-randomized controlled studies of interventions | ||||||||||||||||
| Trials | Álvarez-Contreras 2021 [33] | Budrys 2021 [38] | CTO KUGH registry 2018 | Choo 2019 [41] | Gong 2021 [42] | Guo 2021 [43] | ||||||||||
| Study characteristics | ||||||||||||||||
| Study period | 2010–2014 | 06/2014–12/2018 | 01/2004–11/2015 | 01/2004–12/2010 | 06/2017–10/2019 | 01/2007–12/2018 | ||||||||||
| Study design | Retrospective CS, monocentric, Spain | Retrospective CS, monocentric, Lithuania | Retrospective CS, PSM, monocentric, South Korea | Retrospective CS, PSM, monocentric, South Korea | Retrospective CS, monocentric, China | Retrospective CS, PSM, monocentric, China | ||||||||||
| Included patients | 959 | 378 | 316 | 528 | 563 | 804 | ||||||||||
| CTO definition | TIMI 0, >3 months | - | TIMI 0, vessel diameter >2.5 mm | TIMI 0, >3 months | TIMI 0, ≥3 months | TIMI 0, ≥3 months | ||||||||||
| Indication for treatment | - | - | - | - | Stable Angina, acute coronary syndrome | - | ||||||||||
| Exclusion of prior CABG | No | No | . | Yes | No | Yes | ||||||||||
| Exclusion of patients with concomitant ACS | No | Yes | Yes | No | No | Yes | ||||||||||
| Inclusion of failed revascularization in PCI group Intention-to-treat principle | No | Yes | No | No | No | Yes | ||||||||||
| Comparator treatment | OMT | OMT | OMT | OMT | OMT | OMT | ||||||||||
| Follow-up period, median | 4.3 years | 3.6 years ‡ | 4 years ‡ | 2.2 years | 1 year | 2.6 years | ||||||||||
| Baseline characteristics of patients included | ||||||||||||||||
| PCI | Patients, n | 240 | 163 | 265 | 264 | 300 | 402 | |||||||||
| OMT | Patients, n | 719 | 215 | 265 | 264 | 263 | 402 | |||||||||
| PCI | Age, median | 62.8 [10.8] ‡ | 65.9 [11.3] ‡ | 64.2 [9.8] | 61.5 [9.8] ‡ | 63.0 [10] | - | |||||||||
| OMT | Age, median | 69.6 [10.8] ‡ | 69.2 [9.4] ‡ | 64.5 [10.3] | 61.5 [10.5] ‡ | 67.0 [11] | - | |||||||||
| PCI | Male patients, n | 203 (84.6) | 115 (70.6) | 198 (74.7) | 199 (75.4) | 248 (82.7) | - | |||||||||
| OMT | Male patients, n | 595 (82.8) | 165 (76.7) | 195 (73.5) | 201 (76.1) | 208 (79.1) | - | |||||||||
| PCI | Hypertension, n | 163 (67.9) | 148 (90.8) | 170 (64.1) | 156 (59.1) | 220 (73.3) | - | |||||||||
| OMT | Hypertension, n | 547 (76.1) | 197 (91.6) | 166 (62.6) | 159 (60.2) | 193 (73.4) | - | |||||||||
| PCI | Diabetes, n | 79 (32.9) | 51 (31.3) | 113 (42.6) | 117 (44.3) | 119 (39.7) | - | |||||||||
| OMT | Diabetes, n | 323 (44.9) | 48 (22.3) | 119 (44.9) | 121 (45.8) | 140 (53.2) | - | |||||||||
| PCI | Smoking, n | 49 (20.4) | - | 136 (51.3) | 71 (26.9) | 200 (66.7) | - | |||||||||
| OMT | Smoking, n | 239 (33.2) | - | 146 (55.0) | 64 (24.2) | 171 (65.0) | - | |||||||||
| PCI | Dyslipidemia, n | 156 (65.0) | - | 83 (31.3) | - | 162 (54.0) | - | |||||||||
| OMT | Dyslipidemia, n | 486 (67.6) | - | 81 (30.5) | - | 157 (59.7) | - | |||||||||
| PCI | LVEF, mean | 49 [13] | - | 51.0 [11.9] | 54.8 [11.3] | 61 [10] | - | |||||||||
| OMT | LVEF, mean | 44 [14] | - | 49.2 [12.6] | 53.6 [12.3] | 58 [11] | - | |||||||||
| PCI | MVD, n | 180 (75.0) | - | 188 (70.9) | - | - | - | |||||||||
| OMT | MVD, n | 581 (80.8) | - | 195 (73.5) | - | - | - | |||||||||
| PCI | CTO location RCA, n | 106 (44.2) | - | 118 (44.5) | 124 (47.0) | - | - | |||||||||
| OMT | CTO location RCA, n | 370 (51.5) | - | 123 (46.4) | 118 (44.7) | - | - | |||||||||
| PCI | CTO location LAD, n | 65 (27.1) | - | 79 (29.8) | 84 (31.8) | - | - | |||||||||
| OMT | CTO location LAD, n | 137 (19.1) | - | 81 (30.5) | 80 (30.3) | - | - | |||||||||
| PCI | CTO location LCX, n | 43 (17.9) | - | 92 (34.7) | 66 (25.0) | - | - | |||||||||
| OMT | CTO location LCX, n | 132 (18.4) | - | 81 (30.5) | 72 (27.3) | - | - | |||||||||
| PCI | CTO location other, n | 27 (11.3) | - | 2 (0.7) | 0 (0) | - | - | |||||||||
| OMT | CTO location other, n | 80 (11.1) | - | 1 (0.3) | 0 (0) | - | - | |||||||||
| PCI | J-CTO score, median | - | - | - | - | - | - | |||||||||
| OMT | J-CTO score, median | - | - | - | - | - | - | |||||||||
| Procedural aspects in PCI group | ||||||||||||||||
| Number of PCI procedures/attempts | - | - | 265 | - | - | - | ||||||||||
| Successful CTO-PCI, n | - | 143 (87.7) | 265 (100) | - | - | - | ||||||||||
| Antegrade approach, n | - | - | - | - | - | - | ||||||||||
| Retrograde approach, n | - | - | - | - | - | - | ||||||||||
| Fluoroscopy time, (min), mean | - | - | - | - | - | - | ||||||||||
| Contrast volume (mL), mean | - | - | - | - | - | - | ||||||||||
| (1b) Non-randomized controlled studies of interventions continued | ||||||||||||||||
| Trials | Kook 2021 [46] | Ladwiniec 2015 [47] | SAMSUNG CTO registry | IRCTO, Tomasello | Qin 2022 [32] | Wu 2022 [53] | Yan 2020 [54] | |||||||||
| Study characteristics | ||||||||||||||||
| Study period | 03/2008–12/2014 | 01/2002–12/2007 | 03/2003–02/2012 | 03/2008–03/2009 | 07/2011–07/2019 | 01/2012–12/2020 | 01/2007–12/2017 | |||||||||
| Study design | Retrospective CS, three centers, South Korea | Retrospective CS, PSM, monocentric, United Kingdom | Retrospective CS, PSM, monocentric, South Korea, Samsung Medical Center | Retrospective CS, PSM, multicentric, Italy | Retrospective CS, PSM, monocentric, China | Retrospective CS, monocentric, China | Retrospective CS, PSM, monocentric, China | |||||||||
| Included patients | 2173 | 588 | 912 | 1238 | 216 | 261 | 236 | |||||||||
| CTO definition | TIMI 0, >3 months | TIMI 0, ≥3 months | TIMI 0, >3 months | TIMI 0, >3 months | TIMI 0, ≥3 months, ≥2.5 mm | TIMI 0, ≥3 months, | TIMI 0, ≥3 months | |||||||||
| Indication for treatment | Angina, proof of myocardial ischemia | - | Angina, proof of myocardial ischemia | - | Acute coronary syndrome | Angina Myocardium viability | Angina, silent ischemia | |||||||||
| Exclusion of prior CABG | Yes | Yes | Yes | Yes | Yes | No | Yes | |||||||||
| Exclusion of patients with concomitant ACS | - | No | Yes (ST-segment elevation myocardial infarction) | No | No | - | Yes | |||||||||
| Inclusion of failed revascularization in PCI group Intention-to-treat principle | No | Yes | Yes | Yes | Yes | No | Yes | |||||||||
| Comparator treatment | OMT | OMT | OMT | OMT | OMT | OMT | OMT | |||||||||
| Follow-up period, median | 1138 days | 5 years | 7.9 years | 1 year | 946 days | 38 months | 45 months | |||||||||
| Baseline characteristics of patients included | ||||||||||||||||
| PCI | Patients, n | 1341 | 294 | 456 | 619 | 108 | 135 | 118 | ||||||||
| OMT | Patients, n | 832 | 294 | 456 | 619 | 108 | 126 | 118 | ||||||||
| PCI | Age, median | - | 64.3 [10.0] ‡ | 64.6 [10.1] ‡ | 68.1 [10.3] ‡ | 61.7 [12.4] ‡ | 64.8 [9.0] ‡ | 62.0 | ||||||||
| OMT | Age, median | - | 63.9 [10.2] ‡ | 64.6 [11.7] ‡ | 68.5 [12.5] ‡ | 62.5 [12.0] ‡ | 68.4 [9.6] ‡ | 58.0 | ||||||||
| PCI | Male patients, n | - | 220 (74.8) | 370 (81.1) | 515 (83.2) | 97 (89.8) | 105 (77.8) | 90 (76.3) | ||||||||
| OMT | Male patients, n | - | 220 (74.8) | 354 (77.6) | 525 (84.8) | 97 (89.8) | 95 (75.4) | 93 (78.8) | ||||||||
| PCI | Hypertension, n | - | 159 (54.1) | 302 (66.2) | 489 (79.0) | 73 (67.6) | 91 (67.4) | 84 (71.2) | ||||||||
| OMT | Hypertension, n | - | 157 (53.4) | 301 (66.0) | 486 (78.5) | 74 (68.5) | 80 (63.5) | 79 (66.9) | ||||||||
| PCI | Diabetes, n | - | 62 (21.1) | 218 (47.8) | 194 (31.3) | 37 (34.3) | 51 (37.8) | - | ||||||||
| OMT | Diabetes, n | - | 53 (18.0) | 211 (46.3) | 182 (29.4) | 35 (32.4) | 37 (42.5) | - | ||||||||
| PCI | Smoking, n | - | 198 (67.4) | 141 (30.9) | 264 (42.6) | 40 (37.0) | 98 (72.6) | 68 (57.6) | ||||||||
| OMT | Smoking, n | - | 203 (69.1) | 131 (28.7) | 274 (44.3) | 40 (37.0) | 84 (87.9) | 66 (55.9) | ||||||||
| PCI | Dyslipidemia, n | - | 146 (49.7) | 113 (24.8) | 390 (63.0) | 12 (11.1) | - | 59 (50.0) | ||||||||
| OMT | Dyslipidemia, n | - | 135 (45.9) | 115 (25.2) | 390 (63.0) | 7 (6.5) | - | 61 (51.7) | ||||||||
| PCI | LVEF, mean | - | - | 55.5 [11.7] | - | 53.4 [9.5] | 30.9 [6.9] | 62.0 † | ||||||||
| OMT | LVEF, mean | - | - | 55.4 [11.7] | - | 52.3 [10.3] | 31.4 [6.6] | 61.5 † | ||||||||
| PCI | MVD, n | - | 158 (53.8) | 360 (78.9) | - | - | 121 (89.6) | - | ||||||||
| OMT | MVD, n | - | 150 (51.1) | 360 (78.9) | - | - | 116 (92.1) | - | ||||||||
| PCI | CTO location RCA, n | - | - | 239 (52.4) | - | 47 (43.5) | 54 (40.0) | 54 (45.8) | ||||||||
| OMT | CTO location RCA, n | - | - | 239 (52.4) | - | 47 (43.5) | 51 (40.5) | 49 (41.5) | ||||||||
| PCI | CTO location LAD, n | - | - | 132 (28.9) | - | 21 (19.4) | 51 (37.8) | 33 (28.0) | ||||||||
| OMT | CTO location LAD, n | - | - | 132 (28.9) | - | 17 (15.7) | 46 (36.5) | 36 (30.5) | ||||||||
| PCI | CTO location LCX, n | - | - | 157 (34.4) | - | 40 (37.0) | 30 (22.2) | 31 (26.3) | ||||||||
| OMT | CTO location LCX, n | - | - | 158 (34.6) | - | 45 (41.7) | 29 (23.0) | 33 (28.0) | ||||||||
| PCI | CTO location other, n | - | - | 0 (0) | - | 0 (0) | 0 (0) | 0 (0) | ||||||||
| OMT | CTO location other, n | - | - | 0 (0) | - | 0 (0) | 0 (0) | 0 (0) | ||||||||
| PCI | J-CTO score, median | - | - | - | - | - | 2.5 [0.9] ‡ | - | ||||||||
| OMT | J-CTO score, median | - | - | - | - | - | 2.7 [0.8] ‡ | - | ||||||||
| Procedural aspects in PCI group | ||||||||||||||||
| Number of PCI procedures/attempts | - | 294 | - | - | - | 172 | - | |||||||||
| Successful CTO-PCI, n | - | 177 (60.2) | - | - | - | 135 (78.5) | - | |||||||||
| Antegrade approach, n | - | - | - | - | - | - | - | |||||||||
| Retrograde approach, n | - | - | - | - | - | - | - | |||||||||
| Fluoroscopy time, (min), mean | - | - | - | - | - | - | - | |||||||||
| Contrast volume (mL), mean | - | - | - | - | - | - | - | |||||||||
| (a) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Randomization | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcomes | Selection of the Reported Results | Overall Risk of Bias | ||||||||
| EXPLORE | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | ||||||||
| REVASC | Some concerns | Low risk | Low risk | Low risk | Some concerns | Some concerns | ||||||||
| IMPACTOR | Some concerns | High risk | Some concerns | Some concerns | Some concerns | High risk | ||||||||
| DECISION | Low risk | High risk | Low risk | Low risk | Some concerns | High risk | ||||||||
| EURO-CTO | Low risk | Some concerns | Some concerns | Low risk | Low risk | Some concerns | ||||||||
| COMET-CTO | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | ||||||||
| (b) | ||||||||||||||
| Risk of Bias | Confounding | Patient Selection | Classification of Intervention | Deviations from the Intended Interventions | Missing Data | Outcome Measurement | Selection of the Reported Results | Overall Risk of Bias | ||||||
| Álvarez-Contreras 2021 [33] | Critical | Moderate | Moderate | Serious | Moderate | Low | Moderate | Critical | ||||||
| Budrys 2021 [38] | Serious | Moderate | Low | Low | Low | Low | Moderate | Serious | ||||||
| CTO KUGH registry 2018 | Moderate | Moderate | Moderate | Serious | Low | Low | Moderate | Serious | ||||||
| Choo 2019 [41] | Moderate | Moderate | Low | Serious | Low | Low | Moderate | Serious | ||||||
| Gong 2021 [42] | Serious | Moderate | Moderate | Serious | Moderate | Low | Moderate | Serious | ||||||
| Guo 2021 [43] | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate | ||||||
| Kook 2021 [46] | Serious | Moderate | Moderate | Serious | Low | Low | Moderate | Serious | ||||||
| Ladwiniec 2015 [47] | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate | ||||||
| SAMSUNG CTO registry | Moderate | Moderate | Low | Low | Moderate | Low | Moderate | Moderate | ||||||
| IRCTO | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate | ||||||
| Qin 2022 [32] | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate | ||||||
| Wu 2022 [53] | Serious | Moderate | Moderate | Serious | Moderate | Low | Moderate | Serious | ||||||
| Yan 2020 [54] | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macherey-Meyer, S.; Salem, K.; Heyne, S.; Meertens, M.M.; Finke, K.; Mauri, V.; Baldus, S.; Adler, C.; Lee, S. Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis. J. Clin. Med. 2024, 13, 2919. https://doi.org/10.3390/jcm13102919
Macherey-Meyer S, Salem K, Heyne S, Meertens MM, Finke K, Mauri V, Baldus S, Adler C, Lee S. Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis. Journal of Clinical Medicine. 2024; 13(10):2919. https://doi.org/10.3390/jcm13102919
Chicago/Turabian StyleMacherey-Meyer, Sascha, Khalid Salem, Sebastian Heyne, Max Maria Meertens, Karl Finke, Victor Mauri, Stephan Baldus, Christoph Adler, and Samuel Lee. 2024. "Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis" Journal of Clinical Medicine 13, no. 10: 2919. https://doi.org/10.3390/jcm13102919
APA StyleMacherey-Meyer, S., Salem, K., Heyne, S., Meertens, M. M., Finke, K., Mauri, V., Baldus, S., Adler, C., & Lee, S. (2024). Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis. Journal of Clinical Medicine, 13(10), 2919. https://doi.org/10.3390/jcm13102919







