NGF, EPO, and IGF-1 in the Male Reproductive System
Abstract
1. Introduction
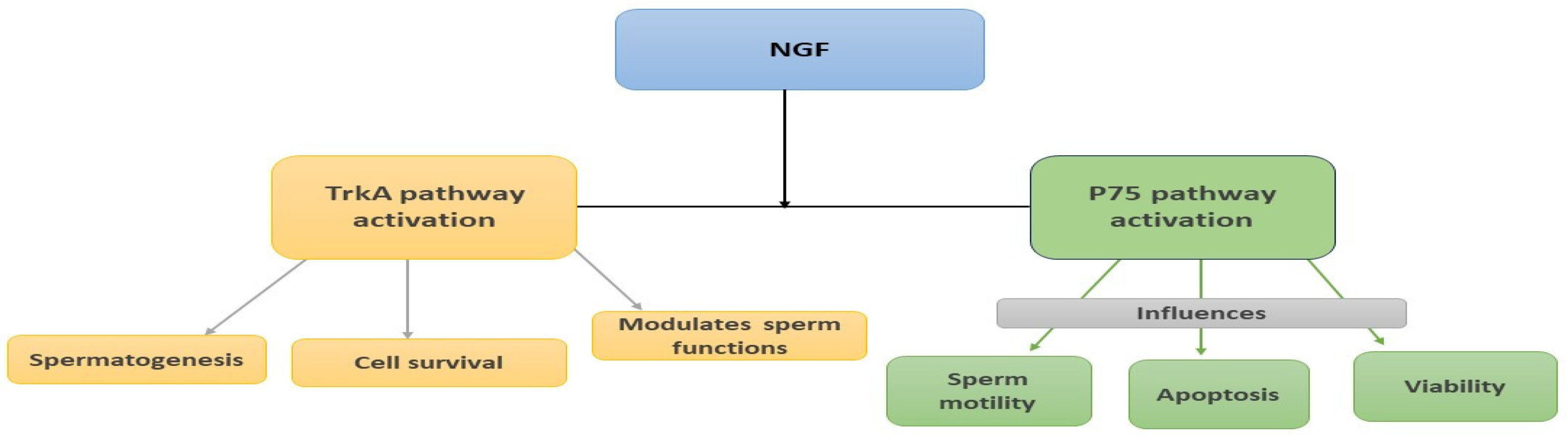
2. Nerve Growth Factor (NGF)
2.1. Introduction
2.2. NGF in the Male Reproductive System of Animal Models
2.2.1. Expression Patterns of NGF and Its Receptors across Species
2.2.2. Role of NGF in the Male Reproductive System
| References | Expression of NGF/Receptors | Cell-Type/Ort | Species |
|---|---|---|---|
| [28] | NGF/TrkA | Testis and Epididymis | Mouse and Rat |
| [29] | NGF | Leydig and Sertoli cells and peritubular myoid cells, but not the germ cells | Mouse |
| TrkA | Nongerm cells | ||
| p75 | Sertoli cells, peritubular myoid cells/temporarily expressed during the germ cell development | ||
| [30] | NGF | Leydig cells, primary spermatocytes, and pachytene spermatocytes | Adult rats |
| TrkA | Elongate spermatids | ||
| p75 | Sertoli cells, Leydig cells, pachytene spermatocytes, and elongate spermatids | ||
| [31] | NGF, TrKA | Seminiferous tubules | Rat |
| p75 | Tubules and in all the germ cells | ||
| [32] | NGF | Germ cells from pachytene spermatocytes and spermatids | Rat |
| TrkA, p75 | Sertoli cells and germ cells | ||
| [33] | NGF | Leydig cells and germinal cells at all stages, from primary spermatocytes to mature spermatozoids | Rat |
| TrkA | Spermatozoa and spermatids (Adults) Leydig cells (Newborn rats) | ||
| p75 | Spermatogonia (Adults) Cellular layer that surrounds the seminiferous tubules (Newborn rats) | ||
| [34] | NGF/TrkA/p75 | Leydig cells, Sertoli cells, and spermatogonia at various stages Epithelial cells, stromal tissues of the caudal epididymis, and seminal vesicle | Adult Japanese monkey |
| [35] | NGF | Ejaculated sperm/sperm head and tail | Bovines |
| TrkA | Ejaculated sperm/acrosomal cap, nucleus, and tail regions | ||
| [36] | NGF | Leydig cells, pachytene and preleptotene spermatocytes, and elongated spermatids | Golden hamster |
| TrkA | Sertoli cells | ||
| p75 | Ubiquitous distribution in the testis | ||
| [37] | NGF, TrkA, and p75 | Testis, prostate gland, and seminal vesicle | Rabbits |
| [38] | NGF | Prostate/middle piece of ejaculated sperm | Llamas |
| TrkA | Epithelial and muscular cells of testis, epididymis, bulbourethral glands, prostate/epididymal sperm, and middle piece of ejaculated sperm | ||
| [50] | NGF, TrkA, and p75 | Fetal testis between 14 and 19 wk of gestation | Human |
| NGF | Sertoli and interstitial cells | ||
| p75 | Peritubular cells | ||
| [51] | NGF, TrkA, and p75 | Adult human testis: prenatal testicular development Leydig cells (fetal and adult human testes) | Human |
| [52] | NGF and TrkA | Human sperm (proteins) | Human |
| TrkA | Spermatozoa (mRNA) |
2.3. NGF in the Male Reproductive System of Humans
2.3.1. Expression Sites
2.3.2. Impact of NGF on Sperm Parameters
2.3.3. Therapeutic Implications of NGF in Male Infertility
2.4. Summary and Future Directions
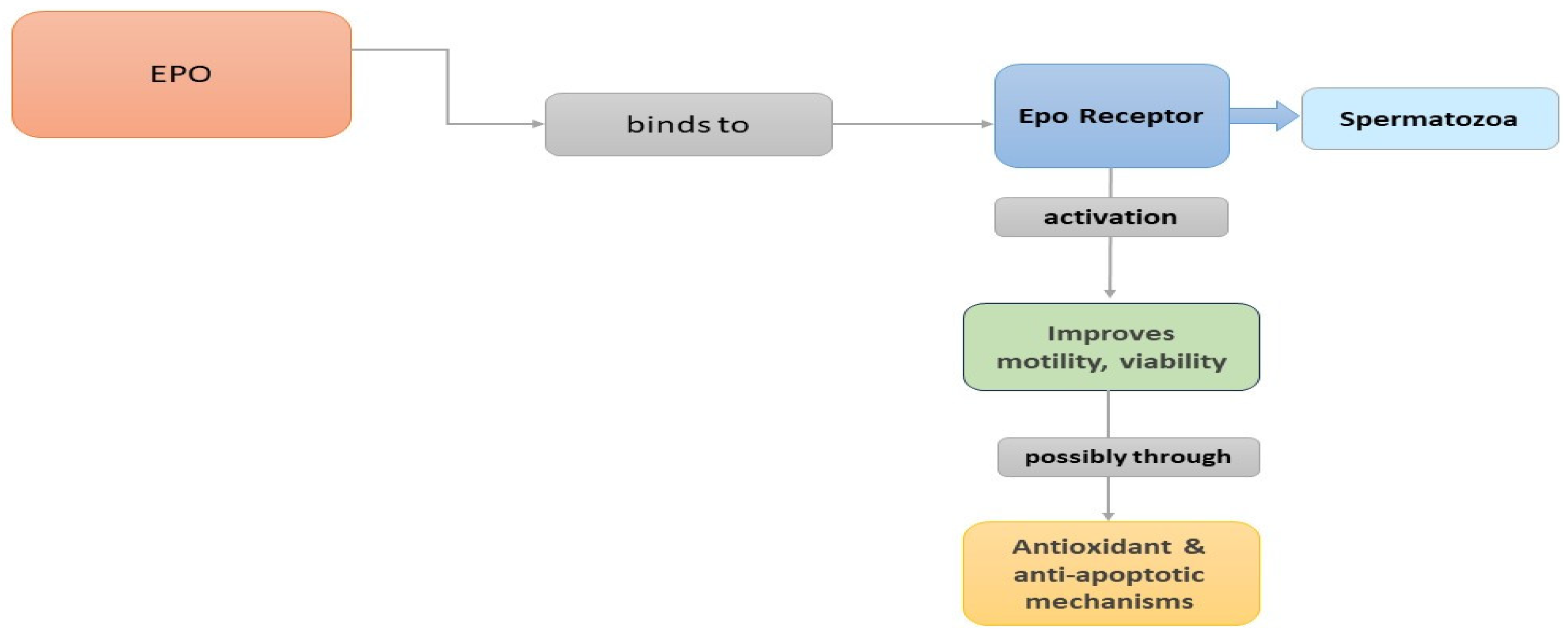
3. Erythropoietin (EPO)
3.1. Introduction
3.2. Expression Patterns and Actions of EPO
3.3. EPO in the Male Reproductive System of Animal Models
3.4. EPO in the Male Reproductive System of Humans
| References | Expression of Protein/Receptors | Cell Type/Ort | Species |
|---|---|---|---|
| [91] | EPO (rHuEPO), EPOR | Leydig cells | Rat |
| [93] | EPO (mRNA) | Sertoli cells, Peritubular myoid cells | Rat |
| [95] | EPOR | Leydig cells | Rat |
| [96] | EPO | Epididymis | Mouse |
| [104] | EPO | Testicular germ cells | Human |
| [107] | EPO | Seminal plasma | Human |
| [108] | EPOR | Spermatozoa plasma membrane | Human |
3.5. Therapeutic Implications of EPO in Male Reproductive Disorders
3.6. Summary and Future Directions
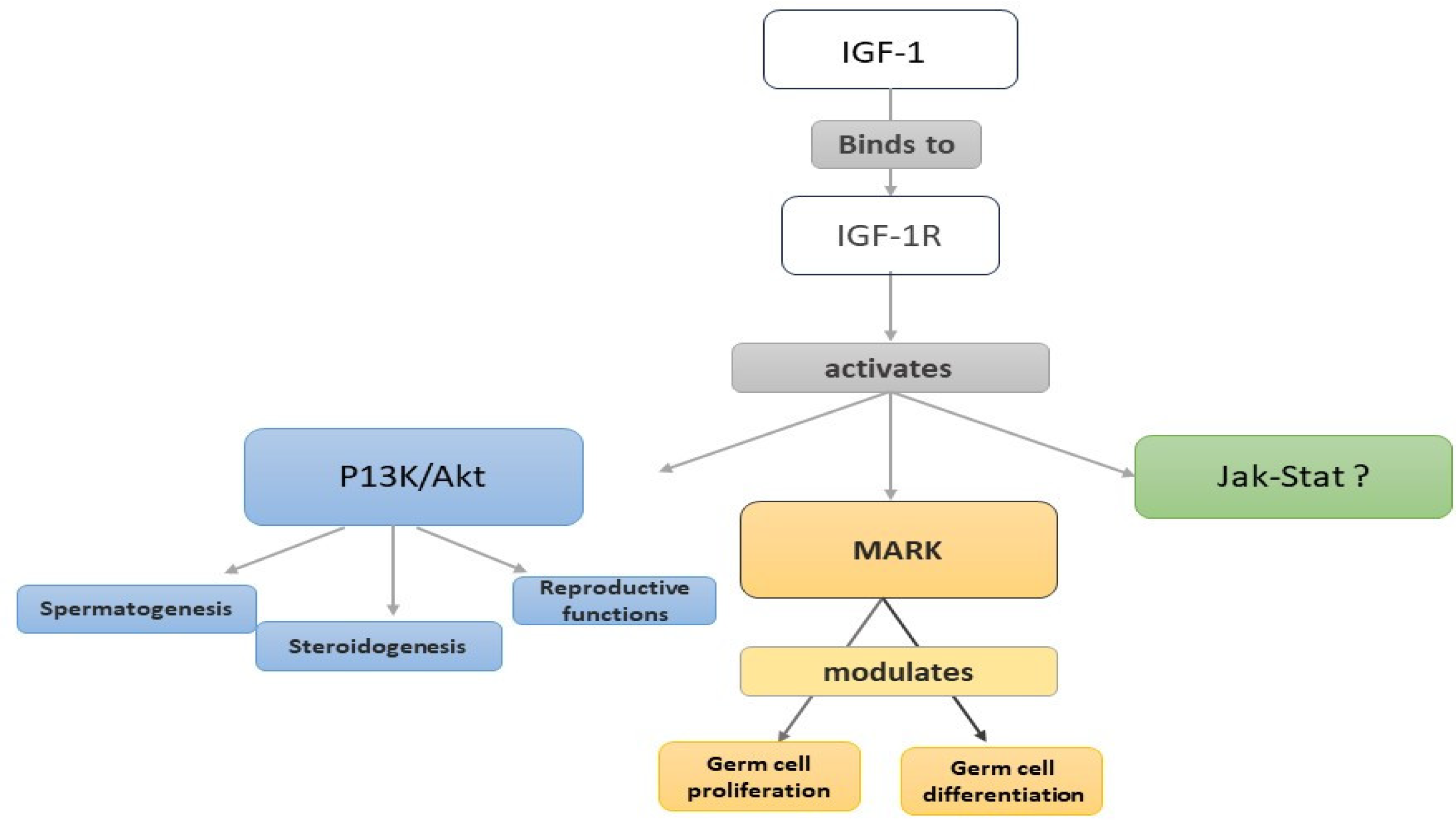
4. Insulin-like Growth Factor-1 (IGF-1)
4.1. Introduction
4.2. IGF-1 in the Male Reproductive System of Animal Models
Expression Sites and Actions
4.3. IGF-1 in the Human Male Reproductive System
4.3.1. Expression of the IGF-1 System
4.3.2. Mechanisms of Action of IGF-1 in Sperm Physiology
4.3.3. Impact of IGF-1 on Sperm Capacitation, Motility, and Vitality
| References | Expression of Protein/Receptors/IGFBPs | Cell-Type/Ort | Species |
|---|---|---|---|
| [121,122] | IGF-1 | Seminal plasma | Human |
| [124] | IFG-1/IGFR-1 | Ejaculate sperm/Sperm cells— acrosomal region | Bovines |
| [125] | IGFR-1 | Mature sperm—equatorial region, acrosomal region, midsection, and tail | Rabbits |
| [129] | IGFR-1 | Leydig cells | Rat |
| [135] | IGF-1 | Sertoli cells, primary spermatocytes, Leydig cells | Human |
| IGFR-1 | Secondary spematocytes, early spermatids, Sertoli cells | ||
| [136] | IGF-1/IGFR-1 | Ejaculates | Human |
| [137] | IGFR-1 | Sperm cells—equatorial region, acrosomal region (lower density) | Human |
| [138] | IGF-1/IGFBP-2, IGFBP-4 | Ejaculates | Human |
| [139] | IGFR-I | Germinal epithelium | Human |
| IGFBP-2, IGFBP-3 IGFBP-4, IGFBP-5, IGFBP-6 | Normal testicular tissue/germinal epithelium | ||
| [140] | IGFBP-2, IGFBR-4, IGFBP-3, IGFBP-5, PSA | Seminal plasma | Human |
| [152] | IGFR-1 | Preimplantation embryos | Human |
4.3.4. Therapeutic Potential of IGF-1
4.4. Summary and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotton, L.M.; O’Bryan, M.K.; Hinton, B.T. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr. Rev. 2008, 29, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Asadi, E.; Moawad, A.R.; Mikaeili, S.; Amidi, F.; Adutwum, E.; Safa, M.; Sobhani, A. Supplementation of freezing and thawing media with brain-derived neurotrophic factor protects human sperm from freeze-thaw-induced damage. Fertil. Steril. 2016, 106, 1658–1665.e4. [Google Scholar] [CrossRef]
- Hefti, F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 1986, 6, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Razavi, S.; Ghasemi, N.; Mardani, M.; Esfandiari, E.; Salehi, H.; Zarkesh-Esfahani, H. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv. Biomed. Res. 2015, 4, 53. [Google Scholar] [CrossRef]
- Lewin, G.R.; Barde, Y. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Barde, Y. The nerve growth factor family. Prog. Growth Factor Res. 1990, 2, 237–248. [Google Scholar] [CrossRef]
- Hallböök, F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opin. Neurobiol. 1999, 9, 616–621. [Google Scholar] [CrossRef]
- Tessarollo, L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998, 9, 125–137. [Google Scholar] [CrossRef]
- Di Rocco, M.; Soligo, M.; Manni, L.; Aloe, L. Nerve growth factor: Early studies and recent clinical trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef]
- Binder, D.K. Neurotrophins in the dentate gyrus. Prog. Brain Res. 2007, 163, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve Growth Factor as a Signaling Molecule for Nerve Cells and also for the Neuroendocrine-Immune Systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Di Rocco, M.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Di Rocco, M.; Bianchi, P.; Soligo, M.; Guaragna, M.; Barbaro, S.P.; Aloe, L. Nerve growth factor: Basic studies and possible therapeutic applications. Growth Factors 2013, 31, 115–122. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Murray-Rust, J.; Ibáñez, C.F.; McDonald, N.Q.; Lapatto, R.; Blundell, T.L. Nerve growth factor: Structure/function relationships. Protein Sci. 1994, 3, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Meakin, S.O.; Shooter, E.M. The nerve growth factor family of receptors. Trends Neurosci. 1992, 15, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; De Vos, A.M. Nerve growth factor: Structure and function. Cell. Mol. Life Sci. 2001, 58, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, J.; Wang, X.; Yao, G.; Wang, W.; Qi, W.; Kong, K. Multiple Roles of the p75 Neurotrophin Receptor in the Nervous System. J. Int. Med. Res. 2009, 37, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Bindocci, E.; Alleva, E. NGF, Brain and Behavioral Plasticity. Neural Plast. 2012, 2012, 784040. [Google Scholar] [CrossRef]
- Eibl, J.K.; Strasser, B.C.; Ross, G.M. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem. Int. 2012, 61, 1266–1275. [Google Scholar] [CrossRef]
- Holgado-Madruga, M.; Moscatello, D.K.; Emlet, D.R.; Dieterich, R.; Wong, A.J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. USA 1997, 94, 12419–12424. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Barker, P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002, 67, 203–233. [Google Scholar] [CrossRef] [PubMed]
- Skeldal, S.; Coulson, E.J. Signaling and function of death receptors of the tumor necrosis factor receptor superfamily. Encycl. Cell Biol. 2016, 3, 67–75. [Google Scholar] [CrossRef]
- Chao, M.V. The p75 neurotrophin receptor. J. Neurobiol. 1994, 25, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Mahadeo, D.C.; Kaplan, L.; Chao, M.V.; Hempstead, B.L. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 1994, 269, 6884–6891. [Google Scholar] [CrossRef] [PubMed]
- Hantzopoulos, P.; Suri, C.; Glass, D.J.; Goldfarb, M.; Yancopouloš, G.D. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron 1994, 13, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Verdi, J.M.; Birren, S.J.; Ibáñez, C.F.; Persson, H.; Kaplan, D.R.; Benedetti, M.; Chao, M.V.; Anderson, D.J. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron 1994, 12, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Ayer-LeLièvre, C.; Olson, L.; Ebendal, T.; Hallböök, F.; Persson, H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc. Natl. Acad. Sci. USA 1988, 85, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Buchberger, A.; Erck, C. Expression of nerve growth factor and neurotrophin receptors in testicular cells suggest novel roles for neurotrophins outside the nervous system. Reprod. Fertil. Dev. 1996, 8, 1075. [Google Scholar] [CrossRef]
- ChunMei, L.; Gen, W.; Qiang, W.; WanZhu, J.; Chie, F.; Akira, K.S.; Kawaguchi, M.; Taya, K. Expression of Nerve Growth Factor (NGF) and Its Receptors TrKA and p75 in the Reproductive Organs of Adult Male Rats. Zool. Sci. 2005, 22, 933–937. [Google Scholar] [CrossRef]
- Artico, M.; Bronzetti, E.; Saso, L.; Felici, L.; D’Ambrosio, A.; Forte, F.; Del Grande, C.; Ortolani, F. Immunohistochemical profile of some neurotransmitters and neurotrophins in the seminiferous tubules of rats treated by lonidamine. DOAJ (DOAJ Dir. Open Access J.) 2007, 51, 19–24. [Google Scholar]
- Perrard, M.-H.; Vigier, M.; Damestoy, A.; Chapat, C.; Silandre, D.; Rudkin, B.B.; Durand, P. Beta-nerve growth factor participates in an auto/paracine pathway of regulation of the meiotic differentiation of rat spermatocytes. J. Cell Physiol. 2007, 210, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Levanti, M.; Germanà, A.; De Carlos, F.; Ciriaco, E.; Vega, J.A.; Germanà, G. Effects of increased nerve growth factor plasma levels on the expression of TrkA and p75NTR in rat testicles. J. Anat. 2006, 208, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Arai, K.Y.; Shimizu, K.; Kojima, C.; Itoh, M.; Watanabe, G.; Taya, K. Cellular Localization of NGF and its Receptors trkA and p75LNGFR in Male Reproductive Organs of the Japanese Monkey, Macaca fuscata fuscata. Endocr. J. 2006, 29, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Y.; Yi, K.; Ma, Y.; Zhang, W.; Zhou, X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology 2010, 74, 1615–1622. [Google Scholar] [CrossRef]
- Jin, W.; Tanaka, A.; Watanabe, G.; Matsuda, H.; Taya, K. Effect of NGF on the motility and acrosome reaction of golden hamster spermatozoa in vitro. J. Reprod. Dev. 2010, 56, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Zerani, M.; Leonardi, L.; Pistilli, A.; Arruda-Alencar, J.M.; Stabile, A.M.; Rende, M.; Castellini, C.; Petrucci, L.; Parillo, F.; et al. Gene expression and localization of NGF and its cognate receptors NTRK1 and NGFR in the sex organs of male rabbits. Reprod. Domest. Anim. 2015, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Sari, L.M.; Zampini, R.; Argañaraz, M.E.; Carretero, M.I.; Fumuso, F.G.; Barraza, D.E.; Ratto, M.H.; Apichela, S.A. Expression of β-NGF and high-affinity NGF receptor (TrKA) in llama (Lama glama) male reproductive tract and spermatozoa. Mol. Reprod. Dev. 2018, 85, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Bosco, A.D.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Role of NGF on sperm traits: A review. Theriogenology 2020, 150, 210–214. [Google Scholar] [CrossRef]
- Russo, M.A.; Giustizieri, M.L.; Farini, D.; Siracusa, G. Expression of neurotrophin receptors in the developing and adult testis. PubMed 1995, 100 (Suppl. S1), 543–551. [Google Scholar]
- Cupp, A.S.; Kim, G.H.; Skinner, M.K. Expression and action of neurotropin-3 and nerve growth factor in embryonic and early postnatal rat testis development. Biol. Reprod. 2000, 63, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Giustizieri, M.L.; Favale, A.; Fantini, M.; Campagnolo, L.; Konda, D.; Germano, F.; Farini, D.; Manna, C.; Siracusa, G. Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis1. Biol. Reprod. 1999, 61, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, X. The potential roles of neurotrophins in male reproduction. Reproduction 2013, 145, R89–R95. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Cotozzolo, E.; Pistilli, A.; Rende, M.; Bartolini, D.; Di Sante, G.; Menchetti, L.; Bosco, A.D.; Stabile, A.M. The effect of interaction NGF/P75NTR in sperm cells: A rabbit model. Cells 2022, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Fanfarillo, F.; Tarani, L.; Blaconà, G.; Tarani, F.; Barbato, C.; Minni, A.; Ralli, M.; Francati, S.; Greco, A.; et al. NGF and the Male Reproductive System: Potential Clinical Applications in Infertility. Int. J. Mol. Sci. 2022, 23, 13127. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Cao, M.; Yuan, C.; Huang, Y.-W.; Zhao, Z.; Zhang, B.; Wang, X.; Wei, Y.; Fan, W.; Zhou, X.; et al. Effect of nerve growth factor on the proliferation in newborn bovine testicular Sertoli cells. Reproduction 2020, 160, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.; Spencer, S.D.; Nishimura, M.; Chen, K.S.; Pitts-Meek, S.; Armaninl, M.P.; Ling, L.H.; McMahon, S.B.; Shelton, D.L.; Levinson, A.D.; et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994, 76, 1001–1011. [Google Scholar] [CrossRef]
- Liebl, D.J.; Klesse, L.J.; Tessarollo, L.; Wohlman, T.; Parada, L.F. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc. Natl. Acad. Sci. USA 2000, 97, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Sari, L.M.; Zampini, R.; Del Pino, F.G.; Argañaraz, M.E.; Ratto, M.H.; Apichela, S.A. Effects of NGF Addition on Llama (Lama glama) Sperm Traits After Cooling. Front. Vet. Sci. 2021, 7, 610597. [Google Scholar] [CrossRef]
- Robinson, L.; Townsend, J.; Anderson, R.A. The human fetal testis is a site of expression of neurotrophins and their receptors: Regulation of the germ cell and peritubular cell population. J. Clin. Endocrinol. Metab. 2003, 88, 3943–3951. [Google Scholar] [CrossRef]
- Müller, D.; Davidoff, M.; Bargheer, O.; Paust, H.J.; Pusch, W.; Koeva, Y.; Ježek, D.; Holstein, A.F.; Middendorff, R. The expression of neurotrophins and their receptors in the prenatal and adult human testis: Evidence for functions in Leydig cells. Histochem. Cell Biol. 2006, 126, 199–211. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Wang, C.; Zhou, X. Absence of nerve growth factor and comparison of tyrosine kinase receptor A levels in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clin. Chim. Acta 2010, 411, 1482–1486. [Google Scholar] [CrossRef]
- Stabile, A.M.; Pistilli, A.; Moretti, E.; Bartolini, D.; Ruggirello, M.; Rende, M.; Castellini, C.; Mattioli, S.; Ponchia, R.; Tripodi, S.; et al. A possible role for nerve growth factor and its receptors in human sperm pathology. Biomedicines 2023, 11, 3345. [Google Scholar] [CrossRef]
- Abdulrahman, S.J.; Al-Ali, I.A.; Hassan, T.A.A.; Qassim, A.M. Correlation of nerve growth factor with antioxidants and sperm parameters among Iraqi infertile males. Int. J. Res. Pharm. Sci. 2019, 10, 1307–1313. [Google Scholar] [CrossRef]
- Shi, C.; Lin, K.J.; Xu, X.; Zhang, S.; Wang, N.; Fan, M. Evidence for the involvement of NGF in human sperm motility. J. Biomed. Sci. Eng. 2012, 5, 534–541. [Google Scholar] [CrossRef][Green Version]
- Lin, K.J.; Ding, X.; Shi, C.; Zeng, D.; QuZong, S.; Liu, S.; Wu, Y.; Gesang, L.; Fan, M.; Zhao, Y. Nerve growth factor promotes human sperm motility in vitro by increasing the movement distance and the number of A grade spermatozoa. Andrologia 2014, 47, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, B.; Tiptiri-Kourpeti, A.; Metallinou, C. IGF-I and NGFβ enhance in vitro progressive motility and vitality of human spermatozoa. Reprod. Med. Biol. 2021, 20, 361–367. [Google Scholar] [CrossRef]
- Saeednia, S.; Bahadoran, H.; Amidi, F.; Asadi, M.H.; Naji, M.; Fallahi, P.; Nejad, N.A. Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process. Iran. J. Basic Med. Sci. 2015, 18, 292–299. [Google Scholar] [PubMed]
- Saeednia, S.; Nashtaei, M.S.; Bahadoran, H.; Aleyasin, A.; Amidi, F. Effect of nerve growth factor on sperm quality in asthenozoosprmic men during cryopreservation. Reprod. Biol. Endocrinol. 2016, 14, 29. [Google Scholar] [CrossRef]
- Lacombe, C. Plenary lecture. The molecular biology erythropoietin. Nephrol. Dial. Transplant. 1999, 14, 22–28. [Google Scholar] [CrossRef]
- Jacobs, K.; Shoemaker, C.B.; Rudersdorf, R.; Neill, S.D.; Kaufman, R.J.; Mufson, A.; Seehra, J.; Jones, S.; Hewick, R.M.; Fritsch, E.F.; et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 1985, 313, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Suggs, S.; Lin, C.-W.; Browne, J.K.; Smalling, R.; Egrie, J.C.; Chen, K.K.; Fox, G.M.; Martin, F.; Stabinsky, Z. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1985, 82, 7580–7584. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.S. The erythropoietin receptor. Semin. Oncol. 2001, 28, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rossert, J.; Eckardt, K. Erythropoietin receptors: Their role beyond erythropoiesis. Nephrol. Dial. Transplant. 2005, 20, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.; Kovács, K.; Scheithauer, B.W. Erythropoietin: A Hormone with Multiple Functions. Pathobiology 2011, 78, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Bunn, H.F. Erythropoietin. Cold Spring Harb. Perspect. Med. 2013, 3, a011619. [Google Scholar] [CrossRef] [PubMed]
- Livnah, O.; Stura, E.A.; Middleton, S.A.; Johnson, D.L.; Jolliffe, L.K.; Wilson, I.A. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 1999, 283, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Philo, J.S.; Aoki, K.H.; Arakawa, T.; Narhi, L.O.; Wen, J. Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: One High-Affinity and one Low-Affinity interaction. Biochemistry 1996, 35, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.; Reid, S.W.J.; Li, C.; Cheetham, J.; Aoki, K.H.; Liu, B.; Zhan, H.; Osslund, T.D.; Chirino, A.J.; Zhang, J.; et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 1998, 395, 511–516. [Google Scholar] [CrossRef]
- Watowich, S.S.; Hilton, D.J.; Lodish, H.F. Activation and inhibition of erythropoietin receptor function: Role of receptor dimerization. Mol. Cell. Biol. 1994, 14, 3535–3549. [Google Scholar] [CrossRef]
- Watowich, S.S.; Yoshimura, A.; Longmore, G.D.; Hilton, D.J.; Yoshimura, Y.; Lodish, H.F. Homodimerization and constitutive activation of the erythropoietin receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Watowich, S.S. The erythropoietin receptor: Molecular structure and hematopoietic signaling pathways. J. Investig. Med. 2011, 59, 1067–1072. [Google Scholar] [CrossRef]
- Darnell, J.; Kerr, I.M.; Stark, G.S. JAK-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Hibi, M.; Hirano, T. Signal TransductionThrough cytokine receptors. Int. Rev. Immunol. 1998, 17, 75–102. [Google Scholar] [CrossRef]
- Ingley, E. Integrating novel signaling pathways involved in erythropoiesis. IUBMB Life 2012, 64, 402–410. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Karamouzis, M.V.; Papavassiliou, A.G. Selective modulation of the erythropoietic and tissue-protective effects of erythropoietin: Time to reach the full therapeutic potential of erythropoietin. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1776, 1–9. [Google Scholar] [CrossRef]
- Fisher, J.W. Erythropoietin: Physiology and Pharmacology update. Exp. Biol. Med. 2003, 228, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lappin, T.; Maxwell, A.P.; Johnston, P.G. EPO’s alter ego: Erythropoietin has multiple actions. Stem Cells 2002, 20, 485–492. [Google Scholar] [CrossRef]
- Dame, C.; Juul, S.E.; Christensen, R.D. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Neonatology 2001, 79, 228–235. [Google Scholar] [CrossRef]
- Smith, K.; Bleyer, A.J.; Little, W.C.; Sane, D.C. The cardiovascular effects of erythropoietin. Cardiovasc. Res. 2003, 59, 538–548. [Google Scholar] [CrossRef]
- Yasuda, Y.; Fujita, Y.; Musha, T.; Tanaka, H.; Shiokawa, S.; Nakamatsu, K.; Mori, S.; Matsuo, T.; Nakamura, Y. Expression of Erythropoietin in Human Female Reproductive Organs. Ital. J. Anat. Embryol. 2001, 106 (Suppl. S2), 215–222. [Google Scholar]
- Lessan-Pezeshki, M.; Ghazizadeh, S. Sexual and reproductive function in end-stage renal disease and effect of kidney transplantation. Asian J. Androl. 2008, 10, 441–446. [Google Scholar] [CrossRef]
- Brines, M.; Cerami, A. Discovering erythropoietin’s extra-hematopoietic functions: Biology and clinical promise. Kidney Int. 2006, 70, 246–250. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Kang, J.; Maiese, K. Erythropoietin: Cytoprotection in vascular and neuronal cells. Curr. Drug Targets 2003, 3, 141–154. [Google Scholar] [CrossRef]
- Masuda, S.; Nagao, M.; Sasaki, R. Erythropoietic, neurotrophic, and angiogenic functions of erythropoietin and regulation of erythropoietin production. Int. J. Hematol. 1999, 70, 1–6. [Google Scholar]
- Jelkmann, W. Molecular Biology of Erythropoietin. Intern. Med. 2004, 43, 649–659. [Google Scholar] [CrossRef]
- Jelkmann, W. Effects of erythropoietin on brain function. Curr. Pharm. Biotechnol. 2005, 6, 65–79. [Google Scholar] [CrossRef]
- Brines, M.; Patel, N.S.A.; Villa, P.; Brines, C.M.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C.; et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930. [Google Scholar] [CrossRef]
- Brines, M. The Therapeutic Potential of Erythropoiesis-Stimulating Agents for Tissue Protection: A tale of two receptors. Blood Purif. 2010, 29, 86–92. [Google Scholar] [CrossRef]
- Mioni, R.; Gottardello, F.; Bordon, P.; Montini, G.; Foresta, C. Evidence for specific binding and stimulatory effects of recombinant human erythropoietin on isolated adult rat Leydig cells. Eur. J. Endocrinol. 1992, 127, 459–465. [Google Scholar] [CrossRef]
- Elliott, S.; Pham, E.; Macdougall, I.C. Erythropoietins: A common mechanism of action. Exp. Hematol. 2008, 36, 1573–1584. [Google Scholar] [CrossRef]
- Magnanti, M.; Gandini, O.; Giuliani, L.; Gazzaniga, P.; Marti, H.H.; Gradilone, A.; Frati, L.; Aglianò, A.M.; Gassmann, M. Erythropoietin expression in primary rat Sertoli and peritubular myoid cells. Blood 2001, 98, 2872–2874. [Google Scholar] [CrossRef]
- Foresta, C.; Mioni, R.; Bordon, P.; Gottardello, F.; Nogara, A.; Rossato, M. Erythropoietin and testicular steroidogenesis: The role of second messengers. Eur. J. Endocrinol. 1995, 132, 103–108. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kanzaki, M.; Kamidono, S.; Fujisawa, M. Effect of erythropoietin on Leydig cell is associated with the activation of Stat5 pathway. Mol. Cell. Endocrinol. 2004, 213, 193–198. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yanase, H.; Iwanaga, T.; Sasaki, R.; Nagao, M. Epididymis is a novel site of erythropoietin production in mouse reproductive organs. Biochem. Biophys. Res. Commun. 2002, 296, 145–151. [Google Scholar] [CrossRef]
- Collares, T.; Campos, V.F.; Urtiaga, G.; De Leon, P.M.M.; Amaral, M.; Hartleben, C.P.; McBride, A.J.A.; Dellagostin, O.A.; Deschamps, J.C.; Seixas, F.K. Erythropoietin non-viral gene therapy does not affect motility, viability, morphology or concentration of rabbit sperm. Animal 2013, 7, 778–783. [Google Scholar] [CrossRef]
- Akman, O.; Özkanlar, Y.E.; Özkanlar, S.; Oruç, E.; Ulaş, N.; Ziypak, T.; Lehimcioğlu, N.C.; Türkeli, M.; Uçar, Ö. Erythropoietin hormone and ACE inhibitor protect the sperm parameters of adult male rats against doxorubicin toxicity. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 805–812. [Google Scholar]
- Shinoda, K.; Mitsumori, K.; Yasuhara, K.; Uneyama, C.; Oda, H.; Hirose, M.; Uehara, M. Doxorubicin induces male germ cell apoptosis in rats. Arch. Toxicol. 1999, 73, 274–281. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Hou, M.; Chrysis, D.; Nurmio, M.; Parvinen, M.; Eksborg, S.; Söder, O.; Jahnukainen, K. Doxorubicin Induces Apoptosis in Germ Line Stem Cells in the Immature Rat Testis and Amifostine Cannot Protect against This Cytotoxicity. Cancer Res. 2005, 65, 9999–10005. [Google Scholar] [CrossRef]
- Çeribaşı, A.O.; Sakin, F.; Türk, G.; Sönmez, M.; Ateşşahín, A. Impact of ellagic acid on adriamycin-induced testicular histopathological lesions, apoptosis, lipid peroxidation and sperm damages. Exp. Toxicol. Pathol. 2012, 64, 717–724. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. [Malondialdehyde (MDA) as a lipid peroxidation marker]. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Ascensao, J.; Gaylis, F.; Bronson, D.L.; Ee, F.; Zanjani, E. Erythropoietin production by a human testicular germ cell line. Blood 1983, 62, 1132–1134. [Google Scholar] [CrossRef][Green Version]
- Eschbach, J.W.; Egrie, J.C.; Downing, M.R.; Browne, J.K.; Adamson, J.W. Correction of the Anemia of End-Stage Renal Disease with Recombinant Human Erythropoietin. N. Engl. J. Med. 1987, 316, 73–78. [Google Scholar] [CrossRef]
- Foresta, C.; Mioni, R.; Bordon, P.; Miotto, D.; Montini, G.; Varotto, A. Erythropoietin stimulates testosterone production in man. J. Clin. Endocrinol. Metab. 1994, 78, 753–756. [Google Scholar] [CrossRef]
- Temma, K.; Shimoya, K.; Hashimoto, K.; Zhang, Q.; Koyama, M.; Murata, Y. Detection of erythropoietin in human seminal plasma. Fertil. Steril. 2004, 81, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Tuğ, N.; Kılıç, Ü.; Karateke, A.; Yılmaz, B.; Ugur, M.; Kılıç, E. Erythropoietin receptor-like immunostaining on human spermatozoa. Reprod. Biomed. Online 2010, 21, 718–720. [Google Scholar] [CrossRef][Green Version]
- Tuğ, N.; Altunkaynak, M.E.; Aktaş, R.G.; Kılıç, Ü.; Yılmaz, B.; Çam, Ç.; Karateke, A. Does erythropoietin affect motility of spermatozoa? Arch. Gynecol. Obstet. 2009, 281, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, B.; Tiptiri-Kourpeti, A.; Metalinou, C. Erythropoitein Increases In vitro Motility and vitality of human spermatozoa. In Vivo 2021, 35, 2669–2673. [Google Scholar] [CrossRef]
- Herington, A.C.; Cornell, H.J.; Kuffer, A.D. Recent advances in the biochemistry and physiology of the insulin-like growth factor/somatomedin family. Int. J. Biochem. 1983, 15, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Rechler, M.M.; Nissley, S.P. The nature and regulation of the receptors for Insulin-Like growth factors. Annu. Rev. Physiol. 1985, 47, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Zapf, J.; Schmid, C.; Froesch, E.R. 1 Biological and immunological properties of insulin-like growth factors (IGF) I and II. Clin. Endocrinol. Metab. 1984, 13, 3–30. [Google Scholar] [CrossRef]
- Monzavi, R.; Cohen, P. IGFs and IGFBPs: Role in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, J.; Matijević, T.; Knežević, J. Biological & physiological aspects of action of insulin-like growth factor peptide family. Indian J. Med. Res. 2007, 125, 511–522. [Google Scholar] [PubMed]
- Novosyadlyy, R.; LeRoith, D. Insulin-Like growth factors. In Encyclopedia of Cancer; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1877–1882. [Google Scholar] [CrossRef]
- Rajah, R.; Valentinis, B.; Cohen, P. Insulin-like Growth Factor (IGF)-binding Protein-3 Induces Apoptosis and Mediates the Effects of Transforming Growth Factor-β1 on Programmed Cell Death through a p53- and IGF-independent Mechanism. J. Biol. Chem. 1997, 272, 12181–12188. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, R.D. “Physiology of Insulin-like Growth Factor 1.” Edited by Peter J. Snyder, MD, and Kathryn A. Martin, MD. MD Section Editor. UpToDate. Available online: https://www.uptodate.com/contents/physiology-of-insulin-like-growth-factor-1 (accessed on 18 February 2024).
- Laviola, L.; Natalicchio, A.; Giorgino, F. The IGF-I signaling pathway. Curr. Pharm. Des. 2007, 13, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, F.; Takahashi, S. 40 YEARS OF IGF1: IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Glander, H.-J.; Kratzsch, J.; Weisbrich, C.; Birkenmeier, G. Andrology: Insulin-like growth factor-I and 2-macroglobulin in seminal plasma correlate with semen quality. Hum. Reprod. 1996, 11, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.B.; Naz, R.K. Modulation of insulin-like growth factor-1 in the seminal plasma of infertile men. Andrology 1999, 20, 118–125. [Google Scholar] [CrossRef]
- Baker, J.C.; Hardy, M.P.; Zhou, J.; Bondy, C.A.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henricks, D.M.; Kouba, A.J.; Lackey, B.R.; Boone, W.R.; Gray, S.L. Identification of Insulin-Like growth factor I in bovine seminal plasma and its receptor on spermatozoa: Influence on sperm motility1. Biol. Reprod. 1998, 59, 330–337. [Google Scholar] [CrossRef]
- Minelli, A.; Liguori, L.; Collodel, G.; Lattaioli, P.; Castellini, C. Effects of the purified IGF-I complex on the capacitation and acrosome reaction of rabbit spermatozoa. J. Exp. Zool. 2001, 290, 311–317. [Google Scholar] [CrossRef]
- Hirai, M.; Boersma, A.; Hoeflich, A.; Wolf, E.; Föll, J.; Aumüller, R.; Braun, J. Objectively Measured Sperm Motility and Sperm Head Morphometry in Boars (Sus scrofa): Relation to Fertility and Seminal Plasma Growth Factors. Andrology 2001, 22, 104–110. [Google Scholar] [CrossRef]
- Macpherson, M.L.; Simmen, R.C.M.; Simmen, F.A.; Hernández, J.G.; Sheerin, B.R.; Varner, D.D.; Loomis, P.R.; Cadario, M.E.; Miller, C.; Brinsko, S.P.; et al. Insulin-Like Growth Factor-I and Insulin-Like Growth Factor Binding Protein-2 and -5 in Equine Seminal Plasma: Association with Sperm Characteristics and Fertility1. Biol. Reprod. 2002, 67, 648–654. [Google Scholar] [CrossRef]
- Ovesen, P.; Flyvbjerg, A.; Ørskov, H. Insulin-like growth factor I (IGF-I) and IGF binding proteins in seminal plasma before and after vasectomy in normal men. Fertil. Steril. 1995, 63, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Haskell, J.F.; Vinson, N.; Terracio, L. Characterization of Insulin and Insulin-Like Growth Factor I receptors of purified leydig cells and their role in steroidogenesis in primary culture: A comparative study*. Endocrinology 1986, 119, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Champion, Z.J.; Vickers, M.H.; Gravance, C.G.; Breier, B.H.; Casey, P.J. Growth hormone or insulin-like growth factor-I extends longevity of equine spermatozoa in vitro. Theriogenology 2002, 57, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.; Reddy, I.J.; Nandi, S.; Rao, S.B.N.; Ravindra, J.P. Influence of IGF-I on buffalo (Bubalus bubalis) spermatozoa motility, membrane integrity, lipid peroxidation and fructose uptake in vitro. Anim. Reprod. Sci. 2009, 113, 60–70. [Google Scholar] [CrossRef]
- Selvaraju, S.; Nandi, S.; Subramani, T.; Raghavendra, B.S.; Rao, S.B.N.; Ravindra, J.P. Improvement in buffalo (Bubalus bubalis) spermatozoa functional parameters and fertility in vitro: Effect of insulin-like growth factor-I. Theriogenology 2010, 73, 1–10. [Google Scholar] [CrossRef]
- Zangerônimo, M.G.; Silva, D.M.; Murgas, L.D.S.; De Sousa, R.V.; Rocha, L.C.S.; Pereira, B.A.; Faria, B.G.; Veras, G.C. Identification of insulin-like growth factor-I in boar seminal plasma and its influence on sperm quality. Arch. Zootec. 2013, 62, 411–418. [Google Scholar] [CrossRef][Green Version]
- Mendez, M.F.B.; Zangerônimo, M.G.; Rocha, L.C.S.; Faria, B.G.; Pereira, B.A.; Fernandes, C.D.; Chaves, B.R.; Murgas, L.D.S.; De Sousa, R.V. Effect of the addition of IGF-I and vitamin E to stored boar semen. Animal 2013, 7, 793–798. [Google Scholar] [CrossRef]
- Vannelli, B.; Barni, T.; Orlando, C.; Natali, A.; Serio, M.; Balboni, G. Insulin-like growth factor-1 (IGF-I) and IGF-I receptor in human testis: An immunohistochemical study. Fertil. Steril. 1988, 49, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Luengo, S.; Fernández, P.; Romeu, A. Insulin growth factors may be implicated in human sperm capacitation. Fertil. Steril. 2005, 83, 1064–1066. [Google Scholar] [CrossRef]
- Naz, R.K.; Padman, P. Identification of insulin-like growth factor (IGF)-1 receptor in human sperm cell. Arch. Androl. 1999, 43, 153–159. [Google Scholar] [CrossRef]
- Nikolić-Judith, A.; Nedić, O.R.; Mihailović, N.; Baričević, I. Insulin-like growth factor-I and -II and their binding proteins in human ejaculates. Jugosl. Med. Biohemija 2003, 22, 221–227. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Bondy, C.A. Anatomy of the insulin-like growth factor system in the human testis. Fertil. Steril. 1993, 60, 897–904. [Google Scholar] [CrossRef]
- Lee, K.-O.; Ng, S.-C.; Lee, P.-S.; Bongso, A.; Taylor, E.A.; Lin, T.; Ratnam, S.S. Effect of growth hormone therapy in men with severe idiopathic oligozoospermia. Eur. J. Endocrinol. 1995, 132, 159–162. [Google Scholar] [CrossRef]
- Neirijnck, Y.; Papaioannou, M.D.; Nef, S. The Insulin/IGF system in mammalian sexual development and reproduction. Int. J. Mol. Sci. 2019, 20, 4440. [Google Scholar] [CrossRef]
- Du, Y.; Chi, X.; Wang, Y.; Cai, X.; Zeng, W.; Huo, Y.; Zhang, M.; Wang, Z.; Guo, Z.; Qiu, J.; et al. Advancements in the ERK1/2 signaling pathway affecting male reproduction. Front. Biosci. 2024, 29, 23. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Q.; Huang, S.; Zhou, T.; Guo, Y.; Wang, G.; Guo, X.; Zhou, Z.; Sha, J. Quantitative phosphoproteomics analysis reveals a key role of insulin Growth Factor 1 receptor (IGF1R) tyrosine kinase in human sperm capacitation*. Mol. Cell. Proteom. 2015, 14, 1104–1112. [Google Scholar] [CrossRef]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miao Zr, M.; Lin, T.; Ta, B.; Zhou, X.J.; Cohen, P.; Lee, K.-O. Effect of insulin-like growth factors (IGFs) and IGF-binding proteins on in vitro sperm motility. Clin. Endocrinol. 1998, 49, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.A.M.; Porchia, L.M.; Camargo, F.N.; López-Bayghen, E. The use of insulin-like growth factor 1 improved the parameters of the seminogram in a patient with severe oligoasthenoteratozoospermia. SAGE Open Med. Case Rep. 2019, 7, 2050313X1983415. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.-S.; Lee, J.S.; Seo, J.T. Serum and seminal plasma insulin-like growth factor-1 in male infertility. Clin. Exp. Reprod. Med. 2016, 43, 97. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Abo-Azma, S.M.; Fayed, S.M.; Mostafa, T. Seminal plasma cotinine and insulin-like growth factor-I in idiopathic oligoasthenoteratozoospermic smokers. BJU Int. 2008, 103, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Getpook, C.; Wirotkarun, S. Sperm motility stimulation and preservation with various concentrations of follicular fluid. J. Assist. Reprod. Genet. 2007, 24, 425–428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, C.; Chao, H.; Lee, S.L.; Wei, Y. Modification of human sperm function by human follicular fluid: A review. Int. J. Androl. 1993, 16, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ralt, D.; Manor, M.; Cohen-Dayag, A.; Tur-Kaspa, I.; Ben-Shlomo, I.; Makler, A.; Yuli, I.; Dor, J.; Blumberg, S.; Mashiach, S.; et al. Chemotaxis and chemokinesis of human spermatozoa to follicular factors1. Biol. Reprod. 1994, 50, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Lighten, A.D.; Hardy, K.; Winston, R.M.L.; Moore, G.E. Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol. Reprod. Dev. 1997, 47, 134–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metallinou, C.; Staneloudi, C.; Nikolettos, K.; Asimakopoulos, B. NGF, EPO, and IGF-1 in the Male Reproductive System. J. Clin. Med. 2024, 13, 2918. https://doi.org/10.3390/jcm13102918
Metallinou C, Staneloudi C, Nikolettos K, Asimakopoulos B. NGF, EPO, and IGF-1 in the Male Reproductive System. Journal of Clinical Medicine. 2024; 13(10):2918. https://doi.org/10.3390/jcm13102918
Chicago/Turabian StyleMetallinou, Chryssa, Chrysovalanto Staneloudi, Konstantinos Nikolettos, and Byron Asimakopoulos. 2024. "NGF, EPO, and IGF-1 in the Male Reproductive System" Journal of Clinical Medicine 13, no. 10: 2918. https://doi.org/10.3390/jcm13102918
APA StyleMetallinou, C., Staneloudi, C., Nikolettos, K., & Asimakopoulos, B. (2024). NGF, EPO, and IGF-1 in the Male Reproductive System. Journal of Clinical Medicine, 13(10), 2918. https://doi.org/10.3390/jcm13102918






