Intra-Individual Relationship between Heart Rate Variability and the Underlying Heart Rate in Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Postural Provocations and ECG Recordings
2.3. QRS Detection
2.4. Heart Rate and Heart Rate Variability
- SDNN—standard deviation of all normal-to-normal RR intervals (note that the exclusion of ectopic beats meant that all the RR intervals were between QRS complexes classified as normal sinus rhythm beats), and
- RMSSD—root mean square of the differences between successive RR intervals.
- LF/HF ratio—obtained as a proportion of the absolute values of the LF and HF components, and
- Quasi-normalised HF components, expressed as qnHF = HF/(LF + HF).
2.5. HRV/Heart Rate Relationship
2.6. Statistics and Data Presentation
3. Results
3.1. Population
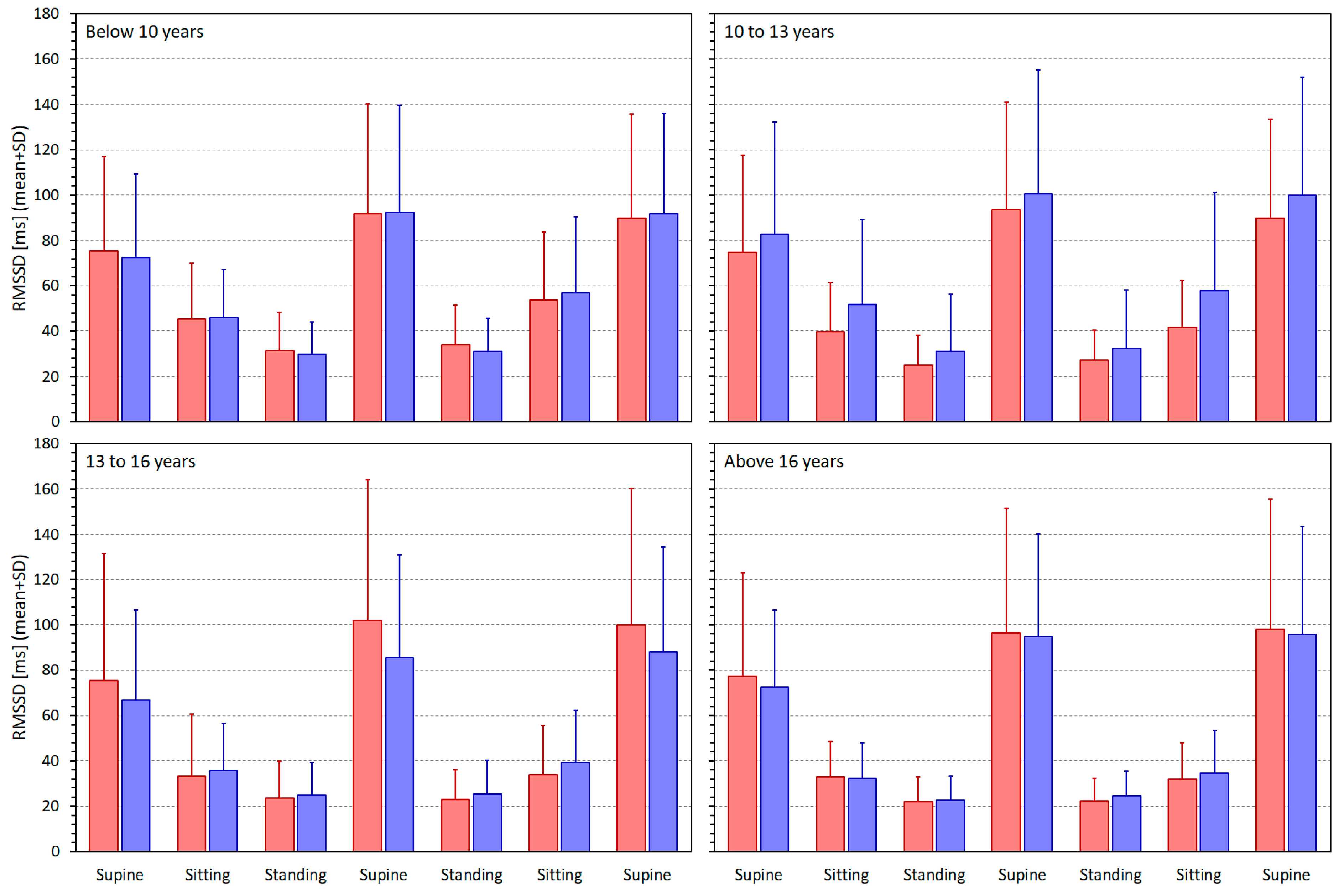
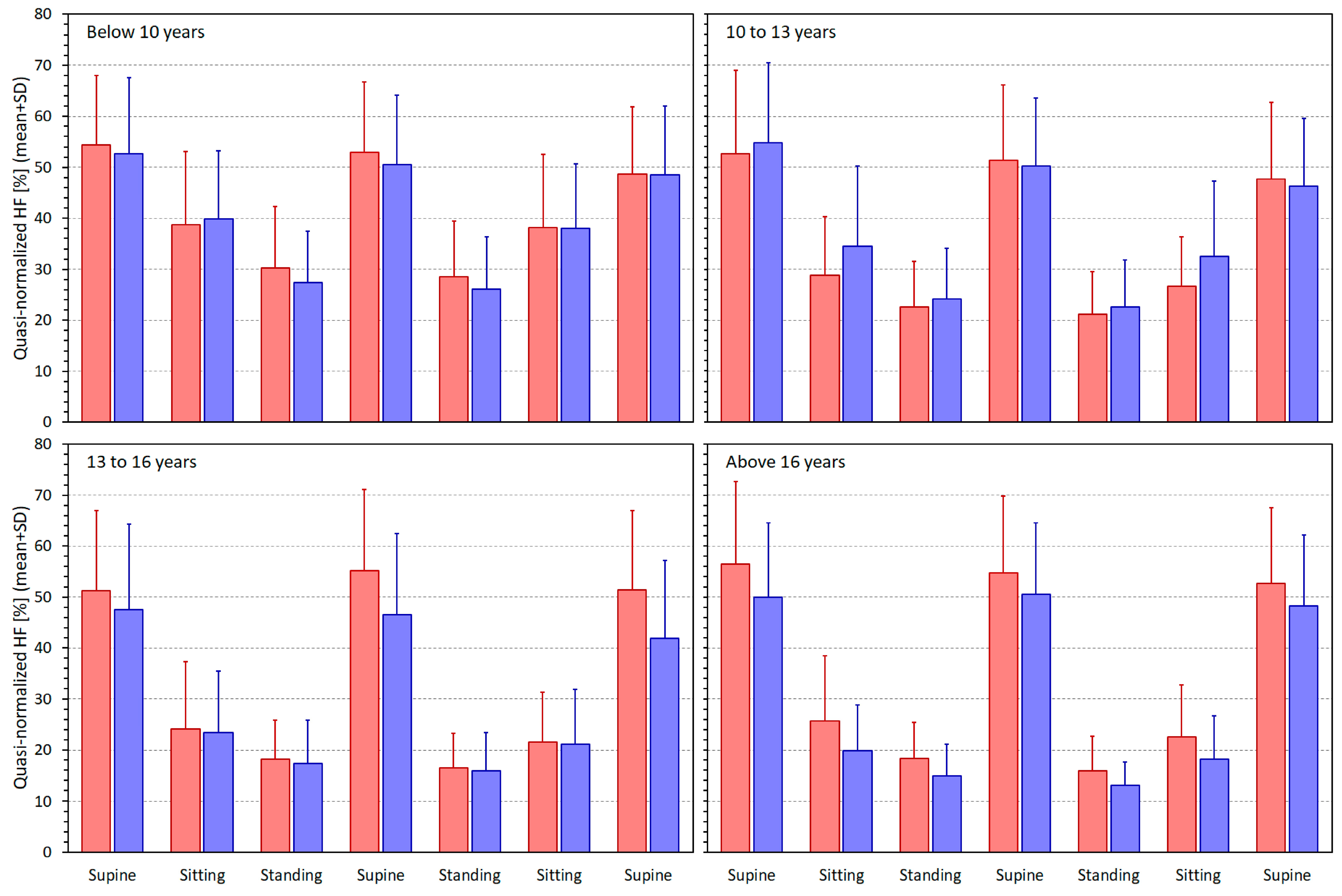
3.2. Heart Rate and Heart Rate Variability Indices
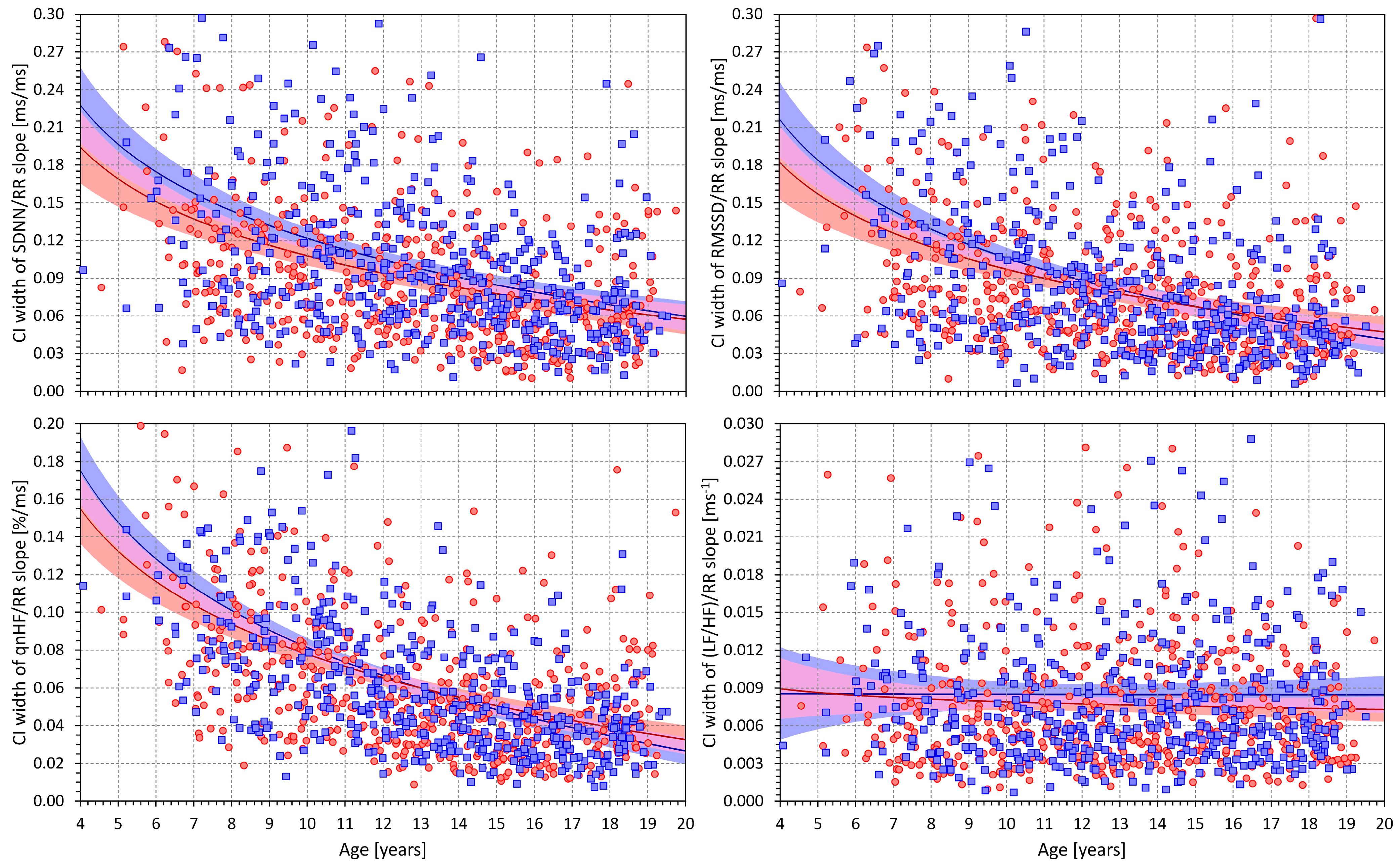
3.3. HRV/RR Relationship in the Complete Population
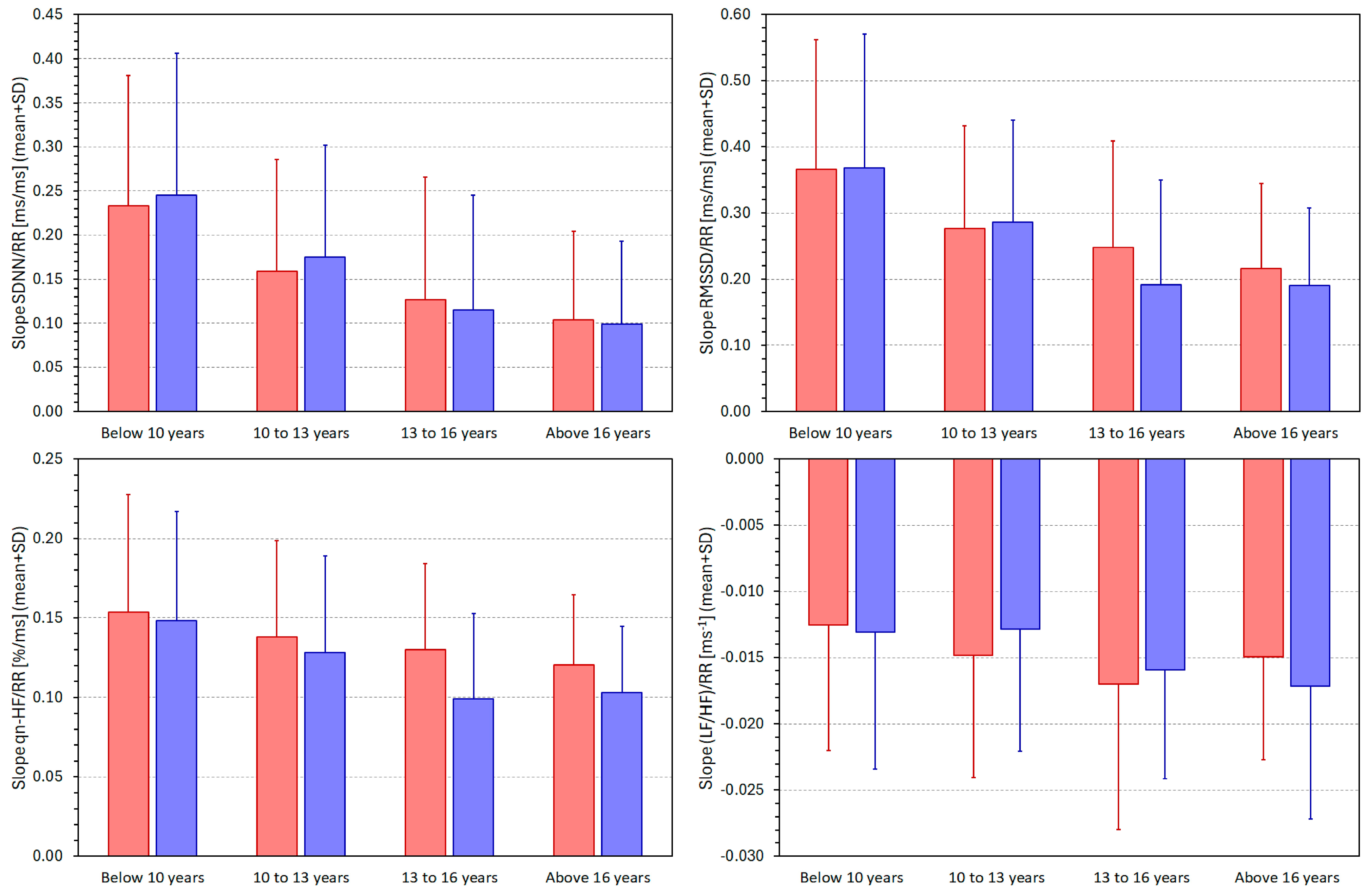
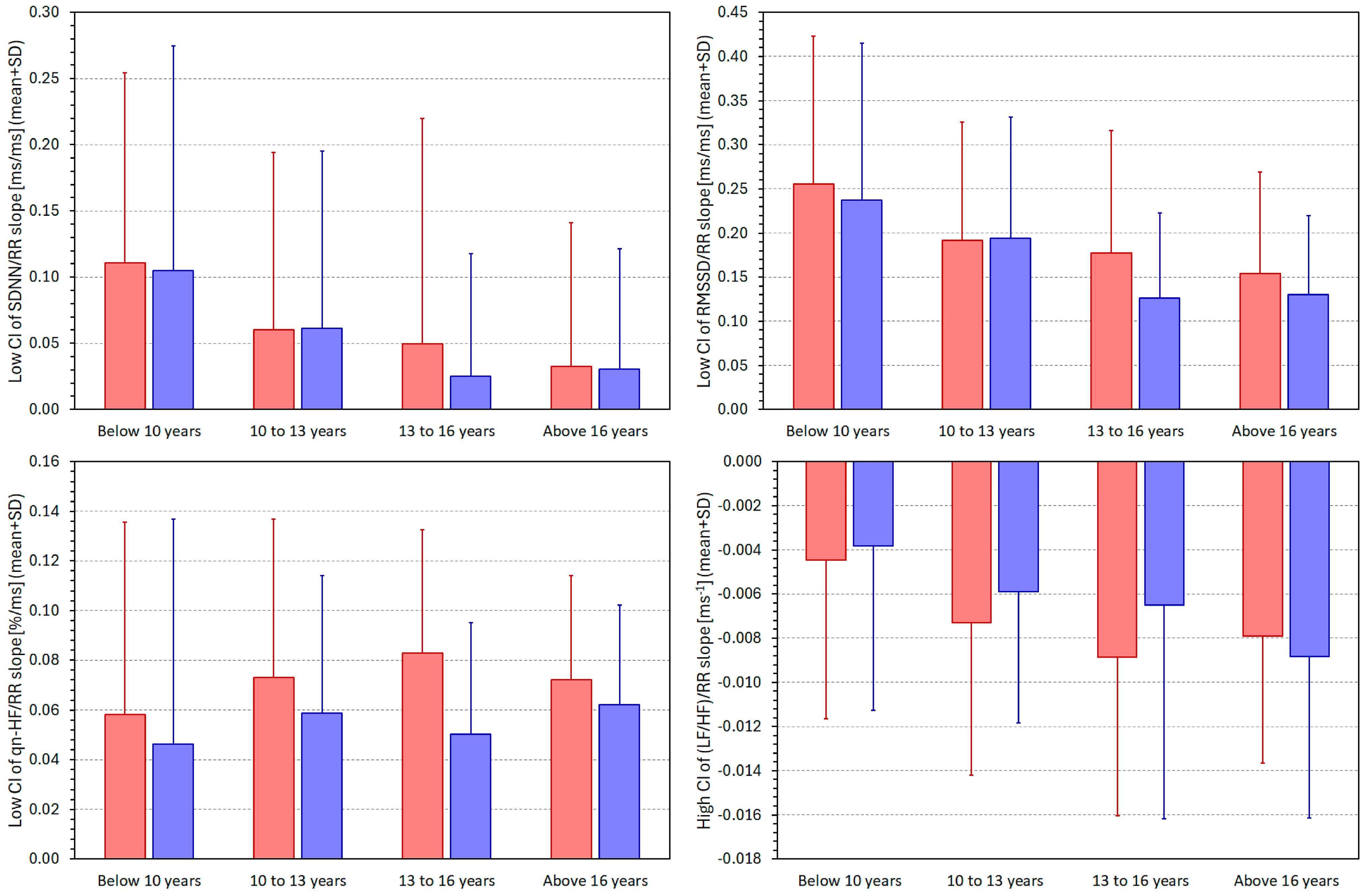
3.4. HRV/RR Relationship in Population Quartiles
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability, origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis, joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–562. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Farrell, T.; Cripps, T.; Camm, A.J. Heart rate variability in relation to prognosis after myocardial infarction, selection of optimal processing techniques. Eur. Heart J. 1989, 10, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Zuanetti, G.; Neilson, J.M.; Latini, R.; Santoro, E.; Maggioni, A.P.; Ewing, D.J. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era. The GISSI-2 results. Gruppo Italiano per lo Studio della Sopravvivenza nell’ Infarto Miocardico. Circulation 1996, 94, 432–436. [Google Scholar] [CrossRef]
- Giordano, M.; Manzella, D.; Paolisso, G.; Caliendo, A.; Varricchio, M.; Giordano, C. Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol. Dial. Transplant. 2001, 16, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.J.; D’Elia, J.A.; Weinrauch, L.A.; Lerman, I.; Gaur, A. Marked abnormalities in heart rate variability are associated with progressive deterioration of renal function in type I diabetic patients with overt nephropathy. Int. J. Cardiol. 2002, 86, 281–287. [Google Scholar] [CrossRef]
- Urbancic-Rovan, V.; Meglic, B.; Stefanovska, A.; Bernjak, A.; Azman-Juvan, K.; Kocijancic, A. Incipient cardiovascular autonomic imbalance revealed by wavelet analysis of heart rate variability in Type 2 diabetic patients. Diabet. Med. 2007, 24, 18–26. [Google Scholar] [CrossRef]
- Panina, G.; Khot, U.N.; Nunziata, E.; Cody, R.J.; Binkley, P.F. Assessment of autonomic tone over a 24-hour period in patients with congestive heart failure, relation between mean heart rate and measures of heart rate variability. Am. Heart J. 1995, 129, 748–753. [Google Scholar] [CrossRef]
- Panina, G.; Khot, U.N.; Nunziata, E.; Cody, R.J.; Binkley, P.F. Role of spectral measures of heart rate variability as markers of disease progression in patients with chronic congestive heart failure not treated with angiotensin-converting enzyme inhibitors. Am. Heart J. 1996, 131, 153–157. [Google Scholar] [CrossRef]
- Nikolic, V.N.; Jevtovic-Stoimenov, T.; Stokanovic, D.; Milovanovic, M.; Velickovic-Radovanovic, R.; Pesic, S.; Stoiljkovic, M.; Pesic, G.; Ilic, S.; Deljanin-Ilic, M.; et al. An inverse correlation between TNF alpha serum levels and heart rate variability in patients with heart failure. J. Cardiol. 2013, 62, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.T.; Rodrigues, S.; Campelo, M.; Dias, D.; Rego, R.; Rocha, H.; Sá, F.; Tavares-Silva, M.; Pinto, R.; Pestana, G.; et al. Heart rate variability in patients with refractory epilepsy, The influence of generalized convulsive seizures. Epilepsy Res. 2021, 178, 106796. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, K.W.; Yang, E.; Kim, S. The Association of nocturnal seizures and interictal cardiac/central autonomic function in frontal lobe epilepsy, Heart rate variability and central autonomic network analysis. Neuropsychiatr. Dis. Treat. 2023, 19, 2081–2091. [Google Scholar] [CrossRef]
- Buchman, T.G.; Stein, P.K.; Goldstein, B. Heart rate variability in critical illness and critical care. Curr. Opin. Crit. Care 2002, 8, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.W.; Barrett-Jolley, R.; Krige, A.; Welters, I.D. Heart rate variability: Measurement and emerging use in critical care medicine. J. Intensive Care Soc. 2020, 21, 148–157. [Google Scholar] [CrossRef]
- Wójcik, M.; Siatkowski, I. The effect of cranial techniques on the heart rate variability response to psychological stress test in firefighter cadets. Sci. Rep. 2023, 13, 7780. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.U.C.; Serfaty, F.M.; Cunha, M.R.; Oigman, W.; Tarvainen, M.P.; Neves, M.F. Heart rate variability and perception of mental stress among medical students and residents at an emergency department. Am. J. Emerg. Med. 2023, 78, 12–17. [Google Scholar] [CrossRef]
- Qin, H.; Fietze, I.; Mazzotti, D.R.; Steenbergen, N.; Kraemer, J.F.; Glos, M.; Wessel, N.; Song, L.; Penzel, T.; Zhang, X. Obstructive sleep apnea heterogeneity and autonomic function, a role for heart rate variability in therapy selection and efficacy monitoring. J. Sleep. Res. 2024, 33, e14020. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R.; Allen, J.R.; Robinson, J.B.; Earley, R.L.; Fedewa, M.V.; Bragg, A.; Keith, C.M.; Wingo, J.E. Heart rate variability and training load among national collegiate athletic association division 1 college football players throughout spring camp. J. Strength. Cond. Res. 2018, 32, 3127–3134. [Google Scholar] [CrossRef]
- Watanabe, T.; Sugiyama, Y.; Sumi, Y.; Watanabe, M.; Takeuchi, K.; Kobayashi, F.; Kono, K. Effects of vital exhaustion on cardiac autononomic nervous functions assessed by heart rate variability at rest in middle-aged male workers. Int. J. Behav. Med. 2002, 9, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Copie, X.; Staunton, A.; Malik, M. Numeric processing of Lorenz plots of R-R intervals from long-term ECGs. Comparison with time-domain measures of heart rate variability for risk stratification after myocardial infarction. J. Electrocardiol. 1995, 28, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Mäkikallio, T.; Ulm, K.; Hnatkova, K.; Schömig, A.; Huikuri, H.; Bunde, A.; et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction, cohort study. Lancet 2006, 367, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Schroeder, R.; Truebner, S.; Goernig, M.; Figulla, H.R.; Schirdewan, A. Comparison of nonlinear methods symbolic dynamics, detrended fluctuation, and Poincare plot analysis in risk stratification in patients with dilated cardiomyopathy. Chaos 2007, 17, 015120. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Zabel, M.; Lombardi, F.; Malik, M.; e-Health, Digital Rhythm Study Group of the European Heart Rhythm Association. Measurement of cardiovascular autonomic function: Where to go from here? Int. J. Cardiol. 2017, 249, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Huikuri, H.; Schmidt, G.; Malik, M.; e-Rhythm Study Group of European Heart Rhythm Association. Short-term heart rate variability: Easy to measure, difficult to interpret. Heart Rhythm 2018, 15, 1559–1560. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J. Components of heart rate variability—What they really mean and what we really measure. Am. J. Cardiol. 1993, 72, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Copie, X.; Hnatkova, K.; Staunton, A.; Fei, L.; Camm, A.J.; Malik, M. Predictive power of increased heart rate versus depressed left ventricular ejection fraction and heart rate variability for risk stratification after myocardial infarction. Results of a two-year follow-up study. J. Am. Coll. Cardiol. 1996, 27, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.E.; Poloniecki, J.D.; Camm, A.J.; Malik, M. Relation of mean heart rate and heart rate variability in patients with left ventricular dysfunction. Am. J. Cardiol. 1999, 84, 225–228. [Google Scholar] [CrossRef]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef]
- Bailón, R.; Serrano, P.; Laguna, P. Influence of time-varying mean heart rate in coronary artery disease diagnostic performance of heart rate variability indices from exercise stress testing. J. Electrocardiol. 2011, 44, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Zabel, M. CrossTalk proposal, Heart rate variability is a valid measure of cardiac autonomic responsiveness. J. Physiol. 2019, 597, 2595–2598. [Google Scholar] [CrossRef]
- Boyett, M.; Wang, Y.; D’Souza, A. CrossTalk opposing view, Heart rate variability as a measure of cardiac autonomic responsiveness is fundamentally flawed. J. Physiol. 2019, 597, 2599–2601. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Zabel, M. Rebuttal. J. Physiol. 2019, 597, 2603–2604. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Pluta, W. Alterations of an average heart rate change heart rate variability due to mathematical reasons. Int. J. Cardiol. 2008, 128, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J. Why should one normalize heart rate variability with respect to average heart rate. Front. Physiol. 2013, 4, 306. [Google Scholar] [CrossRef] [PubMed]
- Carter, R., 3rd; Hinojosa-Laborde, C.; Convertino, V.A. Heart rate variability in patients being treated for dengue viral infection, new insights from mathematical correction of heart rate. Front. Physiol. 2014, 5, 46. [Google Scholar] [CrossRef]
- Sacha, J. Interaction between heart rate and heart rate variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, J.S.; Sacha, J.; Jeleń, P.J.; Zieliński, J.; Przybylski, J. Heart rate and respiratory rate influence on heart rate variability repeatability, Effects of the correction for the prevailing heart rate. Front. Physiol. 2016, 7, 356. [Google Scholar] [CrossRef]
- de Geus, E.J.C.; Gianaros, P.J.; Brindle, R.C.; Jennings, J.R.; Berntson, G.G. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 2019, 56, e13287. [Google Scholar] [CrossRef]
- Maltsev, A.V.; Monfredi, O.; Maltsev, V.A. Universal inverse-square relationship between heart rate variability and heart rate originating in cardiac pacemaker cells. JACC Clin. Electrophysiol. 2022, 8, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, J.S.; Sacha, J.; Jeleń, P.J.; Pawłowski, M.; Werner, B.; Dąbrowski, M.J. Interaction between heart rate variability and heart rate in pediatric population. Front. Physiol. 2015, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Bobkowski, W.; Stefaniak, M.E.; Krauze, T.; Gendera, K.; Wykretowicz, A.; Piskorski, J.; Guzik, P. Measures of heart rate variability in 24-h ECGs depend on age but not gender of healthy children. Front. Physiol. 2017, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Pahlm, O.; Sórnmo, L. Software QRS detection in ambulatory monitoring—A review. Med. Biol. Eng. Comput. 1984, 22, 289–297. [Google Scholar] [CrossRef]
- Kors, J.A.; Talmon, J.L.; van Bemmel, J.H. Multilead ECG analysis. Comput. Biomed. Res. 1986, 19, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, I.K.; Christov, I.I. Electrocardiogram signal preprocessing for automatic detection of QRS boundaries. Med. Eng. Phys. 1999, 21, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.U.; Hennig, C.; Orglmeister, R. The principles of software QRS detection. IEEE Eng. Med. Biol. Mag. 2002, 21, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Arzeno, N.M.; Poon, C.S.; Deng, Z.D. Quantitative analysis of QRS detection algorithms based on the first derivative of the ECG. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Hnatkova, K.; Šišáková, M.; Smetana, P.; Toman, O.; Huster, K.M.; Novotný, T.; Schmidt, G.; Malik, M. Sex differences in heart rate responses to postural provocations. Int. J. Cardiol. 2019, 297, 126–134. [Google Scholar] [CrossRef]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef]
- Nagai, N.; Moritani, T. Effect of physical activity on autonomic nervous system function in lean and obese children. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, Y.; Nakayasu, K.; Nomura, M.; Nakaya, Y.; Saito, K.; Ito, S. The relationship between autonomic nervous activity and physical activity in children. Pediatr. Int. 2005, 47, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Khattab, K.; Brugger, N.; Eser, P.; Saner, H.; Wilhelm, M. High-volume sports club participation and autonomic nervous system activity in children. Eur. J. Clin. Investig. 2013, 43, 821–828. [Google Scholar] [CrossRef]
- Harteveld, L.M.; Nederend, I.; Ten Harkel, A.D.J.; Schutte, N.M.; de Rooij, S.R.; Vrijkotte, T.G.M.; Oldenhof, H.; Popma, A.; Jansen, L.M.C.; Suurland, J.; et al. Maturation of the cardiac autonomic nervous system activity in children and adolescents. J. Am. Heart Assoc. 2021, 10, e017405. [Google Scholar] [CrossRef]
- Oketa-Onyut Julu, P. Normal autonomic neurophysiology of postural orthostatic tachycardia and recommended physiological assessments in postural orthostatic tachycardia syndrome. Physiol. Rep. 2020, 8, e14465. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Mochizuki, G.; Lakhani, B.; McIlroy, W.E. Autonomic contributions in postural control, a review of the evidence. Rev. Neurosci. 2014, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Kurths, J.; Kleiner, H.J.; Witt, A.; Wessel, N.; Saparin, P.; Osterziel, K.J.; Schurath, R.; Dietz, R. The application of methods of non-linear dynamics for the improved and predictive recognition of patients threatened by sudden cardiac death. Cardiovasc. Res. 1996, 31, 419–433. [Google Scholar] [CrossRef]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Mäkikallio, T.H.; Huikuri, H.V.; Hintze, U.; Videbaek, J.; Mitrani, R.D.; Castellanos, A.; Myerburg, R.J.; Møller, M.; DIAMOND Study Group (Danish Investigations of Arrhythmia and Mortality ON Dofetilide). Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am. J. Cardiol. 2001, 87, 178–182. [Google Scholar] [CrossRef]
- Peng, C.K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Barthel, P.; Bauer, A.; Müller, A.; Huster, K.M.; Kanters, J.K.; Paruchuri, V.; Yang, X.; Ulm, K.; Malik, M.; Schmidt, G. Spontaneous baroreflex sensitivity, prospective validation trial of a novel technique in survivors of acute myocardial infarction. Heart Rhythm. 2012, 9, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Perlingeiro, P.; Hachul, D.T.; Gomes-Santos, I.L.; Tsutsui, J.M.; Negrao, C.E.; De Matos, L.D. Predominance of intrinsic mechanism of resting heart rate control and preserved baroreflex sensitivity in professional cyclists after competitive training. PLoS ONE 2016, 11, e0148036. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, D.; Dommasch, M.; Barthel, P.; Müller, A.; Dirschinger, R.J.; Hapfelmeier, A.; Huster, K.M.; Laugwitz, K.L.; Malik, M.; Schmidt, G. Assessment of mean respiratory rate from ECG recordings for risk stratification after myocardial infarction. J. Electrocardiol. 2014, 47, 700–704. [Google Scholar] [CrossRef]
- Helliesen, P.J.; Cook, C.D.; Friedlander, L.; Agathon, S. Studies of respiratory physiology in children. I. Mechanics of respiration and lung volumes in 85 normal children 5 to 17 years of age. Pediatrics 1958, 22, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Leicht, A.S.; Hirning, D.A.; Allen, G.D. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp. Physiol. 2003, 88, 441–446. [Google Scholar] [CrossRef]
- Hill, L.K.; Watkins, L.L.; Hinderliter, A.L.; Blumenthal, J.A.; Sherwood, A. Racial differences in the association between heart rate variability and left ventricular mass. Exp. Physiol. 2017, 102, 764–772. [Google Scholar] [CrossRef]
- Reed, K.E.; Warburton, D.E.; Whitney, C.L.; McKay, H.A. Differences in heart rate variability between Asian and Caucasian children living in the same Canadian community. Appl. Physiol. Nutr. Metab. 2006, 31, 277–282. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šišáková, M.; Helánová, K.; Hnatkova, K.; Andršová, I.; Novotný, T.; Malik, M. Intra-Individual Relationship between Heart Rate Variability and the Underlying Heart Rate in Children and Adolescents. J. Clin. Med. 2024, 13, 2897. https://doi.org/10.3390/jcm13102897
Šišáková M, Helánová K, Hnatkova K, Andršová I, Novotný T, Malik M. Intra-Individual Relationship between Heart Rate Variability and the Underlying Heart Rate in Children and Adolescents. Journal of Clinical Medicine. 2024; 13(10):2897. https://doi.org/10.3390/jcm13102897
Chicago/Turabian StyleŠišáková, Martina, Kateřina Helánová, Katerina Hnatkova, Irena Andršová, Tomáš Novotný, and Marek Malik. 2024. "Intra-Individual Relationship between Heart Rate Variability and the Underlying Heart Rate in Children and Adolescents" Journal of Clinical Medicine 13, no. 10: 2897. https://doi.org/10.3390/jcm13102897




