Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cardiorespiratory Polygraphy
2.3. CPAP Setup and Follow Up
2.4. Statistical Analysis
3. Results
3.1. Comparison of the Adherent and Non-Adherent Groups at 24 Months
3.2. Factors Associated with Adherence at 24 Months
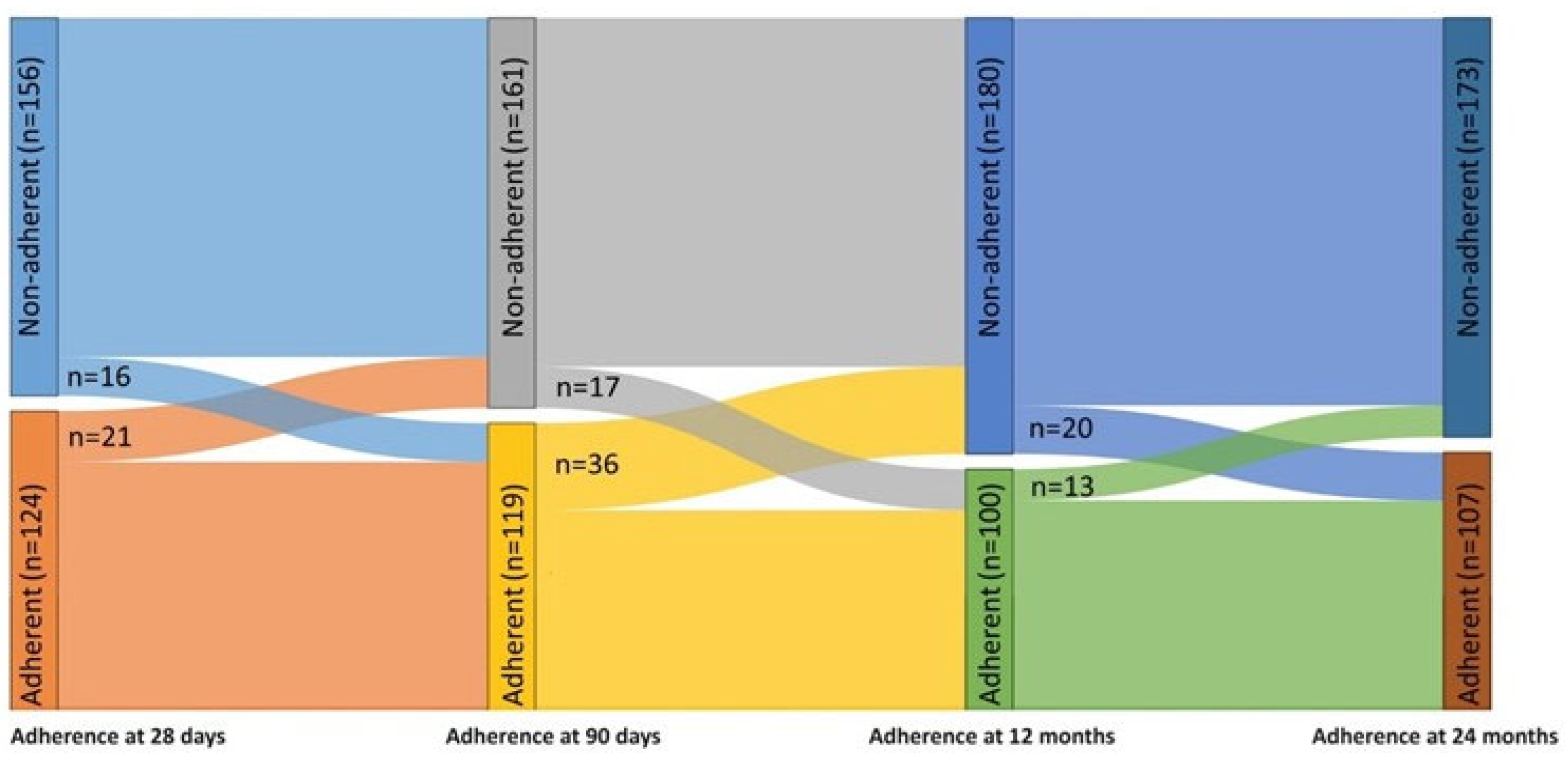
3.3. Changes in Adherence to CPAP over 24 Months
3.4. Patient-Reported Reasons for Non-Adherence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Obstructive Sleep Apnoea/Hypopnoea Syndrome and Obesity Hypoventilation Syndrome in over 16s. National Institute for Health and Care Excellence (NICE). Available online: https://www.nice.org.uk/guidance/ng202 (accessed on 4 September 2021).
- Judson, S.D.; Munster, V.J. Nosocomial Transmission of Emerging Viruses via Aerosol-Generating Medical Procedures. Viruses 2019, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Grote, L.; McNicholas, W.T.; Hedner, J. Sleep apnoea management in Europe during the COVID-19 pandemic: Data from the European Sleep Apnoea Database (ESADA). Eur. Respir. J. 2020, 55, 2001323. [Google Scholar] [CrossRef]
- Turnbull, C.D.; Allen, M.; Appleby, J.; Brown, R.; Bryan, N.; Cooper, A.; Cooper, B.G.; Gillooly, C.; Davidson, J.; Farley, H.; et al. COVID-19-related changes in outpatient CPAP setup pathways for OSA are linked with decreased 30-day CPAP usage. Thorax 2022, 77, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Oyefeso, O.; Koeckerling, D.; Mudalige, N.L.; Pan, D. COVID-19: Community CPAP and NIV should be stopped unless medically necessary to support life. Thorax 2020, 75, 367. [Google Scholar] [CrossRef]
- Bikov, A.; Khalil, S.; Gibbons, M.; Bentley, A.; Jones, D.; Bokhari, S. A Fully Remote Diagnostic and Treatment Pathway in Patients with Obstructive Sleep Apnoea during the COVID-19 Pandemic: A Single Centre Experience. J. Clin. Med. 2021, 10, 4310. [Google Scholar] [CrossRef]
- Meurling, I.J.; Birdseye, A.; Gell, R.; Sany, E.; Brown, R.; Higgins, S.; Muza, R.; O’Regan, D.; Leschziner, G.; Steier, J.; et al. The effect of a change from face-to-face to remote positive airway pressure education for patients with sleep apnoea during the coronavirus disease-2019 pandemic: A prospective cohort study. J. Thorac. Dis. 2023, 15, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head Neck Surg. 2016, 45, 43. [Google Scholar] [CrossRef]
- Sawyer, A.M.; Gooneratne, N.S.; Marcus, C.L.; Ofer, D.; Richards, K.C.; Weaver, T.E. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Med. Rev. 2011, 15, 343–356. [Google Scholar] [CrossRef]
- Stradling, J.R.; Hardinge, M.; Smith, D.M. A novel, simplified approach to starting nasal CPAP therapy in OSA. Respir. Med. 2004, 98, 155–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanchina, M.; Lincoln, J.; Prenda, S.; Holt, M.; Leon, I.; Donat, W.; Corrao, W.; Jabbour, E.; Koenig, S.; Malhotra, A. The impact of different CPAP delivery approaches on nightly adherence and discontinuation rate in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rudie, E.; Dorsey, C.; Caswell, K.; Blase, A.; Sert Kuniyoshi, F.; Benjafield, A.V.; Sullivan, S.S. Pilot study of positive airway pressure usage, patient journey and program engagement for users of a digital obstructive sleep apnea program. Front. Digit. Health 2023, 5, 1043578. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, O.C.; Allam, J.S.; Samarghandi, A.; Anand, N.; Fields, B.G.; Dholakia, S.A.; Venkateshiah, S.B.; Eisenstein, R.; Ciavatta, M.M.; Collop, N.A. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J. Clin. Sleep Med. 2020, 16, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yi, H.; Pi, M.; Zhang, C.; Keenan, B.T.; Glick, H.A.; Dong, X.; Pack, A.I.; Han, F.; Kuna, S.T. Telemedicine management of obstructive sleep apnea disorder in China: A randomized, controlled, non-inferiority trial. Sleep Breath. Schlaf Atm. 2024. ahead of print. [Google Scholar] [CrossRef]
- Chai-Coetzer, C.L.; Luo, Y.M.; Antic, N.A.; Zhang, X.L.; Chen, B.Y.; He, Q.Y.; Heeley, E.; Huang, S.G.; Anderson, C.; Zhong, N.S.; et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep 2013, 36, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Pataka, A.; Mauroudi, E.; Moniaki, V.; Fanaridis, M.; Schiza, S.E. Impact of the COVID-19 pandemic on positive airway pressure adherence and patients’ perspectives in Greece: The role of telemedicine. J. Clin. Sleep Med. 2023, 19, 1743–1751. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.; Chong Neto, H.; Bikov, A.; Kowal, K.; Steiropoulos, P.; Labor, M.; Cherrez-Ojeda, I.; Badellino, H.; Emelyanov, A.; Garcia, R.; et al. Impact of the COVID-19 pandemic on the management of chronic noninfectious respiratory diseases. Expert Rev. Respir. Med. 2021, 15, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Smith, D.; Tippett, V.; Stradling, J.R. Predictors of long-term compliance with continuous positive airway pressure. Thorax 2010, 65, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Martinez-Alonso, M.; Sanchez-de-la-Torre, M.; Barbe, F. Long-term adherence to continuous positive airway pressure therapy in non-sleepy sleep apnea patients. Sleep Med. 2016, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Kurtz, D.; Petiau, C.; Sforza, E.; Trautmann, D. Long-term compliance with CPAP therapy in obstructive sleep apnea patients and in snorers. Sleep 1996, 19 (Suppl. S9), S136–S143. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Day, R.; Hudgel, D.; Stefadu, Y.; Parks, M.; Syron, M.L.; Roth, T. Sleep during titration predicts continuous positive airway pressure compliance. Sleep 2003, 26, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.K.; Shapiro, C.M. Factors that influence CPAP adherence: An overview. Sleep Breath. 2010, 14, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Cowen, J.; Harrison, S.; Thom, L.; Thomas, M.; Sedano, J.; Stephens, P.; Lip, G.Y.H.; Craig, S. Use of historical remote monitoring data to determine predictors of CPAP non-compliance in patients with OSA. Sleep Breath. 2023, 27, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, E.M.; Jobe, S.L.; Oldstone, L.M.; Scharf, S.M.; Johnson, A.M.; Albrecht, J.S. Lower socioeconomic status and co-morbid conditions are associated with reduced continuous positive airway pressure adherence among older adult medicare beneficiaries with obstructive sleep apnea. Sleep 2020, 43, zsaa122. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, I.; Shukla, D.; Greenfield, S. Impact of COVID-19 on the digital divide: A rapid review. BMJ Open 2021, 11, e053440. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D.; Freedland, K.E.; Carney, R.M.; Duntley, S.P.; Stepanski, E.J. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom. Med. 2007, 69, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.E.; Seale, L.; Bartle, I.E.; Watkins, A.J.; Ebden, P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004, 27, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, E.M.; Cole, K.V.; Dexter, R.B.; Malhotra, A.; Cistulli, P.A.; Sterling, K.L.; Pépin, J.L. Depression and comorbid obstructive sleep apnea: Association between positive airway pressure adherence, occurrence of self-harm events, healthcare resource utilization, and costs. J. Affect. Disord. 2024, 349, 254–261. [Google Scholar] [CrossRef] [PubMed]
- McArdle, N.; Kingshott, R.; Engleman, H.M.; Mackay, T.W.; Douglas, N.J. Partners of patients with sleep apnoea/hypopnoea syndrome: Effect of CPAP treatment on sleep quality and quality of life. Thorax 2001, 56, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Grote, L. Lessons for sleep medicine learned from the COVID-19 pandemic. Breathe 2022, 18, 220146. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, S.S.; Kelly, J.L.; Little, S.; Pinnock, H.; Morrell, M.J.; Polkey, M.I.; Murphie, P. Virtual consultations for patients with obstructive sleep apnoea: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 220180. [Google Scholar] [CrossRef] [PubMed]
| Adherent (n = 107) | Non-Adherent (n = 173) | p Value | |
|---|---|---|---|
| Age (years) | 52/43–60/ | 51/43–60/ | 0.807 |
| BMI (kg/m2) | 36/30–40/ | 34/30–40/ | 0.259 |
| Gender (males%) | 48 | 47 | 0.891 |
| Chronic airway diseases (%) | 16 | 18 | 0.777 |
| Hypertension (%) | 32 | 27 | 0.451 |
| Ischaemic heart disease (%) | 9 | 10 | 0.762 |
| Cerebrovascular disease (%) | 3 | 1 | 0.326 |
| Atrial fibrillation (%) | 5 | 5 | 0.807 |
| Chronic heart failure (%) | 3 | 4 | 0.732 |
| Diabetes (%) | 12 | 17 | 0.215 |
| GORD (%) | 12 | 15 | 0.391 |
| Anxiety (%) | 7 | 3 | 0.143 |
| Depression (%) | 21 | 12 | 0.040 |
| No comorbidities (%) | 18 | 16 | 0.606 |
| ESS | 11.5/8.0–15.0/ | 12.0/8.0–16.0/ | 0.340 |
| AHI (events/hour) | 42.0/27.0–60.5/ | 31.9/20.5–45.2/ | <0.001 |
| Driver (%) | 90 | 75 | 0.003 |
| Continuously Adherent, n = 62 | Continuously Non-Adherent, n = 118 | Variable Adherence, n = 100 | p Value | |
|---|---|---|---|---|
| Age (years) | 56/48–62/ | 51/40–61/ | 49/42–58/ | 0.032 |
| BMI (kg/m2) | 36/30–40/ | 34/30–40/ | 35/30–40/ | 0.524 |
| Gender (males%) | 44 | 47 | 49 | 0.793 |
| Chronic airway diseases (%) | 18 | 18 | 16 | 0.948 |
| Hypertension (%) | 31 | 28 | 29 | 0.949 |
| Ischaemic heart disease (%) | 5 | 10 | 12 | 0.335 |
| Cerebrovascular disease (%) | 2 | 1 | 3 | 0.460 |
| Atrial fibrillation (%) | 3 | 4 | 8 | 0.440 |
| Chronic heart failure (%) | 2 | 4 | 4 | 0.654 |
| Diabetes (%) | 13 | 19 | 11 | 0.191 |
| GORD (%) | 13 | 16 | 12 | 0.678 |
| Anxiety (%) | 3 | 6 | 5 | 0.426 |
| Depression (%) | 9 | 26 | 15 | 0.013 |
| No comorbidities (%) | 18 | 16 | 17 | 0.946 |
| ESS | 12.0/8.8–15.0/ | 12.0/8.0–16.5/ | 11.0/7.8–16.0/ | 0.824 |
| AHI (events/hour) | 41.4/24.0–59.8/ | 31.0/21.0–43.9/ | 38.9/23.0–54.0/ | 0.019 |
| Driver (%) | 92 | 71 | 85 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikov, A.; Bentley, A.; Csoma, B.; Smith, N.; Morris, B.; Bokhari, S. Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project. J. Clin. Med. 2024, 13, 2891. https://doi.org/10.3390/jcm13102891
Bikov A, Bentley A, Csoma B, Smith N, Morris B, Bokhari S. Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project. Journal of Clinical Medicine. 2024; 13(10):2891. https://doi.org/10.3390/jcm13102891
Chicago/Turabian StyleBikov, Andras, Andrew Bentley, Balazs Csoma, Nicola Smith, Bryn Morris, and Saba Bokhari. 2024. "Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project" Journal of Clinical Medicine 13, no. 10: 2891. https://doi.org/10.3390/jcm13102891
APA StyleBikov, A., Bentley, A., Csoma, B., Smith, N., Morris, B., & Bokhari, S. (2024). Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnoea Set Up in a Complete Remote Pathway: A Single-Centre Service Evaluation Project. Journal of Clinical Medicine, 13(10), 2891. https://doi.org/10.3390/jcm13102891







