Leukocytospermia and/or Bacteriospermia: Impact on Male Infertility
Abstract
:1. Introduction
2. Male Genital Tract Infections
3. Prevalence of Leukocytospermia
4. Prevalence of Bacteriospermia
5. Quantification of Leukocytes in Semen
6. Detection of Bacteriospermia
Comparison of Traditional Semen Cultures versus Microbiological Findings in Semen by PCR Tests
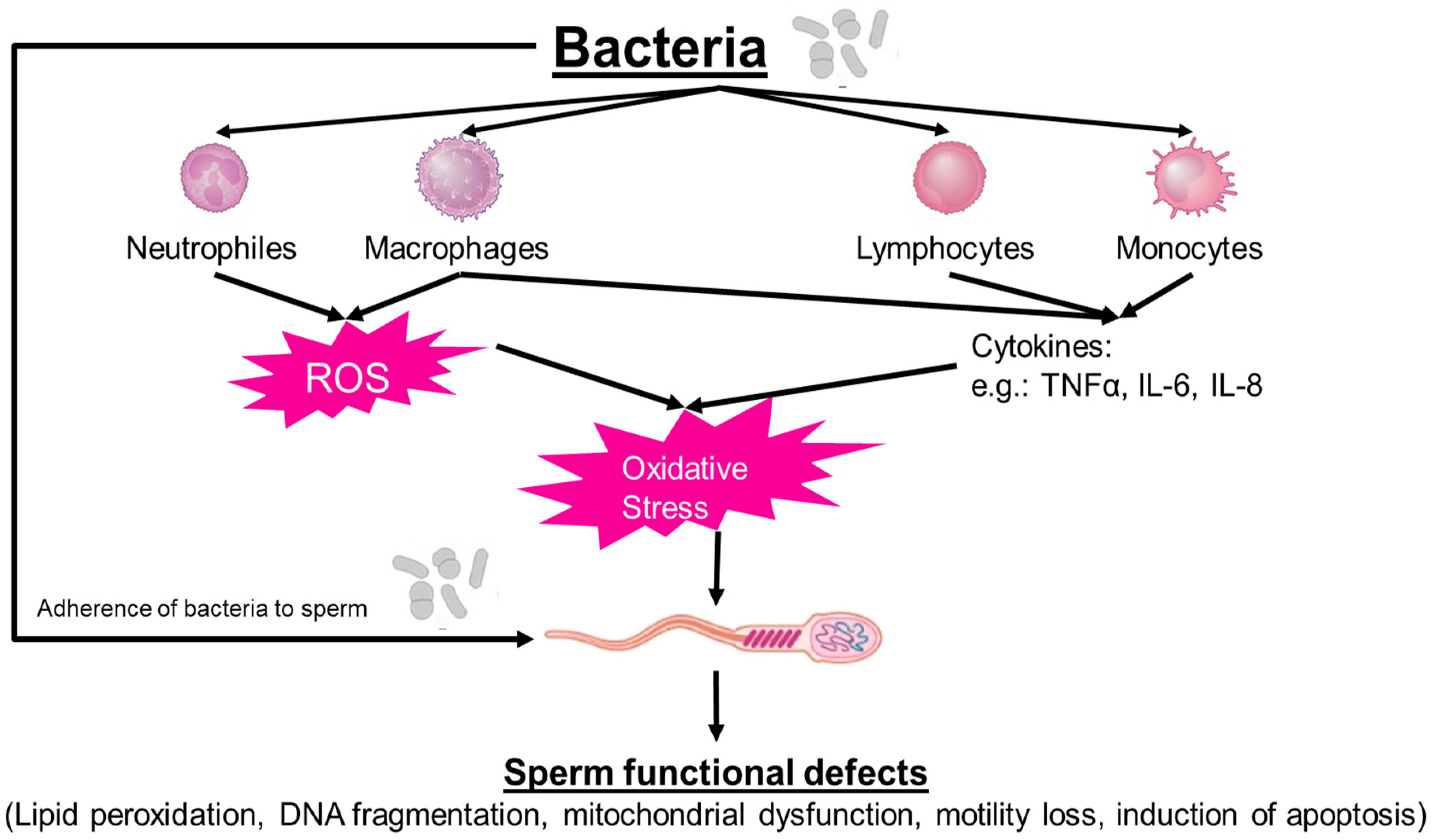
7. Pathogenesis of Male Genital Tract Infection
7.1. Bacteriospermia
7.2. Leukocytospermia
7.3. Impact of Cytokines on Sperm Function
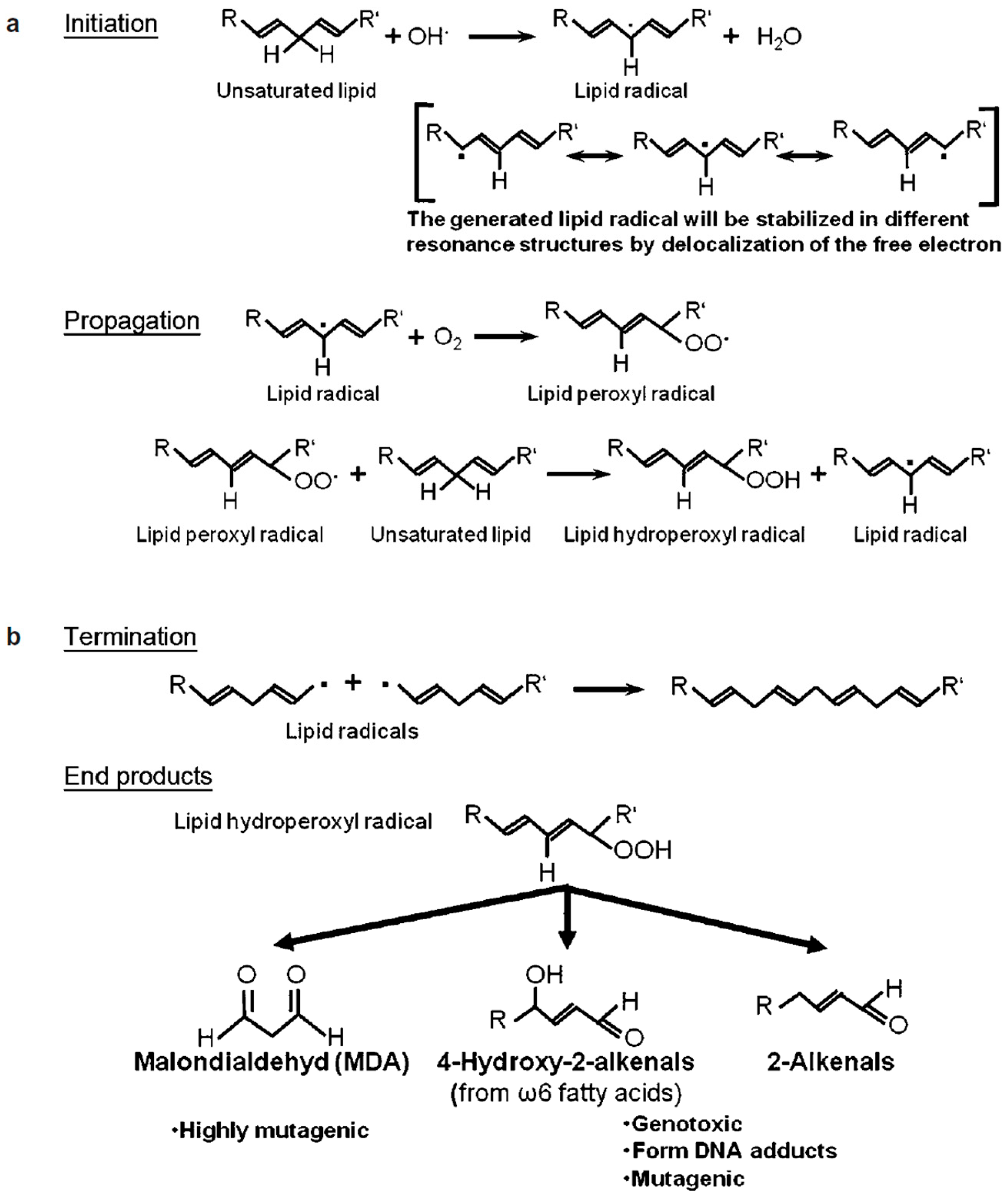
8. Leukocytospermia and Seminal Oxidative Stress
9. Relevance of Bacteriospermia/Leukocytospermia in Assisted Reproductive Technology (ART)
9.1. Relevance of Bacteriospermia
9.2. Relevance of Leukocytospermia
10. Clinical Management of Bacteriospermia
Prevention of Semen Sample Contamination with Skin Commensals, Urethral and General Bacteria
11. Clinical Management of Leukocytospermia
12. Conclusions
Funding
Conflicts of Interest
References
- Evens, E.M. A global perspective on infertility: An under recognized public health issue. Carol. Pap. Int Health 2004, 18, 1–42. [Google Scholar]
- Lutz, W.; O’Neill, B.C.; Scherbov, S. Demographics. Europe’s population at a turning point. Science 2003, 299, 1991–1992. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am. J. Men’s Health 2017, 11, 1279–1304. [Google Scholar] [CrossRef] [PubMed]
- Rutstein, S.O.; Shah, I.H. Infecundity, Infertility, and Childlessness in Developing Countries; DHS Comparative Reports No. 9; ORC Macro and the World Health Organization: Calverton, MD, USA, 2004.
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO.
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Nieschlag, E.; Behre, H.M. Andrology. In Male Reproductive Health and Dysfunction, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000; pp. 5–10. [Google Scholar]
- Huang, B.; Wang, Z.; Kong, Y.; Jin, M.; Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health 2023, 23, 2195. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Jorgensen, N.; Main, K.M.; Rajpert-De Meyts, E.; Leffers, H.; Andersson, A.M.; Juul, A.; Carlsen, E.; Krog Mortensen, G.; Kold-Jensen, T.; et al. Is human fecundity declining? Int. J. Androl. 2006, 29, 2–11. [Google Scholar] [CrossRef]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab. J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.G.; Neal, M.S.; Han, M.S.; Dominguez, M.M. Environmental contaminants and human infertility: Hypothesis or cause for concern? J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 162–176. [Google Scholar] [CrossRef]
- Henkel, R.; Maaß, G.; Jung, A.; Haidl, G.; Schill, W.-B.; Schuppe, H.C. Age-related changes in seminal polymorphonuclear elastase in men with asymptomatic inflammation of the genital tract. Asian J. Androl. 2007, 9, 299–304. [Google Scholar] [CrossRef]
- Weidner, W.; Krause, W.; Ludwig, M. Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum. Reprod. Update 1999, 5, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Diemer, T.; Kopa, Z.; Krausz, C.; Minhas, S.; Tournaye, H. EAU Guidelines on Male Infertility. In Proceedings of the EAU Annual Congress, Barcelona, Spain, 15–19 March 2019; ISBN 978-94-92671-04-2. [Google Scholar]
- Filardo, S.; Skilton, R.J.; O’Neill, C.E.; Di Pietro, M.; Sessa, R.; Clarke, I.N. Growth kinetics of Chlamydia trachomatis in primary human Sertoli cells. Sci. Rep. 2019, 9, 5847. [Google Scholar] [CrossRef] [PubMed]
- Pellati, D.; Mylonakis, I.; Bertoloni, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Armanini, D. Genital tract infections and infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R. Infection in Infertility. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants, 2nd ed.; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 409–424. [Google Scholar]
- Henkel, R. Long-term consequences of sexually transmitted infections on men’s sexual function: A systematic review. Arab. J. Urol. 2021, 19, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Prabha, V. Spermagglutinating Escherichia coli and its role in infertility: In vivo study. Microb. Pathog. 2014, 69–70, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sanocka-Maciejewska, D.; Ciupinska, M.; Kurpisz, M. Bacterial infection and semen quality. J. Reprod. Immunol. 2005, 67, 51–56. [Google Scholar] [CrossRef]
- Monga, M.; Roberts, J.A. Spermagglutination by bacteria: Receptor-specific interactions. J. Androl. 1994, 15, 151–156. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Diemer, T.; Naber, K.G.; Weidner, W. Chronic bacterial prostatitis (NIH type II): Diagnosis, therapy and influence on the fertility status. Andrologia 2008, 40, 100–104. [Google Scholar] [CrossRef]
- Oghbaei, H.; Rezaei, Y.R.; Nikanfar, S.; Zarezadeh, R.; Sadegi, M.; Latifi, Z.; Nouri, M.; Fattahi, A.; Ahmadi, Y.; Bleisinger, N. Effects of bacteria on male fertility: Spermatogenesis and sperm function. Life Sci. 2020, 256, 117891. [Google Scholar] [CrossRef]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with leukocytospermia. BMC Mol. Cell Biol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.; Schulz, M.; Soto, L.; Iglesias, T.; Miska, W.; Sanchez, R. Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertil. Steril. 2005, 83, 808–810. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Kiefer, I.; Beck, C.; Demirakca, T.; Strowitzki, T. Role for tumor necrosis factor alpha (TNF-alpha) and interleukin 1-beta (IL-1beta) determination in seminal plasma during infertility investigation. Fertil. Steril. 2007, 87, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ni, M.; Xing, S.; Yu, Y.; Zhou, Y.; Yang, S.; Li, H.; Zhu, R.; Han, M. Reactive oxygen species secreted by leukocytes in semen induce self-expression of interleukin-6 and affect sperm quality. Am. J. Men’s Health 2020, 14, 1557988320970053. [Google Scholar] [CrossRef]
- Mongioi, L.M.; Alamo, A.; Calogero, A.E.; Compagnone, M.; Giacone, F.; Cannarella, R.; La Vignera, S.; Condorelli, R.A. Evaluation of seminal fluid leukocyte subpopulations in patients with varicocele. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420925719. [Google Scholar] [CrossRef]
- Lemkecher, T.; Dartigues, S.; Vaysse, J.; Kulski, O.; Barraud-Lange, V.; Gattegno, L.; Wolf, J.P. Leucocytospermia, oxidative stress and male fertility: Facts and hypotheses. Gynecol. Obstet. Fertil. 2005, 33, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhu, H.B.; Li, L.L.; Yu, Y.; Zhang, H.G.; Liu, R.Z. Decline of semen quality and increase of leukocytes with cigarette smoking in infertile men. Iran J. Reprod. Med. 2013, 11, 589–596. [Google Scholar]
- Close, C.E.; Roberts, P.L.; Berger, R.E. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J. Urol. 1990, 144, 900–903. [Google Scholar] [CrossRef]
- Onemu, S.O.; Ibeh, I.N. Studies on the significance of positive bacterial semen cultures in male fertility in Nigeria. Int. J. Fertil. Women Med. 2001, 46, 210–214. [Google Scholar]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Kandasamy, B.; Jayachandran, A.L.; Sathiyanarayanan, S.; Tanjore Singaravelu, V.; Krishnamurthy, V.; Elangovan, V. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 2614692. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Karampetsou, N.; Perrea, D.; Konstantopoulos, P.; Daskalakis, G. The impact of bacteriospermia on semen parameters: A Meta-analysis. J. Fam. Reprod. Health 2018, 12, 73–82. [Google Scholar]
- Zeyad, A.; Hamad, M.; Amor, H.; Hammadeh, M.E. Relationships between bacteriospermia, DNA integrity, nuclear protamine alteration, sperm quality and ICSI outcome. Reprod. Biol. 2018, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Roychoudhury, S.; Dey, A.; Jha, N.; Kumar, D.; Roychoudhury, S.; Slama, P.; Kesari, K.K. Bacteriospermia and male infertility: Role of oxidative stress. Adv. Exp. Med. Biol. 2022, 1358, 141–163. [Google Scholar] [PubMed]
- Shash, R.Y.M.; Mohamed, G.A.A.; Shebl, S.E.; Shokr, M.; Soliman, S.A. The impact of bacteriospermia on semen parameters among infertile Egyptian men: A case-control study. Am. J. Men’s Health 2023, 17, 15579883231181861. [Google Scholar] [CrossRef] [PubMed]
- Weidner, W.; Wagenlehner, F.M.; Marconi, M.; Pilatz, A.; Pantke, K.H.; Diemer, T. Acute bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: Andrological implications. Andrologia 2008, 40, 105–112. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization, WHO Press: Geneva, Switzerland, 2010.
- World Health Organization. In WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO.
- World Health Organization. Sexually Transmitted Infections Fact Sheet. 2023. Available online: https://www.who.int/en/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 8 February 2024).
- Comhaire, F.; Verschraegen, G.; Vermeulen, L. Diagnosis of accessory gland infection and its possible role in male infertility. Int. J. Androl. 1980, 3, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Haidl, G.; Haidl, F.; Allam, J.P.; Schuppe, H.C. Therapeutic options in male genital tract inflammation. Andrologia 2019, 51, e13207. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Munoz, G.; Sanchez, R.; Henkel, R.; Gallegos-Avila, G.; Diaz-Gutierrez, O.; Vigil, P.; Vasquez, F.; Kortebani, G.; Mazzolli, A.; et al. Update on the impact of Chlamydia trachomatis infection on male fertility. Andrologia 2004, 36, 1–23. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Mahmoud, A.M.; Depuydt, C.E.; Zalata, A.A.; Christophe, A.B. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: The andrologist’s viewpoint. Hum. Reprod. Update 1999, 5, 393–398. [Google Scholar] [CrossRef]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar] [PubMed]
- Turner, T.T.; Mammen, T.; Kavoussi, P.; Lysiak, J.J.; Costabile, R.A. Cytokine responses to E. coli-induced epididymitis in the rat: Blockade by vasectomy. Urology 2011, 77, 1507.e9–1507.e14. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, M.S.; Girón, J.A.; Nataro, J.P.; Kaper, J.B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 1992, 6, 3427–3437. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.; Schulz, M.; Soto, L.; Sanchez, R. Bacteria induce expression of apoptosis in human spermatozoa. Apoptosis 2005, 10, 105–110. [Google Scholar] [CrossRef]
- Mashaly, M.; Masallat, D.T.; Elkholy, A.A.; Abdel-Hamid, I.A.; Mostafa, T. Seminal Corynebacterium strains in infertile men with and without leucocytospermia. Andrologia 2016, 48, 355–359. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in Female Infertility Via Modulating Sperm Agglutination and Immobilization. Front. Cell. Infect. Microbiol. 2021, 10, 620529. [Google Scholar] [CrossRef] [PubMed]
- Sellami, H.; Znazen, A.; Sellami, A.; Mnif, H.; Louati, N.; Ben Zarrouk, S.; Keskes, L.; Rebai, T.; Gdoura, R.; Hammami, A. Molecular detection of Chlamydia trachomatis and other sexually transmitted bacteria in semen of male partners of infertile couples in Tunisia: The effect on semen parameters and spermatozoa apoptosis markers. PLoS ONE 2014, 9, e98903. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Baccani, I.; Tamburrino, L.; Mattiuz, G.; Nicolò, S.; Bonaiuto, C.; Panico, C.; Vignozzi, L.; Antonelli, A.; Rossolini, G.M.; et al. Effects of common Gram-negative pathogens causing male genitourinary-tract infections on human sperm functions. Sci. Rep. 2021, 11, 19177. [Google Scholar] [CrossRef] [PubMed]
- Șchiopu, P.; Toc, D.A.; Colosi, I.A.; Costache, C.; Ruospo, G.; Berar, G.; Gălbău, S.G.; Ghilea, A.C.; Botan, A.; Pană, A.G.; et al. An overview of the factors involved in biofilm production by the Enterococcus genus. Int. J. Mol. Sci. 2023, 24, 11577. [Google Scholar] [CrossRef]
- Kaur, K.; Prabha, V. Sperm impairment by sperm agglutinating factor isolated from Escherichia coli: Receptor specific interactions. Biomed. Res. Int. 2013, 2013, 548497. [Google Scholar] [CrossRef]
- Paulson, J.D.; Polakoski, K.L. Isolation of a spermatozoal immobilization factor from Escherichia coli filtrates. Fertil. Steril. 1977, 28, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Prabha, V.; Sandhu, R.; Kaur, S.; Kaur, K.; Sarwal, A.; Mavuduru, R.S.; Singh, S.K. Mechanism of sperm immobilization by Escherichia coli. Adv. Urol. 2010, 2010, 240268. [Google Scholar] [CrossRef] [PubMed]
- Prabha, V.; Kaur, S. Isolation and purification of sperm immobilizing/agglutinating factors from bacteria and their corresponding receptors from human spermatozoa. In Chromatography—The Most Versatile Method of Chemical Analysis; de Azevedo Calderon, L., Ed.; IntechOpen: London, UK, 2012; pp. 295–310. [Google Scholar]
- Hosseinzadeh, S.; Pacey, A.A.; Eley, A. Chlamydia trachomatis-induced death of human spermatozoa is caused primarily by lipopolysaccharide. J. Med. Microbiol. 2003, 52, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rennemeier, C.; Frambach, T.; Hennicke, F.; Dietl, J.; Staib, P. Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoa. Infect. Immun. 2009, 77, 4990–4997. [Google Scholar] [CrossRef]
- Ristow, L.C.; Welch, R.A. Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger? Biochim. Biophys. Acta 2016, 1858, 538–545. [Google Scholar] [CrossRef]
- Djordjevic, D.; Lalic, N.; Vukovic, I.; Nale, D.; Perovic, D.; Kisic, D.; Micic, S. Sperm quality and seminal biochemical parameters in infertile men with and without leukocytospermia. Andrology 2018, 7, 197. [Google Scholar]
- Wolff, H. The biologic significance of white blood cells in semen. Fertil. Steril. 1995, 63, 1143–1157. [Google Scholar]
- Kiessling, A.A.; Lamparelli, N.; Yin, H.Z.; Seibel, M.M.; Eyre, R.C. Semen leukocytes: Friends or foes? Fertil. Steril. 1995, 64, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, M.H.; Kim, J.; Baik, S.K.; Koh, S.B.; Park, H.J.; Seo, J.T. Treatment of leukocytospermia in male infertility: A systematic review. World J. Men’s Health 2016, 34, 165–172. [Google Scholar] [CrossRef]
- Kaleli, S.; Ocer, F.; Irez, T.; Budak, E.; Aksu, M.F. Does leukocytospermia associate with poor semen parameters and sperm functions in male infertility? The role of different seminal leukocyte concentrations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 185–191. [Google Scholar] [CrossRef]
- Endtz, A.W. A direct staining method for moist urinary sediment and moist human sperm. Ned. Tijdschr. Geneeskd. 1972, 116, 681–685. [Google Scholar]
- Lackner, J.E.; Herwig, R.; Schmidbauer, J.; Schatzl, G.; Kratzik, C.; Marberger, M. Correlation of leukocytospermia with clinical infection and the positive effect of antiinflammatory treatment on semen quality. Fertil. Steril. 2006, 86, 601–605. [Google Scholar] [CrossRef]
- Brunner, R.J.; Demeter, J.H.; Sindhwani, P. Review of guidelines for the evaluation and treatment of leukocytospermia in male infertility. World J. Men’s Health 2019, 37, 128–137. [Google Scholar] [CrossRef]
- Fedder, J. Nonsperm cells in human semen: With special reference to seminal leukocytes and their possible influence on fertility. Arch. Androl. 1996, 36, 41–65. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.; Virant-Klun, I.; Meden-Vrtovec, H. Semen granulocyte elastase: Its relevance for the diagnosis and prognosis of silent genital tract inflammation. Hum. Reprod. 2000, 15, 1978–1984. [Google Scholar] [CrossRef]
- Lackner, J.E.; Agarwal, A.; Mahfouz, R.; du Plessis, S.S.; Schatzl, G. The association between leukocytes and sperm quality is concentration dependent. Reprod. Biol. Endocrinol. 2010, 8, 12. [Google Scholar] [CrossRef]
- Gambera, L.; Serafini, F.; Morgante, G.; Focarelli, R.; De Leo, V.; Piomboni, P. Sperm quality and pregnancy rate after COX-2 inhibitor therapy of infertile males with abacterial leukocytospermia. Hum. Reprod. 2007, 22, 1047–1051. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pasqualotto, A.E.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J. Androl. 2001, 22, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Punab, M.; Loivukene, K.; Kermes, K.; Mändar, R. The limit of leucocytospermia from the microbiological viewpoint. Andrologia 2003, 35, 271–278. [Google Scholar] [CrossRef]
- Henkel, R.; Kierspel, E.; Stalf, T.; Mehnert, C.; Menkveld, R.; Tinneberg, H.R.; Schill, W.B.; Kruger, T.F. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil. Steril. 2005, 83, 635–642. [Google Scholar] [CrossRef]
- Micillo, A.; Vassallo, M.R.; Cordeschi, G.; D’Andrea, S.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Semen leukocytes and oxidative-dependent DNA damage of spermatozoa in male partners of subfertile couples with no symptoms of genital tract infection. Andrology 2016, 4, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Cottell, E.; Harrison, R.F.; McCaffrey, M.; Walsh, T.; Mallon, E.; Barry-Kinsella, C. Are seminal fluid microorganisms of significance or merely contaminants? Fertil. Steril. 2000, 74, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Weidner, W.; Diemer, T.; Wagenlehner, F.M.E. Male infertility in chronic urogenital infections and inflammation with special reference to ejaculate findings. In Clinical Andrology; Björndahl, L., Giwercman, A., Tournaye, H., Weidner, W., Eds.; Informa Healthcare: New York, NY, USA; London, UK, 2010. [Google Scholar]
- Weidner, W.; Pilatz, A.; Diemer, T.; Schuppe, H.C.; Rusz, A.; Wagenlehner, F. Male urogenital infections: Impact of infection and inflammation on ejaculate parameters. World J. Urol. 2013, 31, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.; Schatzl, G.; Horvath, S.; Kratzik, C.; Marberger, M. Value of counting white blood cells (WBC) in semen samples to predict the presence of bacteria. Eur. Urol. 2006, 49, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, K.; Lacroix, J.M.; Jain, A.; Dumitru, I.; Heritz, D.; Mittelman, M.W. Polymerase chain reaction-based detection of bacteria in semen. Fertil. Steril. 1996, 66, 463–467. [Google Scholar] [CrossRef]
- Willén, M.; Holst, E.; Myhre, E.B.; Olsson, A.M. The bacterial flora of the genitourinary tract in healthy fertile men. Scand J. Urol. Nephrol. 1996, 30, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The seminal microbiome in health and disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Cumming, J.A.; Carrell, D.T. Utility of reflexive semen cultures for detecting bacterial infections in patients with infertility and leukocytospermia. Fertil. Steril. 2009, 91 (Suppl. 4), 1486–1488. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Relevance of assessing the uterine microbiota in infertility. Fertil. Steril. 2018, 110, 337–343. [Google Scholar] [CrossRef]
- Davies, R.; Minhas, S.; Jayasena, C.N. Next-generation sequencing to elucidate the semen microbiome in male reproductive disorders. Medicina 2024, 60, 25. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Cinque, B.; Vassallo, M.R.C.; Mineo, S.; Francavilla, S.; Cifone, M.G.; Francavilla, F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011, 95, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Harkiss, D.; Buckingham, D.W. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol. Reprod. Develop. 1993, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27. [Google Scholar] [PubMed]
- Kaushik, J.K.; Kumar, A.; Duary, R.K.; Mohanty, A.K.; Grover, S.; Batish, V.K. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS ONE 2009, 4, e8099. [Google Scholar] [CrossRef]
- Dardmeh, F.; Alipour, H.; Gazerani, P.; van der Horst, G.; Brandsborg, E.; Nielsen, H.I. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS ONE 2017, 12, e0185964. [Google Scholar] [CrossRef] [PubMed]
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444. [Google Scholar] [CrossRef]
- Helli, B.; Kavianpour, M.; Ghaedi, E.; Dadfar, M.; Haghighian, H.K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum. Fertil. 2022, 25, 499–507. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Genovés, S.; Riesco, M.F.; Martorell, P.; Herráez, M.P.; Ramón, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes. 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Henkel, R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef]
- Ricci, G.; Presani, G.; Guaschino, S.; Simeone, R.; Perticarari, S. Leukocyte detection in human semen using flow cytometry. Hum. Reprod. 2000, 15, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.; Schulz, M.; Vallejos, V.; Henkel, R.; Miska, W.; Sanchez, R. Indirect immunofluorescence using monoclonal antibodies for the detection of leukocytospermia: Comparison with peroxidase staining. Andrologia 2002, 34, 69–73. [Google Scholar] [CrossRef]
- Bunn, T.W.; Sikarwar, A.S. Diagnostics: Conventional versus modern methods. J. Adv. Med. Pharmaceut. Sci. 2016, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Żukowska, M.E. Advanced methods of bacteriological identification in a clinical microbiology laboratory. J. Pre-Clin. Clin. Res. 2021, 15, 68–72. [Google Scholar] [CrossRef]
- Baud, D.; Pattaroni, C.; Vulliemoz, N.; Castella, V.; Marsland, B.J.; Stojanov, M. Sperm microbiota and its impact on semen parameters. Front. Microbiol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Boers, S.A.; Jansen, R.; Hays, J.P. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1059–1070. [Google Scholar] [CrossRef]
- Kiessling, A.A.; Desmarais, B.M.; Yin, H.Z.; Loverde, J.; Eyre, R.C. Detection and identification of bacterial DNA in semen. Fertil. Steril. 2008, 90, 1744–1756. [Google Scholar] [CrossRef]
- Bayasgalan, G.; Naranbat, D.; Tsedmaa, B.; Tsogmaa, B.; Sukhee, D.; Amarjargal, O.; Lhagvasuren, T.; Radnaabazar, J.; Rowe, P.J. Clinical patterns and major causes of infertility in Mongolia. J. Obstet. Gynaecol. Res. 2004, 30, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.J.; Kulkarni, N.; Thirumavalavan, N.; Ramasamy, R. Evaluation and management of male genital tract infections in the setting of male infertility: An updated review. Curr. Opin. Urol. 2023, 33, 180–186. [Google Scholar] [CrossRef]
- Villanueva-Diaz, C.A.; Flores-Reyes, G.A.; Beltran-Zuniga, M.; Echavarría-Sanchez, M.; Ortiz-Ibarra, F.J.; Arredondo-Garcia, J.L. Bacteriospermia and male infertility: A method for increasing the sensitivity of semen culture. Int. J. Fertil. Womens Med. 1999, 44, 198–203. [Google Scholar] [PubMed]
- Jue, J.S.; Ramasamy, R. Significance of positive semen culture in relation to male infertility and the assisted reproductive technology process. Transl. Androl. Urol. 2017, 6, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Saleh, R.A.; Abdoos, M.; Rouzrokh, A.; Nazemian, Z. Positive bacterial culture of semen from infertile men with asymptomatic leukocytospermia. Int. J. Fertil. Womens Med. 2002, 47, 265–270. [Google Scholar] [PubMed]
- Shalika, S.; Dugan, K.; Smith, R.D.; Padilla, S.L. The effect of positive semen bacterial and Ureaplasma cultures on in-vitro fertilization success. Hum. Reprod. 1996, 11, 2789–2792. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Capitani, S.; Figura, N.; Pammolli, A.; Federico, M.G.; Giannerini, V.; Collodel, G. The presence of bacteria species in semen and sperm quality. J. Assist. Reprod. Genet. 2009, 26, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J. Urol. 2021, 205, 36–43. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; Barratt, C.L.; Cooke, I.D. Prospective study of leukocytes and leukocyte subpopulations in semen suggests they are not a cause of male infertility. Fertil. Steril. 1993, 60, 1069–1075. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Capogrosso, P.; Carvalho, J.; Corona, G.; Hatzichristodoulou, G.; Jones, T.H.; Kadioglu, A.; Martinez-Salamanca, J.I.; Minhas, S.; et al. EAU Guidelines on Sexual and Reproductive Health. Edn. In Proceedings of the EAU Annual Congress, Milan, Italy, 10–13 March 2023; ISBN 978-94-92671-19-6. [Google Scholar]
- Ventimiglia, E.; Capogrosso, P.; Boeri, L.; Cazzaniga, W.; Matloob, R.; Pozzi, E.; Chierigo, F.; Abbate, C.; Viganò, P.; Montorsi, F.; et al. Leukocytospermia is not an informative predictor of positive semen culture in infertile men: Results from a validation study of available guidelines. Hum. Reprod. Open 2020, 2020, hoaa039. [Google Scholar] [CrossRef]
- Solomon, M.; Henkel, R. Semen culture and the assessment of genitourinary tract infections. Indian J. Urol. 2017, 33, 188–193. [Google Scholar]
- Gdoura, R.; Kchaou, W.; Znazen, A.; Chakroun, N.; Fourati, M.; Ammar-Keskes, L.; Hammami, A. Screening for bacterial pathogens in semen samples from infertile men with and without leukocytospermia. Andrologia 2008, 40, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, I.N.; Nche, B.T.; Nwagu, I.G.; Nnanna, I.I. Current studies on bacterospermia the leading cause of male infertility: A protégé and potential threat towards man’s extinction. N. Am. J. Med. Sci. 2011, 3, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Farahani, L.; Tharakan, T.; Yap, T.; Ramsay, J.W.; Jayasena, C.N.; Minhas, S. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology 2021, 9, 115–144. [Google Scholar] [CrossRef]
- Ricci, S.; De Giorgi, S.; Lazzeri, E.; Luddi, A.; Rossi, S.; Piomboni, P.; De Leo, V.; Pozzi, G. Impact of asymptomatic genital tract infections on in vitro Fertilization (IVF) outcome. PLoS ONE 2018, 13, e0207684. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Rohr, G.; Ströck, W.; Pohl, S.; Schwalbach, B.; Runnebaum, B. Anaerobes in ejaculates of subfertile men. Hum. Reprod. Update 1995, 1, 462–478. [Google Scholar] [CrossRef]
- Kermes, K.; Punab, M.; Lõivukene, K.; Mändar, R. Anaerobic seminal fluid micro-flora in chronic prostatitis/chronic pelvic pain syndrome patients. Anaerobe 2003, 9, 117–123. [Google Scholar] [CrossRef]
- Damirayakhian, M.; Jeyendran, R.S.; Land, S.A. Significance of semen cultures for men with questionable semen quality. Arch. Androl. 2006, 52, 239–242. [Google Scholar] [CrossRef]
- Yang, S.; Rothman, R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar] [CrossRef]
- Donà, V.; Kasraian, S.; Lupo, A.; Guilarte, Y.N.; Hauser, C.; Furrer, H.; Unemo, M.; Low, N.; Endimiani, A. Multiplex real-time PCR assay with high-resolution melting analysis for characterization of antimicrobial resistance in Neisseria gonorrhoeae. J. Clin. Microbiol. 2016, 54, 2074–2081. [Google Scholar] [CrossRef]
- Rathore, K.; Joseph, B.; Sharma, D.K.; Gaurav, A.; Sharma, S.K.; Milind, M.; Patel, P.; Prakash, C.; Singh, L. Evaluation of multiplex polymerase chain reaction as an alternative to conventional antibiotic sensitivity test. Vet. World 2018, 11, 474–479. [Google Scholar] [CrossRef]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Infectious Diseases Society of America (IDSA). Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 57 (Suppl. 3), S139–S170. [Google Scholar] [CrossRef]
- Pasquaroli, S.; Zandri, G.; Vignaroli, C.; Vuotto, C.; Donelli, G.; Biavasco, F. Antibiotic pressure can induce the viable but non-culturable state in Staphylococcus aureus growing in biofilms. J. Antimicrob. Chemother. 2013, 68, 1812–1817. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Mouraviev, V.; McDonald, M. An implementation of next generation sequencing for prevention and diagnosis of urinary tract infection in urology. Can. J. Urol. 2018, 25, 9349–9356. [Google Scholar]
- Ransom, E.M.; Potter, R.F.; Dantas, G.; Burnham, C.A.D. Genomic prediction of antimicrobial resistance: Ready or not, here it comes! Clin. Chem. 2020, 66, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhang, Y.; Yan, G.; Wang, Y.; Lu, G.; Wu, B.; Chen, W.; Zhou, W. Antimicrobial resistance prediction by clinical metagenomics in pediatric severe pneumonia patients. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Hiergeist, A.; Reischl, U. Priority Program 1656 Intestinal Microbiota Consortium/quality assessment participants; Gessner, A. Multicenter quality assessment of 16S ribosomal DNA-sequencing for microbiome analyses reveals high inter-center variability. Int. J. Med. Microbiol. 2016, 306, 334–342. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, X.; Zhong, X.; Settles, M.; Herring, J.; Wang, L.; Abdo, Z.; Forney, L.J.; Xu, C. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 2013, 100, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Punab, M.; Korrovits, P.; Türk, S.; Ausmees, K.; Lapp, E.; Preem, J.K.; Oopkaup, K.; Salumets, A.; Truu, J. Seminal microbiome in men with and without prostatitis. Int. J. Urol. 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Chen, H.; Luo, T.; Chen, T.; Wang, G. Seminal bacterial composition in patients with obstructive and non-obstructive azoospermia. Exp. Ther. Med. 2018, 15, 2884–2890. [Google Scholar] [CrossRef]
- Witkin, S.S. Immunological aspects of genital chlamydia infections. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, H.; Falk, L.; Jurstrand, M.; Unemo, M. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: Impact on epidemiological surveillance and interventions. APMIS 2004, 112, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Baczynska, A.; Svenstrup, H.F.; Fedder, J.; Birkelund, S.; Christiansen, G. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 2004, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Jephcott, A.E. Microbiological diagnosis of gonorrhoea. Genitourin. Med. 1997, 73, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Shahed, A.R.; Shoskes, D.A. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: Correlation with gram positive bacterial growth and treatment response. J. Androl. 2000, 21, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.F.; Xiao, W.Q.; Zheng, Y.C.; Dong, J.; Zhang, S.M. Increased oxidative stress and oxidative damage associated with chronic bacterial prostatitis. Asian J. Androl. 2006, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Kurpisz, M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: Potential inflammatory markers in semen. Folia Histochem. Cytobiol. 2015, 53, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, G.; Ramos, B.; Santiso, R.; Goyanes, V.; Gosalvez, J.; Fernandez, J.L. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril. 2008, 90, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Sanchez, R.; Soto, L.; Risopatron, J.; Villegas, J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil. Steril. 2010, 94, 619–623. [Google Scholar] [CrossRef]
- Fraczek, M.; Piasecka, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kazienko, A.; Lenart, S.; Laszczynska, M.; Kurpisz, M. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia 2012, 44, 315–329. [Google Scholar] [CrossRef]
- Moretti, E.; Cosci, I.; Spreafico, A.; Serchi, T.; Cuppone, A.M.; Collodel, G. Semen characteristics and inflammatory mediators in infertile men with different clinical diagnoses. Int. J. Androl. 2009, 32, 637–646. [Google Scholar] [CrossRef]
- Rusz, A.; Pilatz, A.; Wagenlehner, F.; Linn, T.; Diemer, T.; Schuppe, H.C.; Lohmeyer, J.; Hossain, H.; Weidner, W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 2012, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of inflammation on male reproductive tract. J. Reprod. Infertil. 2015, 16, 123–129. [Google Scholar] [PubMed]
- Ruggeri, M.; Cannas, S.; Cubeddu, M.; Molicotti, P.; Piras, G.L.; Dessole, S.; Zanetti, S. Bacterial agents as a cause of infertility in humans. New Microbiol. 2016, 39, 206–209. [Google Scholar] [PubMed]
- Sahyoun, H.A.; Shukri, M.H. Laboratory investigation: Sexually transmitted diseases. Clin. Dermatol. 2004, 22, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, H.G. Microbiology of male urethroadnexitis: Diagnostic procedures and criteria for aetiologic classification. Andrologia 1998, 30 (Suppl. 1), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M. Diagnosis and therapy of acute prostatitis, epididymitis and orchitis. Andrologia 2008, 40, 76–80. [Google Scholar] [CrossRef]
- Haidl, G.; Allam, J.P.; Schuppe, H.C. Chronic epididymitis: Impact on semen parameters and therapeutic options. Andrologia 2008, 40, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Diemer, T.; Huwe, P.; Ludwig, M.; Schroeder-Printzen, I.; Michelmann, H.W.; Schiefer, H.G.; Weidner, W. Influence of autogenous leucocytes and Escherichia coli on sperm motility parameters in vitro. Andrologia 2003, 35, 100–105. [Google Scholar] [CrossRef]
- Rose, B.I.; Scott, B. Sperm motility, morphology, hyperactivation, and ionophore-induced acrosome reactions after overnight incubation with mycoplasmas. Fertil. Steril. 1994, 61, 341–348. [Google Scholar] [CrossRef]
- Berktas, M.; Aydin, S.; Yilmaz, Y.; Cecen, K.; Bozkurt, H. Sperm motility changes after coincubation with various uropathogenic microorganisms: An in vitro experimental study. Int. Urol. Nephrol. 2008, 40, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Song, J.; Liu, H.; Bi, W.; Dong, G.; Zhou, T. Characteristic and mechanism of immobilization effect of Staphylococcus aureus on human spermatozoa. Microb. Pathog. 2018, 119, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lenický, M.; Slanina, T.; Kačániová, M.; Galovičová, L.; Petrovičová, M.; Ďuračka, M.; Benko, F.; Kováč, J.; Tvrdá, E. Identification of bacterial profiles and their interactions with selected quality, oxidative, and immunological parameters of turkey semen. Animals 2021, 11, 1771. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Cinque, B.; Filipponi, S.; Mastromarino, P.; Cifone, M.G.; Francavilla, S.; Francavilla, F. Soluble products of Escherichia coli induce mitochondrial dysfunction-related sperm membrane lipid peroxidation which is prevented by lactobacilli. PLoS ONE 2013, 8, e83136. [Google Scholar] [CrossRef]
- El-Mulla, K.F.; Köhn, F.M.; Dandal, M.; el Beheiry, A.H.; Schiefer, H.G.; Weidner, W.; Schill, W.B. In vitro effect of Escherichia coli on human sperm acrosome reaction. Arch. Androl. 1996, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, Y.; Malik, Z.; Breitbart, H. Sperm interaction with bacteria induces the spontaneous acrosome reaction. Theriogenology 2023, 203, 82–88. [Google Scholar] [CrossRef]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef]
- Leisegang, K.; Bouic, P.J.; Henkel, R.R. Metabolic syndrome is associated with increased seminal inflammatory cytokines and reproductive dysfunction in a case-controlled male cohort. Am. J. Reprod. Immunol. 2016, 76, 155–163. [Google Scholar] [CrossRef]
- Martínez, R.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef]
- Eggert-Kruse, W.; Boit, R.; Rohr, G.; Aufenanger, J.; Hund, M.; Strowitzki, T. Relationship of seminal plasma interleukin (IL)-8 and IL-6 with semen quality. Hum. Reprod. 2001, 16, 517–528. [Google Scholar] [CrossRef]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Boockfor, F.R.; Schwarz, L.K. Effects of interleukin-6, interleukin-2, and tumor necrosis factor alpha on transferrin release from Sertoli cells in culture. Endocrinology 1991, 129, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Twillie, D.A.; Eisenberger, M.A.; Carducci, M.A.; Hseih, W.S.; Kim, W.Y.; Simons, J.W. Interleukin-6: A candidate mediator of human prostate cancer morbidity. Urology 1995, 45, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Bates, M.; Vince, G.; Lewis Jones, D.I. The role of cytokine expression in different subgroups of subfertile men. Am. J. Reprod. Immunol. 2009, 62, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kopa, Z.; Wenzel, J.; Papp, G.K.; Haidl, G. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia 2005, 37, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Poynter, M.E.; Baeuerle, P.A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 2007, 28, 1317–1327. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of inflammatory associated impairment of sperm function, spermatogenesis and steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic. Biol. Med. 1995, 18, 487–495. [Google Scholar] [CrossRef]
- Homa, S.T.; Vessey, W.; Perez-Miranda, A.; Riyait, T.; Agarwal, A. Reactive Oxygen Species (ROS) in human semen: Determination of a reference range. J. Assist. Reprod. Genet. 2015, 32, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Koyuturk, M.; Kilic, G.; Alpak, O.; Aytoz, A. Effects of leucocytospermia on semen parameters and outcomes of intracytoplasmic sperm injection. Int. J. Androl. 2005, 28, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Tunc, O. Macrophage activity in semen is significantly correlated with sperm quality in infertile men. Int. J. Androl. 2010, 33, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Y.; Chen, G.; Huang, X.; Yuan, Y.; Wu, X.; Wu, B.; Li, Z.; Shun, F.; Chen, H.; Shi, H. Effects of reactive oxygen species from activated leucocytes on human sperm motility, viability and morphology. Andrologia 2012, 44 (Suppl. 1), 696–703. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Banerjee Mustafi, S.; Chakraborty, P.K.; Dey, R.S.; Raha, S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 2009, 14, 579–589. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Tschopp, J. Mitochondria: Sovereign of inflammation? Eur. J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Solomon, M.C. Leukocytes as a cause of oxidative stress. In Oxidants, Antioxidants, and Impact of the Oxidative Status in Male Reproduction; Henkel, R., Samanta, L., Agarwal, A., Eds.; Elsevier: London, UK, 2018; pp. 37–44. [Google Scholar]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From past to present: The link between reactive oxygen species in sperm and male infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Hargreave, T.B.; Irvine, D.S.; Wu, F.C.W. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J. Androl. 1989, 10, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Irvine, D.S.; Wu, F.C. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am. J. Obstet. Gynecol. 1991, 164, 542–551. [Google Scholar] [CrossRef]
- Zalata, A.A.; Christophe, A.B.; Depuydt, C.E.; Schoonjans, F.; Comhaire, F.H. White blood cells cause oxidative damage to the fatty acid composition of phospholipids of human spermatozoa. Int. J. Androl. 1998, 21, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell. Mol. Biol. Lett. 2003, 8, 391–413. [Google Scholar]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57 (Suppl. 5), 779S–786S. [Google Scholar] [CrossRef]
- Badouard, C.; Ménézo, Y.; Panteix, G.; Ravanat, J.L.; Douki, T.; Cadet, J.; Favier, A. Determination of new types of DNA lesions in human sperm. Zygote 2008, 16, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Gips, H.; Schill, W.-B.; Kruger, T.F. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil. Steril. 2004, 81, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.B.; Kumar, R.; Dada, R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J. Med. Res. 2008, 127, 115–123. [Google Scholar] [PubMed]
- Humaidan, P.; Haahr, T.; Povlsen, B.B.; Kofod, L.; Laursen, R.J.; Alsbjerg, B.; Elbaek, H.O.; Esteves, S.C. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: A pilot study. Int. Braz. J. Urol. 2022, 48, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Vercesi, A.E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 1999, 26, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.; Copeland, W.C.; Day, B.J. Mitochondrial DNA depletion, oxidative stress, and mutation: Mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab. Investig. 2001, 81, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Venkatesh, S.; Kumar, M.; Tanwar, M.; Shasmsi, M.B.; Kumar, R.; Gupta, N.P.; Sharma, R.K.; Talwar, P.; Dada, R. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J. Biochem. Biophys. 2009, 46, 172–177. [Google Scholar]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA fragmentation: A new guideline for clinicians. World J. Men’s Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2’-deoxyguanosine, a marker of oxidative stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell. Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Pradeepa, M.M.; Rao, M.R.S. Chromatin remodeling during mammalian spermatogenesis: Role of testis specific histone variants and transition proteins. Soc. Reprod. Fertil. Suppl. 2007, 63, 1–10. [Google Scholar] [PubMed]
- Ranawat, P.; Bansal, M.P. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: Implications in spermatogenesis. Mol. Cell. Biochem. 2009, 330, 83–95. [Google Scholar] [CrossRef]
- Croteau, D.L.; Stierum, R.H.; Bohr, V.A. Mitochondrial DNA repair pathways. Mutat. Res. 1999, 434, 137–148. [Google Scholar] [CrossRef]
- Folgerø, T.; Bertheussen, K.; Lindal, S.; Torbergsen, T.; Oian, P. Mitochondrial disease and reduced sperm motility. Hum. Reprod. 1993, 8, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Pesole, G.; Gissi, C.; De Chirico, A.; Saccone, C. Nucleotide substitution rate of mammalian mitochondrial genomes. J. Mol. Evol. 1999, 48, 427–434. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar] [CrossRef]
- Bonanno, O.; Romeo, G.; Asero, P.; Pezzino, F.M.; Castiglione, R.; Burrello, N.; Sidoti, G.; Frajese, G.V.; Vicari, E.; D’Agata, R. Sperm of patients with severe asthenozoospermia show biochemical, molecular and genomic alterations. Reproduction 2016, 152, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Spiropoulos, J.; Turnbull, D.M.; Chinnery, P.F. Can mitochondrial DNA mutations cause sperm dysfunction? Mol. Hum. Reprod. 2002, 8, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Caston, R.A.; Demple, B. Risky repair: DNA-protein crosslinks formed by mitochondrial base excision DNA repair enzymes acting on free radical lesions. Free Radic. Biol. Med. 2017, 107, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.G.; Park, H.J.; Kim, J.W.; Jung, J.M.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Kang, M.J.; Wee, G.; Yang, H.Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Sperm mitochondria, the driving force behind human spermatozoa activities: Its functions and dysfunctions—A narrative review. Curr. Mol. Med. 2023, 23, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Siderakis, M.; Tarsounas, M. Telomere regulation and function during meiosis. Chromosome Res 2007, 15, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.G.; Dsouza, R.; Pandya, G.; Kirtonia, A.; Tergaonkar, V.; Lee, S.Y.; Garg, M.; Khattar, E. Role of telomeres and telomeric proteins in human malignancies and their therapeutic potential. Cancers 2020, 12, 1901. [Google Scholar] [CrossRef]
- Armanios, M. The role of telomeres in human disease. Annu. Rev. Genom. Hum. Genet. 2022, 23, 363–381. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Sakaloglou, P.; Bouba, I.; Sofikitis, N.; Georgiou, I. Functional association between telomeres, oxidation and mitochondria. Front. Reprod. Health 2023, 5, 1107215. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Oikawa, S.; Tada-Oikawa, S.; Kawanishi, S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 2001, 40, 4763–4768. [Google Scholar] [CrossRef]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef]
- Thilagavathi, J.; Venkatesh, S.; Dada, R. Telomere length in reproduction. Andrologia 2013, 45, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Darmishonnejad, Z.; Zarei-Kheirabadi, F.; Tavalaee, M.; Zarei-Kheirabadi, M.; Zohrabi, D.; Nasr-Esfahani, M.H. Relationship between sperm telomere length and sperm quality in infertile men. Andrologia 2020, 52, e13546. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Maghsudlu, M.; Sheikhha, M.H. Is sperm telomere length altered in teratozoospermia specimens? A case-control study. Int. J. Reprod. Biomed. 2023, 21, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tan, Y.; Qiu, X.; Luo, H.; Li, Y.; Li, R.; Yang, X. Sperm telomere length as a novel biomarker of male infertility and embryonic development: A systematic review and meta-analysis. Front. Endocrinol. 2023, 13, 1079966. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, V.S.; Shahid, M.; Deo, P.; Fenech, M. Reduced SIRT1 and SIRT3 and lower antioxidant capacity of seminal plasma is associated with shorter sperm telomere length in oligospermic men. Int. J. Mol. Sci. 2024, 25, 718. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, R.; Malhotra, N.; Singh, N.; Dada, R. Mild oxidative stress is beneficial for sperm telomere length maintenance. World J. Methodol. 2016, 6, 163–170. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Chughtai, M.I.; Tan, B.; Omur, A.D.; Tunio, S.A.; Akbar, M.S.; Kalhoro, M.S. Redox mechanisms of environmental toxicants on male reproductive function. Front. Cell. Dev. Biol. 2024, 12, 1333845. [Google Scholar] [CrossRef]
- Sasikumar, S.; Dakshayani, D.; Sarasa, D. An investigation of DNA fragmentation and morphological changes caused by bacteria and fungi in human spermatozoa. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 84–96. [Google Scholar]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas, I.; Bontis, I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef]
- Štšepetova, J.; Baranova, J.; Simm, J.; Parm, Ü.; Rööp, T.; Sokmann, S.; Korrovits, P.; Jaagura, M.; Rosenstein, K.; Salumets, A.; et al. The complex microbiome from native semen to embryo culture environment in human in vitro fertilization procedure. Reprod. Biol. Endocrinol. 2020, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Amato, V.; Papaleo, E.; Pasciuta, R.; Viganò, P.; Ferrarese, R.; Clementi, N.; Sanchez, A.M.; Quaranta, L.; Burioni, R.; Ambrosi, A.; et al. Differential composition of vaginal microbiome, but not of seminal microbiome, is associated with successful intrauterine insemination in couples with idiopathic infertility: A prospective observational study. Open Forum Infect. Dis. 2019, 7, ofz525. [Google Scholar] [CrossRef] [PubMed]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 3, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Koort, K.; Sõsa, K.; Türk, S.; Lapp, E.; Talving, E.; Karits, P.; Rosenstein, K.; Jaagura, M.; Sekavin, A.; Sõritsa, D.; et al. Lactobacillus crispatus-dominated vaginal microbiome and Acinetobacter-dominated seminal microbiome support beneficial ART outcome. Acta Obstet. Gynecol. Scand. 2023, 102, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Khodamoradi, K.; Kuchakulla, M.; Narasimman, M.; Khosravizadeh, Z.; Ali, A.; Brackett, N.; Ibrahim, E.; Ramasamy, R. Laboratory and clinical management of leukocytospermia and hematospermia: A review. Ther. Adv. Reprod. Health 2020, 14, 2633494120922511. [Google Scholar] [CrossRef] [PubMed]
- Velez, D.; Ohlander, S.; Niederberger, C. Pyospermia: Background and controversies. F S Rep. 2021, 2, 2–6. [Google Scholar] [CrossRef]
- Barraud-Lange, V.; Pont, J.C.; Ziyyat, A.; Pocate, K.; Sifer, C.; Cedrin-Durnerin, I.; Fechtali, B.; Ducot, B.; Wolf, J.P. Seminal leukocytes are Good Samaritans for spermatozoa. Fertil. Steril. 2011, 9, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.E.; Märk, I.; Sator, K.; Huber, J.; Sator, M. Effect of leukocytospermia on fertilization and pregnancy rates of artificial reproductive technologies. Fertil. Steril. 2008, 90, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, M.; Oliveira, J.B.; Petersen, C.G.; Mauri, A.L.; Silva, L.F.; Massaro, F.C.; Baruffi, R.L.; Franco, J.G., Jr. The influence of leukocytospermia on the outcomes of assisted reproductive technology. Reprod. Biol. Endocrinol. 2012, 10, 44. [Google Scholar] [CrossRef]
- Ricci, G.; Granzotto, M.; Luppi, S.; Giolo, E.; Martinelli, M.; Zito, G.; Borelli, M. Effect of seminal leukocytes on in vitro fertilization and intracytoplasmic sperm injection outcomes. Fertil. Steril. 2015, 104, 87–93. [Google Scholar] [CrossRef]
- Qiao, X.; Zeng, R.; Yang, Z.; Xu, L.; Ma, Q.; Yang, Y.; Bai, Y.; Yang, Y.; Bai, P. Effects of leukocytospermia on the outcomes of assisted reproductive technology. Andrologia 2022, 54, e14403. [Google Scholar] [CrossRef]
- Castellini, C.; D’Andrea, S.; Martorella, A.; Minaldi, E.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship between leukocytospermia, reproductive potential after assisted reproductive technology, and sperm parameters: A systematic review and meta-analysis of case-control studies. Andrology 2020, 8, 125–135. [Google Scholar] [CrossRef]
- Farber, N.J.; Madhusoodanan, V.K.; Gerkowicz, S.A.; Patel, P.; Ramasamy, R. Reasons that should prompt a referral to a reproductive urologist: Guidelines for the gynecologist and reproductive endocrinologist. Gynecol. Pelvic. Med. 2019, 2, 20. [Google Scholar] [CrossRef]
- Tortolero, I.; Duarte Ojeda, J.M.; Pamplona Casamayor, M.; Alvarez González, E.; Arata-Bellabarba, G.; Regadera, J.; Leiva Galvis, O. The effect of seminal leukocytes on semen quality in subfertile males with and without varicocele. Arch. Esp. Urol. 2004, 57, 921–928. [Google Scholar]
- Pasqualotto, F.F.; Sobreiro, B.; Allamaneni, S.S.; Hallak, J.; Lucon, A.M.; Agarwal, A. Relationship between increased seminal leukocytes and varicocele. Fertil Steril 2005, 84 (Suppl. 1), S418. [Google Scholar] [CrossRef]
- Damirayakhian, M.A.; Perez-Pelaez, M.; Jeyendran, R.S. Antibiotic susceptibility of prostatovesicular fluid isolates. Infertility 1987, 10, 95–101. [Google Scholar]
- Branigan, E.F.; Muller, C.H. Efficacy of treatment and recurrence rate of leukocytospermia in infertile men with prostatitis. Fertil. Steril. 1994, 62, 580–584. [Google Scholar] [CrossRef]
- Pallett, A.; Hand, K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65 (Suppl. 3), iii25–iii33. [Google Scholar] [CrossRef]
- Dooley, M.; Dineen, T.; Sarma, K.; Nolan, A. The psychological impact of infertility and fertility treatment on the male partner. Hum. Fertil. 2014, 17, 203–209. [Google Scholar] [CrossRef]
- De Jonge, C.J.; Gellatly, S.A.; Vazquez-Levin, M.H.; Barratt, C.L.R.; Rautakallio-Hokkanen, S. Male attitudes towards infertility: Results from a global questionnaire. World J. Men’s Health 2023, 41, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Peate, I. Delivering men’s health. Br. J. Nurs. 2023, 32, 705. [Google Scholar] [CrossRef]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Womens Health 2014, 23, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Street, E.; Joyce, A.; Wilson, J. Clinical Effectiveness Group, British Association for Sexual Health and HIV. BASHH UK guideline for the management of epididymo-orchitis, 2010. Int. J. STD AIDS 2011, 22, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F.; Mezei, T.; Pilatz, A.; et al. EAU Guidelines on Urological infections. EAU Guidelines. Edn. In Proceedings of the EAU Annual Congress, Amsterdam, The Netherlands, 17–26 July 2020; ISBN 978-94-92671-07-3. [Google Scholar]
- Naber, K.G.; Madsen, P.O. Antibiotics: Basic concepts. In Textbook of Prostatitis; Nickel, J.C., Ed.; Isis Medical Media: Cambridge, UK, 1999; pp. 83–94. [Google Scholar]
- Naber, K.G.; Weidner, W. Chronic prostatitis-an infectious disease? J. Antimicrob. Chemother. 2000, 46, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Bjerklund Johansen, T.E.; Grüneberg, R.N.; Guibert, J.; Hofstetter, A.; Lobel, B.; Naber, K.G.; Palou Redorta, J.; van Cangh, P.J. The role of antibiotics in the treatment of chronic prostatitis: A consensus statement. Eur. Urol. 1998, 34, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Montag, M.; van der Ven, H.; Haidl, G. Recovery of ejaculated spermatozoa for intracytoplasmic sperm injection after anti-inflammatory treatment of an azoospermic patient with genital tract infection: A case report. Andrologia 1999, 31, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Haidl, G. Management strategies for male factor infertility. Drugs 2002, 62, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Lanzafame, F.M.; La Vignera, S.; Vicari, E.; Calogero, A.E. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Reproductive Medicine Network; et al. The effect of antioxidants on male factor infertility: The Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertil. Steril. 2020, 113, 552–560. [Google Scholar] [CrossRef] [PubMed]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 5, CD007411. [Google Scholar] [CrossRef]
- Agarwal, A.; Cannarella, R.; Saleh, R.; Harraz, A.M.; Kandil, H.; Salvio, G.; Boitrelle, F.; Kuroda, S.; Farkouh, A.; Rambhatla, A.; et al. Impact of antioxidant therapy on natural pregnancy outcomes and semen parameters in infertile men: A systematic review and meta-analysis of randomized controlled trials. World J. Men’s Health 2023, 41, 14–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chandraker, S.; Patel, V.K.; Ramteke, P. Antibacterial activity of medicinal plants against pathogens causing complicated urinary tract infections. Indian J. Pharm. Sci. 2009, 71, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Guillet-Rosso, F.; Fari, A.; Taylor, S.; Forman, R.; Belaisch-Allart, J.; Testart, J.; Frydman, R. Systematic semen culture and its influence on IVF management. Br. J. Obstet. Gynaecol. 1987, 94, 543–547. [Google Scholar] [CrossRef]
- Wong, P.C.; Balmaceda, J.P.; Blanco, J.D.; Gibbs, R.S.; Asch, R.H. Sperm washing and swim-up technique using antibiotics removes microbes from human semen. Fertil. Steril. 1986, 45, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Cottell, E.; Lennon, B.; McMorrow, J.; Barry-Kinsella, C.; Harrison, R.F. Processing of semen in an antibiotic-rich culture medium to minimize microbial presence during in vitro fertilization. Fertil. Steril. 1997, 67, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Krissi, H.; Orvieto, R.; Ashkenazi, J.; Gilboa, Y.; Shalev, J.; Moscovitch, I.; Bar-Hava, I. Effect of contaminated preprocessed semen on fertilization rate and embryo quality in assisted reproductive techniques. Gynecol. Endocrinol. 2004, 18, 63–67. [Google Scholar] [CrossRef]
- Jones, D.M.; Kovacs, G.T.; Harrison, L.; Jennings, M.G.; Baker, H.W. Immobilization of sperm by condoms and their components. Clin. Reprod. Fertil. 1986, 4, 367–372. [Google Scholar]
- Pradiee, J.; O’Brien, E.; Esteso, M.C.; Castaño, C.; Toledano-Díaz, A.; López-Sebastián, A.; Santiago-Moreno, J. Spermiotoxicity of commercial condoms made from polyurethane, polyisoprene and latex, using domestic ruminants as an experimental animal model. Andrologia 2016, 48, 475–480. [Google Scholar] [CrossRef]
- Boucher, P.; Lejeune, H.; Pinatel, M.C.; Gille, Y. Spermoculture: Improvement of the bacteriological quality of samples by direct verbal counseling before semen collection. Fertil. Steril. 1995, 64, 657–660. [Google Scholar] [CrossRef]
- Kim, F.Y.; Goldstein, M. Antibacterial skin preparation decreases the incidence of false-positive semen culture results. J. Urol. 1999, 161, 819–821. [Google Scholar] [CrossRef]
- Garrido, N.; Zuzuarregui, J.L.; Meseguer, M.; Simón, C.; Remohí, J.; Pellicer, A. Sperm and oocyte donor selection and management: Experience of a 10 year follow-up of more than 2100 candidates. Hum. Reprod. 2002, 17, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Rodin, D.M.; Larone, D.; Goldstein, M. Relationship between semen cultures, leukospermia, and semen analysis in men undergoing fertility evaluation. Fertil. Steril. 2003, 79 (Suppl. 3), 1555–1558. [Google Scholar] [CrossRef]
- Barratt, C.L.; Bolton, A.E.; Cooke, I.D. Functional significance of white blood cells in the male and female reproductive tract. Hum. Reprod. 1990, 5, 639–648. [Google Scholar] [CrossRef]
- Trum, J.W.; Mol, B.W.J.; Pannekoek, Y.; Spanjaard, L.; Wertheim, P.; Bleker, O.P.; van der Veen, F. Value of detecting leukocytospermia in the diagnosis of genital tract infection in subfertile men. Fertil. Steril. 1998, 70, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Vicari, E. Effectiveness and limits of antimicrobial treatment on seminal leukocyte concentration and related reactive oxygen species production in patients with male accessory gland infection. Hum. Reprod. 2000, 15, 2536–2544. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Rowe, P.J.; Farley, T.M. The effect of doxycycline in infertile couples with male accessory gland infection: A double blind prospective study. Int. J. Androl. 1986, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Hibi, H.; Katsuno, S.; Miyake, K. Antibiotic and ejaculation treatments improve resolution rate of leukocytospermia in infertile men with prostatitis. Nagoya J. Med. Sci. 1995, 58, 41–45. [Google Scholar]
- Moubasher, A.; Sayed, H.; Mosaad, E.; Mahmoud, A.; Farag, F.; Taha, E.A. Impact of leukocytospermia on sperm dynamic motility parameters, DNA and chromosomal integrity. Cent. European J. Urol. 2018, 71, 470–475. [Google Scholar]
- Skau, P.A.; Folstad, I. Do bacterial infections cause reduced ejaculate quality? A meta-analysis of antibiotic treatment of male infertility. Behav. Ecol. 2003, 14, 40–47. [Google Scholar] [CrossRef]
- Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-inflammatory diets in fertility: An evidence review. Nutrients 2022, 14, 3914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Diao, R.Y.; Duan, Y.G.; Yi, T.H.; Cai, Z.M. In vitro antioxidant effect of curcumin on human sperm quality in leucocytospermia. Andrologia 2017, 49, e12760. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.; Gan, H.; Tian, F.; Cai, X.; Zhen, W.; Song, X.; Duan, Y.G. In vitro antioxidation effect of Quercetin on sperm function from the infertile patients with leukocytospermia. Am. J. Reprod. Immunol. 2019, 82, e13155. [Google Scholar] [CrossRef]
- Vyas, A.; Purohit, A.; Ram, H. Assessment of dose-dependent reproductive toxicity of diclofenac sodium in male rats. Drug Chem. Toxicol. 2019, 42, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Cannarella, R.; Saleh, R.; Boitrelle, F.; Gül, M.; Toprak, T.; Salvio, G.; Arafa, M.; Russo, G.I.; Harraz, A.M.; et al. Impact of Varicocele Repair on Semen Parameters in Infertile Men: A Systematic Review and Meta-Analysis. World J. Men’s Health 2023, 41, 289–310. [Google Scholar] [CrossRef]
- Kowalczyk, A. The role of the natural antioxidant mechanism in sperm cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Li, H.T.; Jiao, M.; Chen, J.; Liang, Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim. Biophys. Sin. 2010, 42, 183–194. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a bioactive micronutrient in the human diet and its cancer chemopreventive activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Chia, S.E.; Ong, C.N.; Chua, L.H.; Ho, L.M.; Tay, S.K. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J. Androl. 2000, 21, 53–57. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawano, N.; Yoshiike, M.; Yoshida, M.; Iwamoto, T.; Morisawa, M. Physiological roles of semenogelin I and zinc in sperm motility and semen coagulation on ejaculation in humans. Mol. Hum. Reprod. 2008, 14, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.B.; Suryakar, A.N.; Huddedar, A.D.; Shukla, P.S. Effect of antioxidants and antibiotics on levels of seminal oxidative stress in leukocytospermic infertile men. Indian J. Clin. Biochem. 2006, 21, 152–156. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Tosti, C.; Morgante, G.; Ponchia, R.; Luddi, A.; Governini, L.; Piomboni, P. Positive effect of a new combination of antioxidants and natural hormone stimulants for the treatment of oligoasthenoteratozoospermia. J. Clin. Med. 2022, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Woodman, R.; Hill, A.; Shehadeh, H.; Lane, M.; Zander-Fox, D. Use of a male antioxidant nutraceutical is associated with superior live birth rates during IVF treatment. Asian J. Androl. 2020, 23, 16–23. [Google Scholar] [CrossRef]
- Kızılay, F.; Altay, B. Evaluation of the effects of antioxidant treatment on sperm parameters and pregnancy rates in infertile patients after varicocelectomy: A randomized controlled trial. Int. J. Impot. Res. 2019, 31, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Leisegang, K.; Majzoub, A.; Henkel, R.; Finelli, R.; Panner Selvam, M.K.; Tadros, N.; Parekh, N.; Ko, E.Y.; Cho, C.L.; et al. Utility of antioxidants in the treatment of male infertility: Clinical guidelines based on a systematic review and analysis of evidence. World J. Men’s Health 2021, 39, 233–290. [Google Scholar] [CrossRef]
- Rolf, C.; Cooper, T.G.; Yeung, C.H.; Nieschlag, E. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: A randomized, placebo-controlled, double-blind study. Hum. Reprod. 1999, 14, 1028–1033. [Google Scholar] [CrossRef]
- Ozer, C. Antioxidant treatment of increased sperm DNA fragmentation: Complex combinations are not more successful. Arch. Ital. Urol. Androl. 2020, 92, 362–365. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Mårdh, P.A.; Colleen, S. Antimicrobial activity of human seminal fluid. Scand. J. Urol. Nephrol. 1975, 9, 17–23. [Google Scholar] [CrossRef]
- Edström, A.M.; Malm, J.; Frohm, B.; Martellini, J.A.; Giwercman, A.; Mörgelin, M.; Cole, A.M.; Sorensen, O.E. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J. Immunol. 2008, 181, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Elyasi, L.; Khaleghi, A.A.; Mohammadi, M. The effect of zinc supplementation on improving sperm parameters in infertile diabetic men. J. Obstet. Gynaecol. India 2023, 73, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Aston, K.; Carrell, D.; DeVilbiss, E.; Sjaarda, L.; Perkins, N.; Mills, J.L.; Chen, Z.; Sparks, A.; Clemons, T.; et al. The impact of zinc and folic acid supplementation on sperm DNA methylation: Results from the folic acid and zinc supplementation randomized clinical trial (FAZST). Fertil. Steril. 2022, 117, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: A randomized clinical trial. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, 3, CD007411. [Google Scholar] [CrossRef]
- Rochdi, C.; Ouadrhiri, M.; Allai, L.; Bellajdel, I.; Mamri, S.; Taheri, H.; Saadi, H.; Mimouni, A.; Choukri, M. Beneficial effects of oral antioxidant supplementation on semen quality parameters, reproductive hormones, and sperm DNA integrity in men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2024. Online ahead of print. [Google Scholar] [CrossRef]


| Pathogen | Disease | Diagnostic Test | Reference |
|---|---|---|---|
| Chlamydia trachomatis | Urethritis Prostatitis Orchitis Epididymitis | PCR C. trachomatis culture with immunofluorescent staining of reticulate bodies | [109,111,113,142,143] |
| Ureaplasma urealyticum | Urethritis Prostatitis | PCR Semen culture | [111,113,142] |
| Ureaplasma parvum | Urethritis | Semen culture PCR | [113] |
| Mycoplasma hominis | Urethritis | PCR, RT-PCR | [111,113,144] |
| Mycoplasma genitalium | Urethritis | PCR | [111] |
| Neisseria gonorrhea | Urethritis Orchitis Epididymitis | PCR Gonococcal culture | [143,145] |
| Gram-positive cocci (e.g., Enterococcus spp.) | Prostatitis | Semen culture | [111,113] |
| Enterobacteriaceae (e.g., Escherichia coli, Klebsiella spp.) | Urethritis Prostatitis Orchitis Epididymitis | Semen culture API 20E test | [111,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henkel, R. Leukocytospermia and/or Bacteriospermia: Impact on Male Infertility. J. Clin. Med. 2024, 13, 2841. https://doi.org/10.3390/jcm13102841
Henkel R. Leukocytospermia and/or Bacteriospermia: Impact on Male Infertility. Journal of Clinical Medicine. 2024; 13(10):2841. https://doi.org/10.3390/jcm13102841
Chicago/Turabian StyleHenkel, Ralf. 2024. "Leukocytospermia and/or Bacteriospermia: Impact on Male Infertility" Journal of Clinical Medicine 13, no. 10: 2841. https://doi.org/10.3390/jcm13102841





