Myopia Is an Ischemic Eye Condition: A Review from the Perspective of Choroidal Blood Flow
Abstract
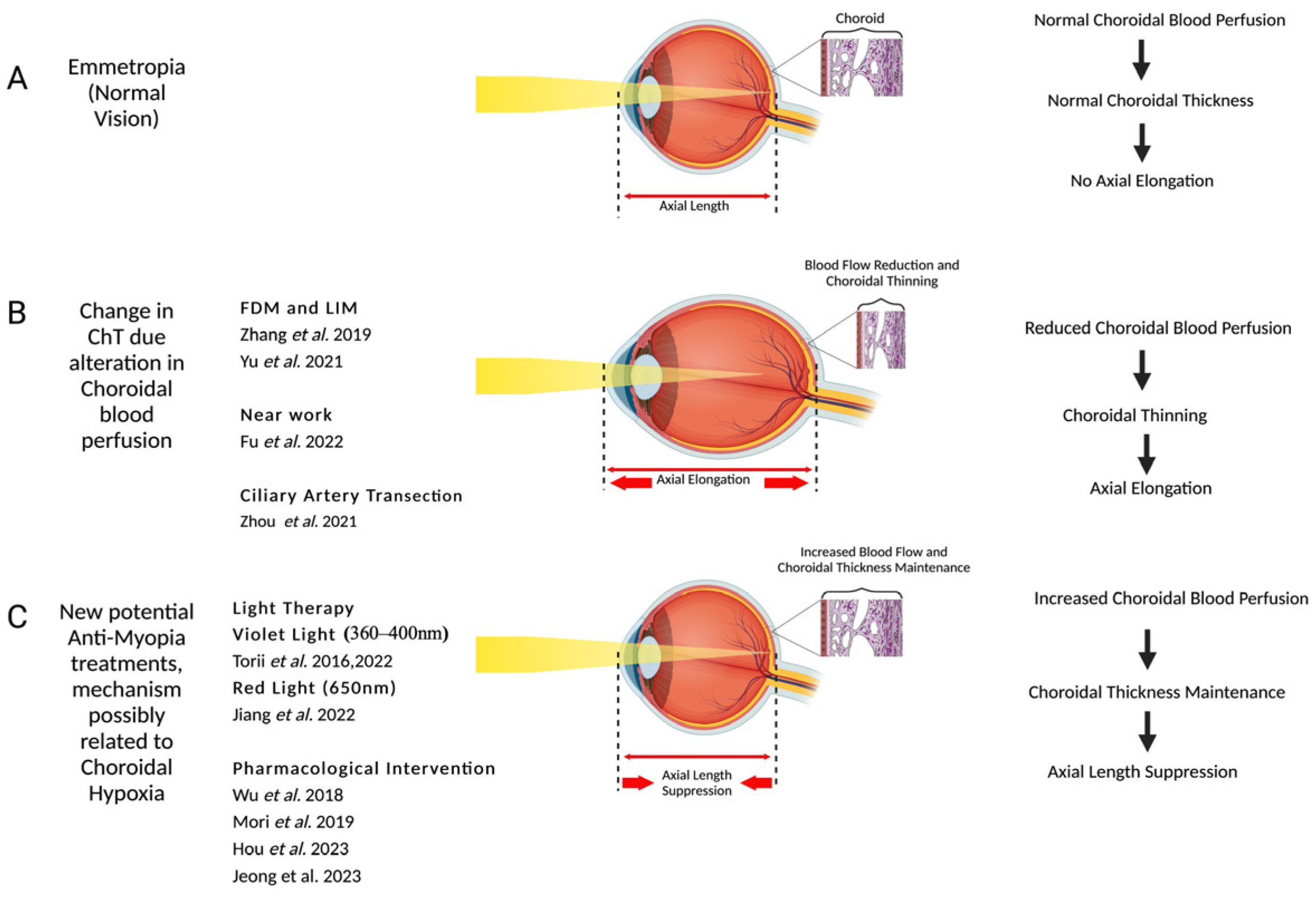
:1. Introduction
2. Choroidal Blood Flow Changes in Myopia
3. Choroidal Change with Near Work
4. Modulation of Choroidal Blood Flow Using Pharmacological Interventions
5. Choroidal Changes with Light Exposure
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Hirai, H. Prevalence of Myopia and Refractive Changes in Students From 3 to 17 Years of Age. Surv. Ophthalmol. 1999, 44, S109–S115. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Bi, H.S.; Wang, S.M.; Hu, Y.Y.; Wu, H.; Sun, W.; Lu, T.L.; Wang, X.R.; Jonas, J.B. Refractive Error, Visual Acuity and Causes of Vision Loss in Children in Shandong, China. The Shandong Children Eye Study. PLoS ONE 2013, 8, e82763. [Google Scholar] [CrossRef]
- Jung, S.-K.; Lee, J.H.; Kakizaki, H.; Jee, D. Prevalence of Myopia and Its Association with Body Stature and Educational Level in 19-Year-Old Male Conscripts in Seoul, South Korea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5579–5583. [Google Scholar] [CrossRef]
- Koh, V.; Yang, A.; Saw, S.M.; Chan, Y.H.; Lin, S.T.; Tan, M.M.H.; Tey, F.; Nah, G.; Ikram, M.K. Differences in Prevalence of Refractive Errors in Young Asian Males in Singapore between 1996–1997 and 2009–2010. Ophthalmic Epidemiol. 2014, 21, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential Lost Productivity Resulting from the Global Burden of Myopia. Ophthalmology 2019, 126, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Hammond, C. High Myopia and Its Risks. Community Eye Health 2019, 32, 5–6. [Google Scholar]
- Yamada, M.; Hiratsuka, Y.; Roberts, C.B.; Pezzullo, M.L.; Yates, K.; Takano, S.; Miyake, K.; Taylor, H.R. Prevalence of Visual Impairment in the Adult Japanese Population by Cause and Severity and Future Projections. Ophthalmic Epidemiol. 2010, 17, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, G.; Zhou, X.; Xu, R.; Wang, S.; Guan, Z.; Lu, J.; Srinivasalu, N.; Shen, M.; Jin, Z.; et al. Changes in Choroidal Thickness and Choroidal Blood Perfusion in Guinea Pig Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3074. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Yang, F.; Yang, Y.; Huang, Q.; Huang, C.; Qu, J.; Zhou, X. Decreased Choroidal Blood Perfusion Induces Myopia in Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Z.; Wang, F.; Peng, X.; He, W.; Chen, D.; Shen, M.; Lu, F.; Jiang, J. Choroidal Thickness and Choriocapillaris Vascular Density in Myopic Anisometropia. Eye Vis. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, C.; Yuan, Y.; Hu, X.; Chen, C.; Zhu, H.; Ke, B. Three-Dimensional Choroidal Vascularity Index in High Myopia Using Swept-Source Optical Coherence Tomography. Curr. Eye Res. 2022, 47, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The Multifunctional Choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed]
- Nag, T.C.; Kumari, C. Chapter 2-Electron Microscopy of the Human Choroid. In Choroidal Disorders; Chhablani, J., Ruiz-Medrano, J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 7–20. ISBN 978-0-12-805313-3. [Google Scholar]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Shin, Y.U.; Cho, H.Y.; Lee, B.R. Measurement of Choroidal Thickness in Normal Eyes Using 3D OCT-1000 Spectral Domain Optical Coherence Tomography. Korean J. Ophthalmol. 2012, 26, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, C.; Wang, X.; Zhou, W.; Reinach, P.; Qu, J. Choroidal Blood Perfusion as a Potential “Rapid Predictive Index” for Myopia Development and Progression. Eye Vis. 2021, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Xu, Y.; Pang, Z.; Mu, G. The Influence of the Choroid on the Onset and Development of Myopia: From Perspectives of Choroidal Thickness and Blood Flow. Acta Ophthalmol. 2021, 99, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, W.; Zhao, F.; Zhou, Q.; Reinach, P.S.; Deng, L.; Ma, L.; Luo, S.; Srinivasalu, N.; Pan, M.; et al. Scleral Hypoxia Is a Target for Myopia Control. Proc. Natl. Acad. Sci. USA 2018, 115, E7091–E7100. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Vincent, S.J.; Alonso-Caneiro, D. Choroidal Thickness in Myopic and Nonmyopic Children Assessed with Enhanced Depth Imaging Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7578. [Google Scholar] [CrossRef]
- Read, S.A.; Cox, R.A.; Alonso-Caneiro, D.; Hopkins, S.; Wood, J.M. Choroidal Thickness in Indigenous Australian Children. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- He, X.; Jin, P.; Zou, H.; Li, Q.; Jin, J.; Lu, L.; Zhao, H.; He, J.; Xu, X.; Wang, M.; et al. Choroidal thickness in healthy Chinese children aged 6 to 12: The Shanghai Children Eye Study. Retina 2017, 37, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; He, X.; Deng, J.; Lv, M.; Jin, J.; Sun, S.; Yao, C.; Zhu, J.; Zou, H.; Xu, X. Choroidal Thickness in 3001 Chinese Children Aged 6 to 19 Years Using Swept-Source OCT. Sci. Rep. 2017, 7, 45059. [Google Scholar] [CrossRef] [PubMed]
- Bidaut-Garnier, M.; Schwartz, C.; Puyraveau, M.; Montard, M.; Delbosc, B.; Saleh, M. Choroidal Thickness Measurement in Children Using Optical Coherence Tomography. Retina 2014, 34, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.K.; Deshpande, R.; Sachdeva, V.; Vidya, S.; Rao, P.S.; Panigati, A.; Mahat, B.; Pappuru, R.R.; Pehere, N.; Pathengay, A. Choroidal Thickness Profile in Healthy Indian Children. Indian J. Ophthalmol. 2015, 63, 474–477. [Google Scholar] [CrossRef]
- Akhtar, Z.; Rishi, P.; Srikanth, R.; Rishi, E.; Bhende, M.; Raman, R. Choroidal Thickness in Normal Indian Subjects Using Swept Source Optical Coherence Tomography. PLoS ONE 2018, 13, e0197457. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Mitamura, Y.; Katome, T.; Shinomiya, K.; Naito, T.; Nagasato, D.; Shimizu, Y.; Tabuchi, H.; Kiuchi, Y. Macular Choroidal Thickness and Volume in Healthy Pediatric Individuals Measured by Swept-Source Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7068–7074. [Google Scholar] [CrossRef]
- Ohsugi, E.; Mitamura, Y.; Shinomiya, K.; Niki, M.; Sano, H.; Nagasawa, T.; Shimizu, Y.; Nagasato, D.; Tabuchi, H. Changes in Choroidal Thickness in Healthy Pediatric Individuals: A Longitudinal Study. Int. J. Ophthalmol. 2018, 11, 1179–1184. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.E.C.; Del Mar, N.; Li, C. Neural Control of Choroidal Blood Flow. Prog. Retin. Eye Res. 2018, 64, 96–130. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A Review of Optical Coherence Tomography Angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, F.; Foo, L.L.; Wong, Q.Y.; Ting, D.; Hoang, Q.V.; Chong, R.; Ang, M.; Wong, C.W. Advances in OCT Imaging in Myopia and Pathologic Myopia. Diagnostics 2022, 12, 1418. [Google Scholar] [CrossRef]
- Yu, T.; Xie, X.; Wei, H.; Shen, H.; Wu, Q.; Zhang, X.; Ji, H.; Tian, Q.; Song, J.; Bi, H. Choroidal Changes in Lens-Induced Myopia in Guinea Pigs. Microvasc. Res. 2021, 138, 104213. [Google Scholar] [CrossRef] [PubMed]
- Junghans, B.M.; Crewther, S.G.; Liang, H.; Crewther, D.P. A Role for Choroidal Lymphatics during Recovery from Form Deprivation Myopia? Optom. Vis. Sci. 1999, 76, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.F.; Fitzgerald, M.E.; Norton, T.T.; Gamlin, P.D.; Hodos, W.; Reiner, A. Reduction in Choroidal Blood Flow Occurs in Chicks Wearing Goggles That Induce Eye Growth toward Myopia. Curr. Eye Res. 1993, 12, 219–227. [Google Scholar] [CrossRef]
- Fitzgerald, M.E.C.; Wildsoet, C.F.; Reiner, A. Temporal Relationship of Choroidal Blood Flow and Thickness Changes during Recovery from Form Deprivation Myopia in Chicks. Exp. Eye Res. 2002, 74, 561–570. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ikuno, Y. Choroidal Filling Delay in Choroidal Neovascularisation Due to Pathological Myopia. Br. J. Ophthalmol. 2010, 94, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Duan, A.; Chan, S.; Wang, X.; Wei, W. Vascular Flow Density in Pathological Myopia: An Optical Coherence Tomography Angiography Study. BMJ Open 2017, 7, e013571. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Nagaoka, T.; Takahashi, A.; Tanano, I.; Tani, T.; Ishibazawa, A.; Yoshida, A. Relationship between Choroidal Thickness and Choroidal Circulation in Healthy Young Subjects. Am. J. Ophthalmol. 2012, 153, 1129–1132.e1. [Google Scholar] [CrossRef]
- Milani, P.; Montesano, G.; Rossetti, L.; Bergamini, F.; Pece, A. Vessel Density, Retinal Thickness, and Choriocapillaris Vascular Flow in Myopic Eyes on OCT Angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1419–1427. [Google Scholar] [CrossRef]
- Hui, W.; Xiaofeng, H.; Hua, X.; Yihan, D.; Yong, T. Assessment of Choroidal Vascularity and Choriocapillaris Blood Perfusion in Chinese Preschool-Age Anisometropic Hyperopic Amblyopia Children. Front. Pediatr. 2022, 10, 1056888. [Google Scholar] [CrossRef]
- Kinoshita, T.; Mitamura, Y.; Shinomiya, K.; Egawa, M.; Iwata, A.; Fujihara, A.; Ogushi, Y.; Semba, K.; Akaiwa, K.; Uchino, E.; et al. Diurnal Variations in Luminal and Stromal Areas of Choroid in Normal Eyes. Br. J. Ophthalmol. 2017, 101, 360–364. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.S.; Kwon, H.J.; Koh, H.J.; Lee, S.C. Association between Choroidal Thickness and Ocular Perfusion Pressure in Young, Healthy Subjects: Enhanced Depth Imaging Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7710–7717. [Google Scholar] [CrossRef]
- Swiatczak, B.; Schaeffel, F.; Calzetti, G. Imposed Positive Defocus Changes Choroidal Blood Flow in Young Human Subjects. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.K.M.; Zhang, H.; Liu, Z.; Tse, D.Y.Y.; Zhou, Y.; Lam, C.S.Y.; To, C.H. Defocus Incorporated Multiple Segments (DIMS) Spectacle Lenses Increase the Choroidal Thickness: A Two-Year Randomized Clinical Trial. Eye Vis. 2023, 10, 39. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Q. Short-Term Effect of Orthokeratology Lens Wear on Choroidal Blood Flow in Children with Low and Moderate Myopia. Sci. Rep. 2022, 12, 17653. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, F.; Oueslati, T.; Delamarre, L.; Castanon, J.; Maurin, C.; Chiambaretta, F.; Baker, J.S.; Ugbolue, U.C.; Zak, M.; Lakbar, I.; et al. Myopia and Near Work: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 875. [Google Scholar] [CrossRef]
- Wallman, J.; Wildsoet, C.; Xu, A.; Gottlieb, M.D.; Nickla, D.L.; Marran, L.; Krebs, W.; Christensen, A.M. Moving the Retina: Choroidal Modulation of Refractive State. Vis. Res. 1995, 35, 37–50. [Google Scholar] [CrossRef]
- Chakraborty, R.; Read, S.A.; Collins, M.J. Monocular Myopic Defocus and Daily Changes in Axial Length and Choroidal Thickness of Human Eyes. Exp. Eye Res. 2012, 103, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Y.; Chen, L.; Dong, M.; Tang, W.; Chen, S.; Qu, J.; Zhou, X.; Zhi, Z. Near Work Induces Myopia in Guinea Pigs. Exp. Eye Res. 2022, 224, 109202. [Google Scholar] [CrossRef]
- Woodman, E.C.; Read, S.A.; Collins, M.J. Axial Length and Choroidal Thickness Changes Accompanying Prolonged Accommodation in Myopes and Emmetropes. Vis. Res. 2012, 72, 34–41. [Google Scholar] [CrossRef]
- Woodman, E.C.; Read, S.A.; Collins, M.J.; Hegarty, K.J.; Priddle, S.B.; Smith, J.M.; Perro, J.V. Axial Elongation Following Prolonged near Work in Myopes and Emmetropes. Br. J. Ophthalmol. 2010, 95, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Woodman-Pieterse, E.C.; Read, S.A.; Collins, M.J.; Alonso-Caneiro, D. Regional Changes in Choroidal Thickness Associated with Accommodation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6414. [Google Scholar] [CrossRef]
- Li, M.; Cheng, H.; Yuan, Y.; Wang, J.; Chen, Q.; Me, R.; Ke, B. Change in Choroidal Thickness and the Relationship with Accommodation Following Myopic Excimer Laser Surgery. Eye 2016, 30, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, S.; Zhang, G.; Chen, Y.; Lei, Y.; Xiang, J.; Xu, R.; Qu, J.; Zhou, X. Increased Choroidal Blood Perfusion Can Inhibit Form Deprivation Myopia in Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2020, 61, 25. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kurihara, T.; Miyauchi, M.; Ishida, A.; Jiang, X.; Ikeda, S.-I.; Torii, H.; Tsubota, K. Oral Crocetin Administration Suppressed Refractive Shift and Axial Elongation in a Murine Model of Lens-Induced Myopia. Sci. Rep. 2019, 9, 295. [Google Scholar] [CrossRef]
- Mori, K.; Torii, H.; Fujimoto, S.; Jiang, X.; Ikeda, S.-I.; Yotsukura, E.; Koh, S.; Kurihara, T.; Nishida, K.; Tsubota, K. The Effect of Dietary Supplementation of Crocetin for Myopia Control in Children: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 1179. [Google Scholar] [CrossRef] [PubMed]
- Giaccio, M. Crocetin from Saffron: An Active Component of an Ancient Spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.M.; Gainer, J.L. The Carotenoid Crocetin Enhances Pulmonary Oxygenation. J. Appl. Physiol. 1988, 65, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Seyde, W.C.; McKernan, D.J.; Laudeman, T.; Gainer, J.L.; Longnecker, D.E. Carotenoid Compound Crocetin Improves Cerebral Oxygenation in Hemorrhaged Rats. J. Cereb. Blood Flow Metab. 1986, 6, 703–707. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Chang, A.; Grigg, J.; McCluskey, P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2015, 56, 2767–2776. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Xiang, M.; He, S.-Y.; Qian, Z.-Y. Protein Kinase C Pathway Is Involved in the Inhibition by Crocetin of Vascular Smooth Muscle Cells Proliferation. Phytotherapy Res. 2010, 24, 1680–1686. [Google Scholar] [CrossRef]
- Lithander, J. Prevalence of Myopia in School Children in the Sultanate of Oman: A Nation-Wide Study of 6292 Randomly Selected Children. Acta Ophthalmol. Scand. 1999, 77, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Montes-Micó, R.; Ferrer-Blasco, T. Distribution of Refractive Errors in Spain. Doc. Ophthalmol. 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Norouzirad, R.; Hashemi, H.; Yekta, A.; Nirouzad, F.; Ostadimoghaddam, H.; Yazdani, N.; Dadbin, N.; Javaherforoushzadeh, A.; Khabazkhoob, M. The Prevalence of Refractive Errors in 6- to 15-Year-Old Schoolchildren in Dezful, Iran. J. Curr. Ophthalmol. 2015, 27, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Mori, K.; Ikeda, S.; Jeong, H.; Torii, H.; Negishi, K.; Kurihara, T.; Tsubota, K. Ginkgo Biloba Extracts Improve Choroidal Circulation Leading to Suppression of Myopia in Mice. Sci. Rep. 2023, 13, 3772. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, H.J.; Chung, W.S.; Kim, C.Y.; Seong, G.J. Short-Term Effects of Ginkgo Biloba Extract on Peripapillary Retinal Blood Flow in Normal Tension Glaucoma. Korean J. Ophthalmol. 2011, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Lee, D.; Jiang, X.; Negishi, K.; Tsubota, K.; Kurihara, T. Topical Application of Bunazosin Hydrochloride Suppresses Myopia Progression with an Increase in Choroidal Blood Perfusion. Investig. Ophthalmol. Vis. Sci. 2023, 64, 15. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ichikawa, M.; Oku, H.; Shimazawa, M.; Araie, M. Bunazosin, a Selective Alpha1-Adrenoceptor Antagonist, as an Anti-Glaucoma Drug: Effects on Ocular Circulation and Retinal Neuronal Damage. Cardiovasc. Drug Rev. 2005, 23, 43–56. [Google Scholar] [CrossRef]
- Jiang, X.; Pardue, M.T.; Mori, K.; Ikeda, S.-I.; Torii, H.; D’Souza, S.; Lang, R.A.; Kurihara, T.; Tsubota, K. Violet Light Suppresses Lens-Induced Myopia via Neuropsin (OPN5) in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2018840118. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chuang, M.-N.; Choi, J.; Chen, H.; Wu, G.; Ohno-Matsui, K.; Jonas, J.B.; Cheung, C.M.G. Update in Myopia and Treatment Strategy of Atropine Use in Myopia Control. Eye 2019, 33, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chia, A.; Lu, Q.S.; Tan, D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology 2016, 123, 391–399. [Google Scholar] [CrossRef]
- Chiang, S.T.-H.; Phillips, J.R. Effect of Atropine Eye Drops on Choroidal Thinning Induced by Hyperopic Retinal Defocus. J. Ophthalmol. 2018, 2018, 8528315. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Zhu, X.; Wallman, J. Effects of Muscarinic Agents on Chick Choroids in Intact Eyes and Eyecups: Evidence for a Muscarinic Mechanism in Choroidal Thinning. Ophthalmic Physiol. Opt. 2013, 33, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.-H.; Turnbull, P.R.K.; Phillips, J.R. Additive Effect of Atropine Eye Drops and Short-Term Retinal Defocus on Choroidal Thickness in Children with Myopia. Sci. Rep. 2020, 10, 18310. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, R.; Zhu, Y.; Zhou, J.; Cui, C. Effect of 0.01% Atropine Eye Drops on Choroidal Thickness in Myopic Children. J. Français D’ophtalmologie 2020, 43, 862–868. [Google Scholar] [CrossRef]
- Ye, L.; Shi, Y.; Yin, Y.; Li, S.; He, J.; Zhu, J.; Xu, X. Effects of Atropine Treatment on Choroidal Thickness in Myopic Children. Investig. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, X.; Xuan, Y.; Wang, M.; Zhou, X.; Qu, X. Short-Term Effects of Atropine 0.01% on the Structure and Vasculature of the Choroid and Retina in Myopic Chinese Children. Ophthalmol. Ther. 2022, 11, 833–856. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sinnott, L.T.; Mutti, D.O.; Mitchell, G.L.; Moeschberger, M.L.; Zadnik, K. Parental History of Myopia, Sports and Outdoor Activities, and Future Myopia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3524–3532. [Google Scholar] [CrossRef]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W.; Mitchell, P. Outdoor Activity Reduces the Prevalence of Myopia in Children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef]
- Dirani, M.; Tong, L.; Gazzard, G.; Zhang, X.; Chia, A.; Young, T.L.; Rose, K.A.; Mitchell, P.; Saw, S.M. Outdoor Activity and Myopia in Singapore Teenage Children. Br. J. Ophthalmol. 2009, 93, 997–1000. [Google Scholar] [CrossRef]
- Wu, P.-C.; Tsai, C.-L.; Wu, H.-L.; Yang, Y.-H.; Kuo, H.-K. Outdoor Activity during Class Recess Reduces Myopia Onset and Progression in School Children. Ophthalmology 2013, 120, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-C.; Chen, C.-T.; Lin, K.-K.; Sun, C.-C.; Kuo, C.-N.; Huang, H.-M.; Poon, Y.-C.; Yang, M.-L.; Chen, C.-Y.; Huang, J.-C.; et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology 2018, 125, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- French, A.N.; Morgan, I.G.; Mitchell, P.; Rose, K.A. Risk Factors for Incident Myopia in Australian Schoolchildren. Ophthalmology 2013, 120, 2100–2108. [Google Scholar] [CrossRef]
- Guggenheim, J.A.; Northstone, K.; McMahon, G.; Ness, A.R.; Deere, K.; Mattocks, C.; Pourcain, B.S.; Williams, C. Time Outdoors and Physical Activity as Predictors of Incident Myopia in Childhood: A Prospective Cohort Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Smith, 3rd; Earl, L.; Hung, L.-F.; Huang, J. Protective Effects of High Ambient Lighting on the Development of Form-Deprivation Myopia in Rhesus Monkeys. Investig. Ophthalmol. Vis. Sci. 2012, 53, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Belkin, M.; Yehezkel, O.; Solomon, A.S.; Polat, U. Dependency between Light Intensity and Refractive Development under Light–Dark Cycles. Exp. Eye Res. 2011, 92, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Feldkaemper, M.; Schaeffel, F. Bright Light Induces Choroidal Thickening in Chickens. Optom. Vis. Sci. 2013, 90, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Schmidt, T.; Podolsky, R.H.; Roberts, R. Melanopsin Phototransduction Contributes to Light-Evoked Choroidal Expansion and Rod L-Type Calcium Channel Function In Vivo. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5314–5319. [Google Scholar] [CrossRef]
- Read, S.A.; Pieterse, E.C.; Alonso-Caneiro, D.; Bormann, R.; Hong, S.; Lo, C.-H.; Richer, R.; Syed, A.; Tran, L. Daily Morning Light Therapy Is Associated with an Increase in Choroidal Thickness in Healthy Young Adults. Sci. Rep. 2018, 8, 8200. [Google Scholar] [CrossRef]
- Chakraborty, R.; Baranton, K.; Spiegel, D.; Lacan, P.; Guillon, M.; Barrau, C.; Villette, T. Effects of Mild- and Moderate-Intensity Illumination on Short-Term Axial Length and Choroidal Thickness Changes in Young Adults. Ophthalmic Physiol. Opt. 2022, 42, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Ostrin, L.A. The Outdoor Environment Affects Retinal and Choroidal Thickness. Ophthalmic Physiol. Opt. 2023, 43, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Ostrin, L.A. Effects of Narrowband Light on Choroidal Thickness and the Pupil. Investig. Ophthalmol. Vis. Sci. 2020, 61, 40. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhakal, R.; Verkicharla, P.K. Short-Term Exposure to Blue Light Shows an Inhibitory Effect on Axial Elongation in Human Eyes Independent of Defocus. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kurihara, T.; Jiang, X.; Kondo, S.; Ueno, Y.; Hayashi, Y.; Lee, D.; Ikeda, S.-I.; Mori, K.; Torii, H.; et al. Suppressive Effects of Violet Light Transmission on Myopia Progression in a Mouse Model of Lens-Induced Myopia. Exp. Eye Res. 2023, 228, 109414. [Google Scholar] [CrossRef] [PubMed]
- Torii, H.; Mori, K.; Okano, T.; Kondo, S.; Yang, H.-Y.; Yotsukura, E.; Hanyuda, A.; Ogawa, M.; Negishi, K.; Kurihara, T.; et al. Short-Term Exposure to Violet Light Emitted from Eyeglass Frames in Myopic Children: A Randomized Pilot Clinical Trial. J. Clin. Med. 2022, 11, 6000. [Google Scholar] [CrossRef] [PubMed]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.; Kondo, S.; Miyauchi, M.; et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine 2017, 15, 210–219. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, D.; Jiang, X.; Negishi, K.; Tsubota, K.; Kurihara, T. Opsin 5 Mediates Violet Light-Induced Early Growth Response-1 Expression in the Mouse Retina. Sci. Rep. 2023, 13, 17861. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Z.; Tan, X.; Kong, X.; Zhong, H.; Zhang, J.; Xiong, R.; Yuan, Y.; Zeng, J.; Morgan, I.G.; et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology 2022, 129, 509–519. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Wang, W.; Zhang, J.; Chen, Y.; Bulloch, G.; Yuan, Y.; Zhang, S.; Xuan, M.; et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology 2023, 130, 286–296. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Guo, J.; Peng, J.; Zhao, P. Retinal Damage After Repeated Low-Level Red-Light Laser Exposure. JAMA Ophthalmol. 2023, 141, 693–695. [Google Scholar] [CrossRef] [PubMed]

| Study Reference | Study Location | Number of Eyes | Age | Mean Axial Length | Subfoveal ChT |
|---|---|---|---|---|---|
| Read et al., 2013 [20] | Australia | 104 eyes | 10–15 years | 22.98 ± 0.82 mm | 337 ± 82 μm |
| Read et al., 2020 [21] | 250 eyes | 4–18 years | 23.13 ± 0.73 mm | 361 ± 74 µm | |
| He et al., 2017 [22] | China | 144 eyes | 6–12 years | 23.59 ± 1.09 mm | 302 ± 63 μm |
| Xiong et al., 2017 [23] | 3001 eyes | 6–19 years | 24.4 ± 1.31 mm | 245 ± 66 μm | |
| Bidaut-Garnier et al., 2014 [24] | France | 348 eyes | 3.5–14.9 years | 22.3 ± 1.05 mm | 341.96 ± 74.7 μm |
| Chabblani et al., 2015 [25] | India | 255 eyes | 5–18 years | 23.55 ± 0.74 mm | 312.1 ± 45.40 μm |
| Akhtar et al., 2018 [26] | 30 eyes | 12–18 years | - | 327 ± 68 μm | |
| Nagasawa et al., 2013 [27] | Japan | 100 eyes | 3–15 years | 23.13 ± 1.37 mm | 260 ± 57.2 μm |
| Ohsugi et al., 2018 [28] | 64 eyes | 3.6–5.8 years | 21.90 ± 0.67 mm | 301.8 ± 8.6 µm |
| Study Reference | Species | Intervention | Change in ChBP | Change in ChT | Refractive Status |
|---|---|---|---|---|---|
| Zhang et al., 2019 [9] | Guinea Pigs | LIM (−4 D Lens) FDM | Decreased Decreased | Decreased Decreased | Myopic Shift |
| Zhou et al., 2021 [10] | Guinea Pigs | Temporal ciliary artery transection. Phenylephrine (Vasoconstrictor) | Decreased Decreased | Decreased Decreased | Myopic Shift |
| Yu et al., 2021 [32] | Guinea Pigs | LIM (−6 D Lens) | Decreased | Decreased | Myopic Shift |
| Fu et al., 2022 [50] | Guinea Pigs | Near work | Decreased | Decreased | Myopic Shift |
| Jiang et al., 2021 [71] | Mice | Violet Light | Not reported | Increased | Myopia Inhibition |
| Jeong et al., 2023 [69] | Mice | Bunazosin hydrochloride | Increased | Increased | Myopia Inhibition |
| Zhou et al., 2020 [55] | Guinea Pigs | Prazosin (Vasodilator) | Increased | Increased | Myopia Inhibition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baksh, J.; Lee, D.; Mori, K.; Zhang, Y.; Torii, H.; Jeong, H.; Hou, J.; Negishi, K.; Tsubota, K.; Kurihara, T. Myopia Is an Ischemic Eye Condition: A Review from the Perspective of Choroidal Blood Flow. J. Clin. Med. 2024, 13, 2777. https://doi.org/10.3390/jcm13102777
Baksh J, Lee D, Mori K, Zhang Y, Torii H, Jeong H, Hou J, Negishi K, Tsubota K, Kurihara T. Myopia Is an Ischemic Eye Condition: A Review from the Perspective of Choroidal Blood Flow. Journal of Clinical Medicine. 2024; 13(10):2777. https://doi.org/10.3390/jcm13102777
Chicago/Turabian StyleBaksh, Jiaul, Deokho Lee, Kiwako Mori, Yan Zhang, Hidemasa Torii, Heonuk Jeong, Jing Hou, Kazuno Negishi, Kazuo Tsubota, and Toshihide Kurihara. 2024. "Myopia Is an Ischemic Eye Condition: A Review from the Perspective of Choroidal Blood Flow" Journal of Clinical Medicine 13, no. 10: 2777. https://doi.org/10.3390/jcm13102777









