Artificial Proteins Designed from G3LEA Contribute to Enhancement of Oxidation Tolerance in E. coli in a Chaperone-like Manner
Abstract
:1. Introduction
2. Results
2.1. Sequence Composition within the HD Domain
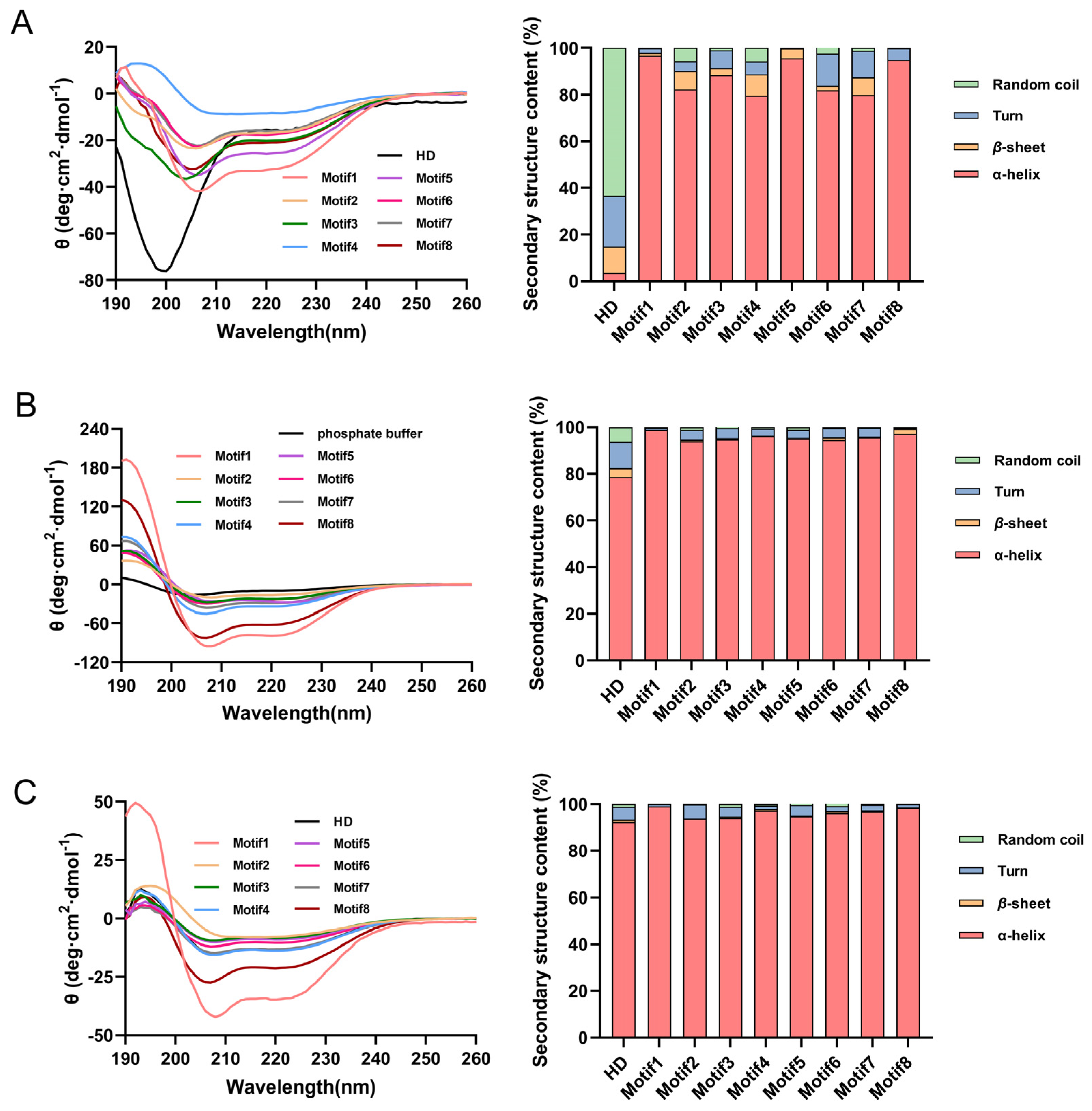
2.2. CD Analysis of Artificial Hydrophilic Proteins
2.3. Artificial Proteins Conferred E. coli Tolerance to Oxidation, Desiccation, Salinity and Freezing
2.4. Artificial Proteins Contributed to Higher Stabilization of the LDH/MDH Activity under Stress Conditions
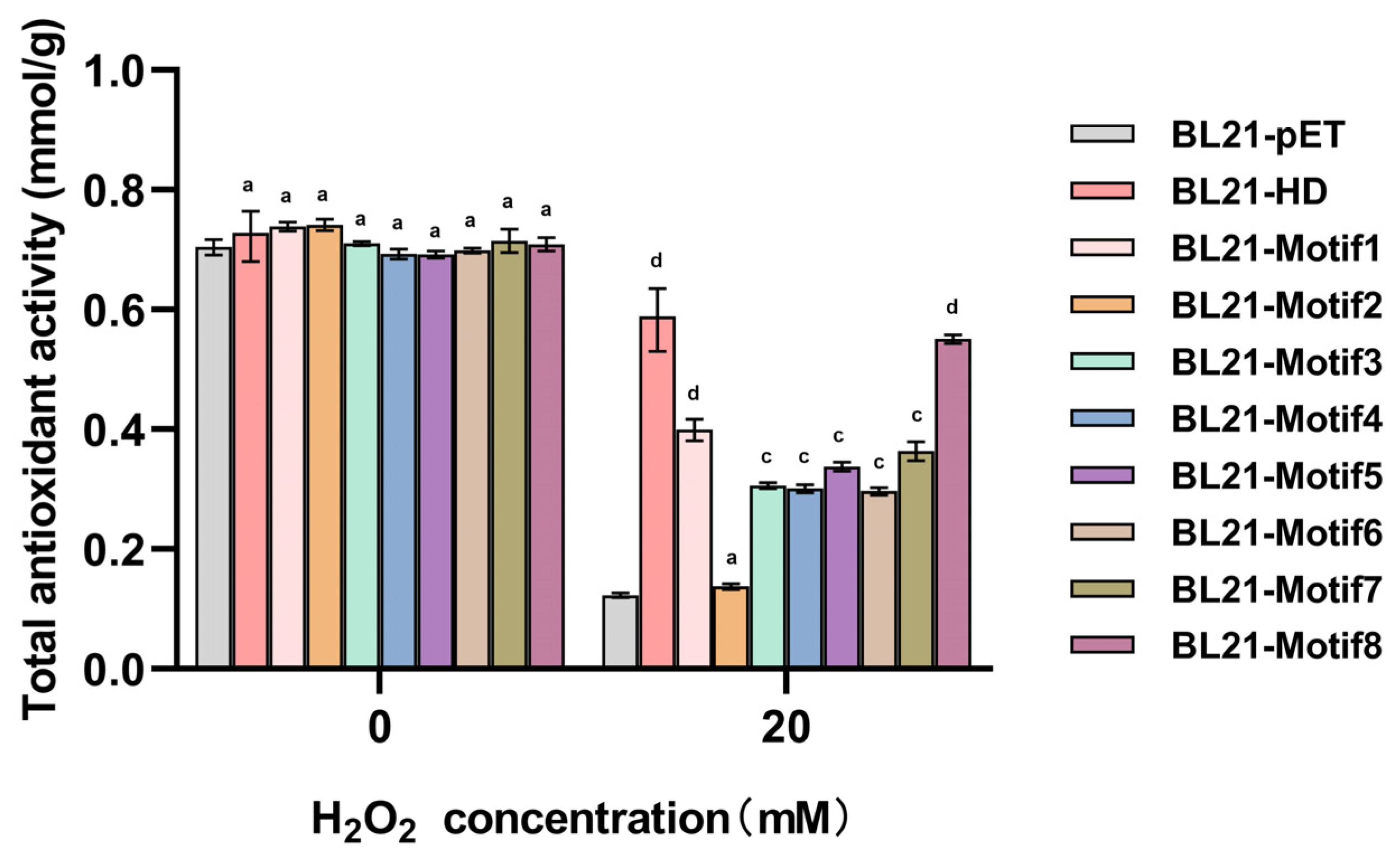
2.5. Artificial Proteins Increased the Total Antioxidant Capacity of E. coli Transformants
2.6. E. coli Containing Artificial Proteins Had Higher CAT Activity and Less MDA Production
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Culture Medium and Conditions
4.3. Protein Induction and Purification
4.4. Circular Dichroism (CD) Spectroscopic Analysis
4.5. Diverse Stresses Tolerance in E. coli
4.6. LDH/MDH Enzymatic Activity Assay In Vitro
4.7. Total Antioxidant Capacity, Catalase Activity and MDA Level Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, H.; He, X.; Zhao, Y.; Lu, S.; Guo, Z. Constitutive expression of a group 3 LEA protein from Medicago falcata (MfLEA3) increases cold and drought tolerance in transgenic tobacco. Plant Cell Rep. 2020, 39, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Furuki, T.; Sakurai, M. Physicochemical Aspects of the Biological Functions of Trehalose and Group 3 LEA Proteins as Desiccation Protectants. Surviv. Strateg. Extrem. Cold Desiccation Adapt. Mech. Appl. 2018, 1081, 271–286. [Google Scholar]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA Proteins During Water Stress: Not Just for Plants Anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, N.; Wang, X.; Meng, X.; Cui, X.; Chen, Z.; Ren, H.; Ma, J.; Liu, H. Late embryogenesis abundant (LEA) gene family in Salvia miltiorrhiza: Identification, expression analysis, and response to drought stress. Plant Signal. Behav. 2021, 16, 1891769. [Google Scholar] [CrossRef] [PubMed]
- Pouchkina-Stantcheva, N.N.; McGee, B.M.; Boschetti, C.; Tolleter, D.; Chakrabortee, S.; Popova, A.V.; Meersman, F.; Macherel, D.; Hincha, D.K.; Tunnacliffe, A. Functional divergence of former alleles in an ancient asexual invertebrate. Science 2007, 318, 268–271. [Google Scholar] [CrossRef]
- Shih, M.D.; Hsieh, T.Y.; Jian, W.T.; Wu, M.T.; Yang, S.J.; Hoekstra, F.A.; Hsing, Y.I.C. Functional studies of soybean (Glycine max L.) seed LEA proteins GmPM6, GmPM11, and GmPM30 by CD and FTIR spectroscopy. Plant Sci. 2012, 196, 152–159. [Google Scholar] [CrossRef]
- Shih, M.D.; Lin, S.D.; Hsieh, J.S.; Tsou, C.H.; Chow, T.Y.; Lin, T.P.; Hsing, Y.I.C. Gene cloning and characterization of a soybean (Glycine max L.) LEA protein, GmPM16. Plant Mol. Biol. 2004, 56, 689–703. [Google Scholar] [CrossRef]
- Wolkers, W.F.; McCready, S.; Brandt, W.F.; Lindsey, G.G.; Hoekstra, F.A. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. BBA-Protein Struct. Mol. Enzymol. 2001, 1544, 196–206. [Google Scholar] [CrossRef]
- Goyal, K.; Tisi, L.; Basran, A.; Browne, J.; Burnell, A.; Zurdo, J.; Tunnacliffe, A. Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J. Biol. Chem. 2003, 278, 12977–12984. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Han, J.; Jiang, S.; Geng, X.; Xue, D.; Chen, Y.; Zhang, C.; Zhou, Z.; Zhang, W.; et al. Functional assessment of hydrophilic domains of late embryogenesis abundant proteins from distant organisms. Microb. Biotechnol. 2019, 12, 752–762. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, M.; Tang, Y.; Han, J.; Gui, Y.; Ge, J.; Jiang, S.; Dai, Q.; Zhang, W.; Lin, M.; et al. Modular Assembly of Ordered Hydrophilic Proteins Improve Salinity Tolerance in Escherichia coli. Int. J. Mol. Sci. 2021, 22, 4482. [Google Scholar] [CrossRef] [PubMed]
- Dure, L., III. A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Dure, L. Occurrence of a repeating 11-mer amino acid sequence motif in diverse organisms. Protein Pept. Lett. 2001, 8, 115–122. [Google Scholar] [CrossRef]
- Furuki, T.; Watanabe, T.; Furuta, T.; Takano, K.; Shirakashi, R.; Sakurai, M. The Dry Preservation of Giant Vesicles Using a Group 3 LEA Protein Model Peptide and Its Molecular Mechanism. Bull. Chem. Soc. Jpn. 2016, 89, 1493–1499. [Google Scholar] [CrossRef]
- Furuki, T.; Shimizu, T.; Chakrabortee, S.; Yamakawa, K.; Hatanaka, R.; Takahashi, T.; Kikawada, T.; Okuda, T.; Mihara, H.; Tunnacliffe, A.; et al. Effects of Group 3 LEA protein model peptides on desiccation-induced protein aggregation. Biochim. Biophys. Acta 2012, 1824, 891–897. [Google Scholar] [CrossRef]
- Furuki, T.; Shimizu, T.; Kikawada, T.; Okuda, T.; Sakurai, M. Salt Effects on the Structural and Thermodynamic Properties of a Group 3 LEA Protein Model Peptide. Biochemistry 2011, 50, 7093–7103. [Google Scholar] [CrossRef]
- Shimizu, T.; Kanamori, Y.; Furuki, T.; Kikawada, T.; Okuda, T.; Takahashi, T.; Mihara, H.; Sakurai, M. Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry 2010, 49, 1093–1104. [Google Scholar] [CrossRef]
- Nishimoto, T.; Takahashi, Y.; Miyama, S.; Furuta, T.; Sakurai, M. Replica exchange molecular dynamics simulation study on the mechanism of desiccation-induced structuralization of an intrinsically disordered peptide as a model of LEA proteins. Biophys. Physicobiol. 2019, 16, 196–204. [Google Scholar] [CrossRef]
- Xue, R.; Liu, Y.; Zheng, Y.; Wu, Y.; Li, X.; Pei, F.; Ni, J. Three-dimensional structure and mimetic-membrane association of consensus 11-amino-acid motif from soybean LEA3 protein. Biopolymers 2012, 98, 59–66. [Google Scholar] [CrossRef]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Sudhof, T.C. A broken alpha -helix in folded alpha -Synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef]
- Gangabadage, C.S.; Najda, A.; Bogdan, D.; Wijmenga, S.S.; Tessari, M. Dependence of the size of a protein-SDS complex on detergent and Na+ concentrations. J. Phys. Chem. B 2008, 112, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Xu, S.; Sheftic, S.R.; Alexandrescu, A.T. Dynamic alpha-helix structure of micelle-bound human amylin. J. Biol. Chem. 2009, 284, 11982–11991. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, T.S.; Bax, A. Comparison of structure and dynamics of micelle-bound human alpha-synuclein and Parkinson disease variants. J. Biol. Chem. 2005, 280, 43179–43187. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and dynamics of micelle-bound human alpha-synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, N.; Fu, M.; Qin, J.; Huang, X. Characterization of OsLEA1a and its inhibitory effect on the resistance of E. coli to diverse abiotic stresses. Int. J. Biol. Macromol. 2016, 91, 1010–1017. [Google Scholar] [CrossRef]
- Janis, B.; Belott, C.; Brockman, T.; Menze, M.A. Functional and Conformational Plasticity of an Animal Group 1 LEA Protein. Biomolecules 2022, 12, 425. [Google Scholar] [CrossRef]
- Xiang, D.J.; Man, L.L.; Zhang, C.L.; Peng, L.; Li, Z.G.; Zheng, G.C. A new Em-like protein from Lactuca sativa, LsEm1, enhances drought and salt stress tolerance in Escherichia coli and rice. Protoplasma 2018, 255, 1089–1106. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Levine, R.L.; Berlett, B.S.; Moskovitz, J.; Mosoni, L.; Stadtman, E.R. Methionine residues may protect proteins from critical oxidative damage. Mech. Ageing Dev. 1999, 107, 323–332. [Google Scholar] [CrossRef]
- Nantapong, N.; Murata, R.; Trakulnaleamsai, S.; Kataoka, N.; Yakushi, T.; Matsushita, K. The effect of reactive oxygen species (ROS) and ROS-scavenging enzymes, superoxide dismutase and catalase, on the thermotolerant ability of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2019, 103, 5355–5366. [Google Scholar] [CrossRef]
- Messner, K.R.; Imlay, J.A. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 2002, 277, 42563–42571. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kang, K.; Gan, L.; Ning, S.; Xiong, J.; Song, S.; Xi, L.; Lai, S.; Yin, Y.; Gu, J.; et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019, 17, 2123–2142. [Google Scholar] [CrossRef] [PubMed]
- Shiraku, M.L.; Magwanga, R.O.; Zhang, Y.; Hou, Y.; Kirungu, J.N.; Mehari, T.G.; Xu, Y.; Wang, Y.; Wang, K.; Cai, X.; et al. Late embryogenesis abundant gene LEA3 (Gh_A08G0694) enhances drought and salt stress tolerance in cotton. Int. J. Biol. Macromol. 2022, 207, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Graether, S.P. The in vitro structure and functions of the disordered late embryogenesis abundant three proteins. Protein Sci. 2021, 30, 678–692. [Google Scholar] [CrossRef]
- Rodriguez-Salazar, J.; Moreno, S.; Espin, G. LEA proteins are involved in cyst desiccation resistance and other abiotic stresses in Azotobacter vinelandii. Cell Stress Chaperones 2017, 22, 397–408. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Zhang, Y.; Wang, W.; Li, R. Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses. Curr. Microbiol. 2010, 60, 373–378. [Google Scholar] [CrossRef]
- Mowla, S.B.; Cuypers, A.; Driscoll, S.P.; Kiddle, G.; Thomson, J.; Foyer, C.H.; Theodoulou, F.L. Yeast complementation reveals a role for an Arabidopsis thaliana late embryogenesis abundant (LEA)-like protein in oxidative stress tolerance. Plant J. 2006, 48, 743–756. [Google Scholar] [CrossRef]
- Kikawada, T.; Nakahara, Y.; Kanamori, Y.; Iwata, K.; Watanabe, M.; McGee, B.; Tunnacliffe, A.; Okuda, T. Dehydration-induced expression of LEA proteins in an anhydrobiotic chironomid. Biochem. Biophys. Res. Commun. 2006, 348, 56–61. [Google Scholar] [CrossRef]
- Pathak, N.; Ikeno, S. In vivo expression of a short peptide designed from late embryogenesis abundant protein for enhancing abiotic stress tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2017, 492, 386–390. [Google Scholar] [CrossRef]
- Furuki, T.; Sakurai, M. Group 3 LEA protein model peptides protect enzymes against desiccation stress. Biochim. Biophys. Acta 2016, 1864, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Cheng, H.; Sun, N.; Liu, S.; Li, S.; Wang, Y.; Zheng, Y.; Uversky, V.N. The effect of phosphorylation on the salt-tolerance-related functions of the soybean protein PM18, a member of the group-3 LEA protein family. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Furuki, T.; Sakurai, M. Group 3 LEA protein model peptides protect liposomes during desiccation. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 2757–2766. [Google Scholar] [CrossRef]
- Besenius, P.; Portale, G.; Bomans, P.H.; Janssen, H.M.; Palmans, A.R.; Meijer, E.W. Controlling the growth and shape of chiral supramolecular polymers in water. Proc. Natl. Acad. Sci. USA 2010, 107, 17888–17893. [Google Scholar] [CrossRef]
- Appel, R.; Fuchs, J.; Tyrrell, S.M.; Korevaar, P.A.; Stuart, M.C.; Voets, I.K.; Schonhoff, M.; Besenius, P. Steric Constraints Induced Frustrated Growth of Supramolecular Nanorods in Water. Chemistry 2015, 21, 19257–19264. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yan, Q.; Chen, Y.; McDonald, J.M.; Song, Y. Trifluoperazine regulation of calmodulin binding to Fas: A computational study. Proteins 2011, 79, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Meersman, F.; Kaminski Schierle, G.S.; Bertoncini, C.W.; McGee, B.; Kaminski, C.F.; Tunnacliffe, A. Catalytic and chaperone-like functions in an intrinsically disordered protein associated with desiccation tolerance. Proc. Natl. Acad. Sci. USA 2010, 107, 16084–16089. [Google Scholar] [CrossRef]
- Dai, J.L.; Gao, K.X.; Yao, T.; Lu, H.Z.; Zhou, C.L.; Guo, M.; Dai, S.; Wang, L.Y.; Xu, H.; Tian, B.; et al. Late embryogenesis abundant group3 protein (DrLEA3) is involved in antioxidation in the extremophilic bacterium Deinococcus radiodurans. Microbiol. Res. 2020, 240, 126559. [Google Scholar] [CrossRef] [PubMed]
- Knox-Brown, P.; Rindfleisch, T.; Günther, A.; Balow, K.; Bremer, A.; Walther, D.; Miettinen, M.S.; Hincha, D.K.; Thalhammer, A. Similar Yet Different–Structural and Functional Diversity among Arabidopsis thaliana LEA_4 Proteins. Int. J. Mol. Sci. 2020, 21, 2794. [Google Scholar] [CrossRef]
- Navarro-Retamal, C.; Bremer, A.; Alzate-Morales, J.; Caballero, J.; Hincha, D.K.; Gonzalez, W.; Thalhammer, A. Molecular dynamics simulations and CD spectroscopy reveal hydration-induced unfolding of the intrinsically disordered LEA proteins COR15A and COR15B from Arabidopsis thaliana. Phys. Chem. Chem. Phys. 2016, 18, 25806–25816. [Google Scholar] [CrossRef]
- Ponnuswamy, P.K.; Gromiha, M.M. On the Conformational Stability of Folded Proteins. J. Theor. Biol. 1994, 166, 63–74. [Google Scholar] [CrossRef]
- Gromiha, M.M.; Selvaraj, S. Inter-residue interactions in protein folding and stability. Prog. Biophys. Mol. Biol. 2004, 86, 235–277. [Google Scholar] [CrossRef] [PubMed]
- Gromiha, M.M.; Selvaraj, S. Influence of medium and long range interactions in different structural classes of globular proteins. J. Biol. Phys. 1997, 23, 151–162. [Google Scholar] [CrossRef]
- Barlow, D.J.; Thornton, J.M. Ion-pairs in proteins. J. Mol. Biol. 1983, 168, 867–885. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.W. Structural and genetic analysis of protein stability. Annu. Rev. Biochem. 1993, 62, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Gromiha, M.M. A simple method for predicting transmembrane alpha helices with better accuracy. Protein Eng. 1999, 12, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shih, M.D.; Huang, L.T.; Wei, F.J.; Wu, M.T.; Hoekstra, F.A.; Hsing, Y.I. OsLEA1a, a new Em-like protein of cereal plants. Plant Cell Physiol. 2010, 51, 2132–2144. [Google Scholar] [CrossRef]
- Jiang, S.J.; Wang, J.; Liu, X.L.; Liu, Y.Y.; Guo, C.; Zhang, L.W.; Han, J.H.; Wu, X.L.; Xue, D.; Gomaa, A.E.; et al. DrwH, a novel WHy domain-containing hydrophobic LEA5C protein from Deinococcus radiodurans, protects enzymatic activity under oxidative stress. Sci. Rep. 2017, 7, 9281. [Google Scholar] [CrossRef]
- Brochier-Armanet, C.; Madern, D. Phylogenetics and biochemistry elucidate the evolutionary link between L-malate and L-lactate dehydrogenases and disclose an intermediate group of sequences with mix functional properties. Biochimie 2021, 191, 140–153. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Hu, X.; Bian, X.; Nian, S. Gastrodin prevents homocysteine-induced human umbilical vein endothelial cells injury via PI3K/Akt/eNOS and Nrf2/ARE pathway. J. Cell Mol. Med. 2021, 25, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, T.T.; Li, T.T.; Tian, Y.Y.; Wang, L.F.; Liu, W.C. Jasmonic acid promotes leaf senescence through MYC2-mediated repression of CATALASE2 expression in Arabidopsis. Plant Sci. 2020, 299, 110604. [Google Scholar] [CrossRef] [PubMed]
- Niu, A.; Bian, W.P.; Feng, S.L.; Pu, S.Y.; Wei, X.Y.; Yang, Y.F.; Song, L.Y.; Pei, D.S. Role of manganese superoxide dismutase (Mn-SOD) against Cr(III)-induced toxicity in bacteria. J. Hazard. Mater. 2021, 403, 123604. [Google Scholar] [CrossRef] [PubMed]



| Name | 0 mM H2O2 | 20 mM H2O2 |
|---|---|---|
| BL21–pET | 5.3039 | 3.0684 |
| BL21–HD | 5.2450 | 4.6380 |
| BL21–Motif1 | 5.2406 | 4.3771 |
| BL21–Motif2 | 5.3995 | 3.7208 |
| BL21–Motif3 | 5.5459 | 3.5968 |
| BL21–Motif4 | 5.4094 | 3.4982 |
| BL21–Motif5 | 5.2206 | 3.5353 |
| BL21–Motif6 | 5.3817 | 3.4173 |
| BL21–Motif7 | 5.1463 | 3.8108 |
| BL21–Motif8 | 5.5093 | 4.2346 |
| Name | 0 mM H2O2 | 20 mM H2O2 |
|---|---|---|
| BL21–pET | 53.5783 | 146.0222 |
| BL21–HD | 51.5800 | 60.2700 |
| BL21–Motif1 | 55.6324 | 66.2613 |
| BL21–Motif2 | 53.8313 | 116.2587 |
| BL21–Motif3 | 52.9995 | 87.9578 |
| BL21–Motif4 | 53.2835 | 82.7497 |
| BL21–Motif5 | 55.7432 | 85.7624 |
| BL21–Motif6 | 51.1800 | 88.9068 |
| BL21–Motif7 | 52.6298 | 73.3786 |
| BL21–Motif8 | 50.9233 | 63.0585 |
| Strains/Plasmids | Relevant Genotype or Description | Source |
|---|---|---|
| Strains | ||
| BL21(DE3) | An E. coli strain with T7 RNA polymerase and without Lon protease | Vazyme biotech co. |
| BL21–pET | The strain containing plasmid pET28a | This study |
| BL21–HD | The strain containing plasmid 28a–HD | This study |
| BL21–Motif1 | The strain containing plasmid 28a–Motif1 | This study |
| BL21–Motif2 | The strain containing plasmid 28a–Motif2 | This study |
| BL21–Motif3 | The strain containing plasmid 28a–Motif3 | This study |
| BL21–Motif4 | The strain containing plasmid 28a–Motif4 | This study |
| BL21–Motif5 | The strain containing plasmid 28a–Motif5 | This study |
| BL21–Motif6 | The strain containing plasmid 28a–Motif6 | This study |
| BL21–Motif7 | The strain containing plasmid 28a–Motif7 | This study |
| BL21–Motif8 | The strain containing plasmid 28a–Motif8 | This study |
| Plasmids | ||
| pET28a | carrying N/C–terminal His tag | Laboratory stock |
| 28a–HD | pET28a–derived plasmid carrying the HD gene | This study |
| 28a–Motif1 | pET28a–derived plasmid carrying the Motif1 gene | This study |
| 28a–Motif2 | pET28a–derived plasmid carrying the Motif2 gene | This study |
| 28a–Motif3 | pET28a–derived plasmid carrying the Motif3 gene | This study |
| 28a–Motif4 | pET28a–derived plasmid carrying the Motif4 gene | This study |
| 28a–Motif5 | pET28a–derived plasmid carrying the Motif5 gene | This study |
| 28a–Motif6 | pET28a–derived plasmid carrying the Motif6 gene | This study |
| 28a–Motif7 | pET28a–derived plasmid carrying the Motif7 gene | This study |
| 28a–Motif8 | pET28a–derived plasmid carrying the Motif8 gene | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Jiang, S.; Zhou, Z.; Lin, M.; Wang, J. Artificial Proteins Designed from G3LEA Contribute to Enhancement of Oxidation Tolerance in E. coli in a Chaperone-like Manner. Antioxidants 2023, 12, 1147. https://doi.org/10.3390/antiox12061147
Han J, Jiang S, Zhou Z, Lin M, Wang J. Artificial Proteins Designed from G3LEA Contribute to Enhancement of Oxidation Tolerance in E. coli in a Chaperone-like Manner. Antioxidants. 2023; 12(6):1147. https://doi.org/10.3390/antiox12061147
Chicago/Turabian StyleHan, Jiahui, Shijie Jiang, Zhengfu Zhou, Min Lin, and Jin Wang. 2023. "Artificial Proteins Designed from G3LEA Contribute to Enhancement of Oxidation Tolerance in E. coli in a Chaperone-like Manner" Antioxidants 12, no. 6: 1147. https://doi.org/10.3390/antiox12061147




