Comparison between OCl−-Injection and In Situ Electrochlorination in the Formation of Chlorate and Perchlorate in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analysis Methods
3. Results and Discussion
3.1. Total Residual Oxidant in Direct OCl−-Injection and Electrolysis
3.1.1. Direct OCl−-Injection
3.1.2. Effects of Current Density during Electrolysis
3.2. Formation of ClO3− and ClO4− in Electrolysis and Direct OCl−-Injection
3.2.1. Direct OCl−-Injection
3.2.2. Electrochlorination
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pan, S.Y.; Snyder, S.W.; Packman, A.I.; Lin, Y.J.; Chiang, P.C. Cooling water use in thermoelectric power generation and its associated challenges for addressing water-energy nexus. Water-Energy Nexus 2018, 1, 26–41. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). World Energy Outlook; OECD/IEA: Paris, France, 2016; p. 13. [Google Scholar]
- Pugh, S.J.; Hewitt, G.F.; Müller-Steinhagen, H. Fouling during the use of seawater as coolant—The development of a ‘User Guide’. In Proceedings of the Engineering Conferences International, Heat Exchanger Fouling and Cleaning: Fundamentals and Applications, Santa Fe, NM, USA, 18–22 May 2003. [Google Scholar]
- Satpathy, K.K.; Mohanty, A.K.; Sahu, G.; Biswas, S.; Prasad, M.V.R.; Slvanayagam, M. Biofouling and Its Control in Seawater Cooled Power Plant Cooling Water System—A Review. In Nuclear Power; Tsvetkov, P., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Nanny, M.A. Handbook of chlorination and alternative disinfectants. Fourth edition by George Clifford White. John Wiley and Sons, Inc.: New York, Chichester, Weinheim, Brisbane, Singapore, and Toronto. 1999. xxi + 1569 pp. $195.00. ISBN 0-471-29297-9. J. Am. Chem. Soc. 1999, 121, 8678–8679. [Google Scholar] [CrossRef]
- Masilamoni, G.; Jesudoss, K.S.; Nandakumar, K.; Satapathy, K.K.; Azariah, J.; Nair, K.V.K. Lethal and sub-lethal effects of chlorination on green mussel Perna viridis in the context of biofouling control in a power plant cooling water system. Mar. Environ. Res. 2002, 53, 65–76. [Google Scholar] [CrossRef]
- Russell, L.B. Modelling for cost-effectiveness analysis. Stat. Med. 1999, 18, 3235–3244. [Google Scholar] [CrossRef]
- White, G.C. Handbook of Chlorination and Alternative Disinfectants; John Wiley and Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Asokan, K.; Subramanian, K. Design of a tank electrolyser for in-situ generation of NaClO. In Proceedings of the World Congress on Engineering and Computer Science 2009, San Francisco, CA, USA, 20–22 October 2009. [Google Scholar]
- Berl, E. A new cathodic process for the production of H2O2. J. Electrochem. Soc. 1939, 76, 359–369. [Google Scholar] [CrossRef]
- Viswanathan, K.; Tilak, B.V. Chemical, electrochemical, and technological aspects of sodium chlorate manufacture. J. Electrochem. Soc. 1984, 131, 1551–1559. [Google Scholar] [CrossRef]
- Munichandraiah, N.; Sathyanarayana, S. Kinetics and mechanism of anodic oxidation of chlorate ion to perchlorate ion on lead dioxide electrodes. J. Appl. Electrochem. 1987, 17, 33–48. [Google Scholar] [CrossRef]
- Tasaka, A.; Tojo, T. Anodic oxidation mechanism of hypochlorite ion on platinum electrode in alkaline solution. J. Electrochem. Soc. 1985, 132, 1855–1859. [Google Scholar] [CrossRef]
- Allonier, A.-S.; Khalanski, M.; Camel, V.; Bermond, A. Characterization of chlorination by-products in cooling effluents of coastal nuclear power stations. Mar. Pollut. Bull. 1999, 31, 1232–1241. [Google Scholar] [CrossRef]
- Poornima, E.H.; Rajadurai, M.; Rao, T.S.; Anupkumar, B.; Rajamohan, R.; Narasimhan, S.V.; Rao, V.N.R.; Venugopalan, V.P. Impact of thermal discharge from a tropical coastal power plant on phytoplankton. J. Therm. Biol. 2005, 30, 307–316. [Google Scholar] [CrossRef]
- Nebot, E.; Casanueva, J.F.; Casanueva, T.; Fernández-Bastón, M.M.; Sales, D. In situ experimental study for the optimization of chlorine dosage in seawater cooling systems. Appl. Therm. Eng. 2006, 26, 1893–1900. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Kohil, A.; Frenken, K. Cooling Water for Energy Generation and Its Impact on National-Level Water Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Goleman, W.L.; Carr, J.A.; Anderson, T.A. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environ. Toxicol. Chem. 2002, 21, 590–597. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Chlorite and Chlorate in Drinking-Water; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- United States Environmental Protection Agency (EPA). Chlorine, Total Residual (Spectrophotometric, DPD); EPA-NERL: 330.5; EPA: Cincinnati, OH, USA, 1978.
- Beggs, K.M.H.; Summers, R.S. Character and chlorine reactivity of dissolved organic matter from a mountain pine beetle impacted watershed. Environ. Sci. Technol. 2011, 45, 5717–5724. [Google Scholar] [CrossRef] [PubMed]
- Boccelli, D.L.; Tryby, M.E.; Uber, J.G.; Summers, R.S. A reactive species model for chlorine decay and THM formation under rechlorination conditions. Water Res. 2003, 37, 2654–2666. [Google Scholar] [CrossRef]
- Abarnou, A.; Miossec, L. Chlorinated waters discharged to the marine environment chemistry and environmental impact. An overview. Sci. Total Environ. 1992, 126, 173–197. [Google Scholar] [CrossRef]
- Zeng, J.; Jiang, Z.; Chen, Q.; Zheng, P.; Huang, Y. The decay kinetic of residual chlorine in cooling seawater simulation experiments. Acta Oceanol. Sin. 2009, 28, 54–59. [Google Scholar]
- Venkatnarayanan, S.; Murthy, P.S.; Kirubagaran, R.; Venugopalan, V.P. Effect of chlorination on barnacle larval stages: Implications for biofouling control and environmental impact. Int. Biodeterior. Biodegrad. 2016, 109, 141–149. [Google Scholar] [CrossRef]
- Saidan, M.; Rawajfeh, K.; Nasrallah, S.; Meric, S.; Mashal, A. Evaluation of factors affecting bulk chlorine decay kinetics for Zai water supply system in Jordan: Case Study. Environ. Prot. Eng. 2017, 43, 223–231. [Google Scholar]
- Abdel-Wahab, A.; Khodary, A.; Bensalah, N. Formation of trihalomethanes during seawater chlorination. J. Environ. Prot. 2010, 1, 456–465. [Google Scholar] [CrossRef]
- Yu, H.-W.; Oh, S.-G.; Kim, I.S.; Pepper, I.; Snyder, S.; Jang, A. Formation and speciation of haloacetic acids in seawater desalination using chlorine dioxide as disinfectant. J. Ind. Eng. Chem. 2015, 26, 193–201. [Google Scholar] [CrossRef]
- Brookman, R.M.; Lamsal, R.; Gagnon, G.A. Comparing the formation of bromate and bromoform due to ozonation and UV-TiO2 oxidation in seawater. J. Adv. Oxid. Technol. 2011, 14, 23–30. [Google Scholar] [CrossRef]
- Agus, E.; Sedlak, D.L. Formation and fate of chlorination by-products in reverse osmosis desalination systems. Water Res. 2010, 44, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, W.; Lee, W. Formation of disinfection byproducts upon chlorine dioxide preoxidation followed by chlorination or chloramination of natural organic matter. Chemosphere 2013, 91, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Caroli, S.; Braun, R.D. Tables of standard electrode potentials. J. Electrochem. Soc. 1978, 125, 261C. [Google Scholar] [CrossRef]
- Czarnetzki, L.R.; Janssen, L.J.J. Formation of hypochlorite, chlorate and oxygen during NaCl electrolysis from alkaline solutions at an RuO2/TiO2 anode. J. Appl. Electrochem. 1992, 22, 315–324. [Google Scholar] [CrossRef]
- Jung, Y.J.; Baek, K.W.; Oh, B.S.; Kang, J.-W. An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies. Water Res. 2010, 44, 5345–5355. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, R.T.P.; Santos, C.M.P.; Silva, G.P.; Ferreira, R.J.R.; Oliveira, A.P.; Côrtes, C.E.S.; Faria, R.B. Kinetics and mechanism of chlorate-chloride reaction. J. Braz. Chem. Soc. 2012, 23, 1543–1550. [Google Scholar] [CrossRef]
- Jung, Y.; Hong, E.; Yoon, Y.; Kwon, M.; Kang, J.-W. Formation of bromate and chlorate during ozonation and electrolysis in seawater for ballast water treatment. Ozone Sci. Eng. 2014, 36, 515–525. [Google Scholar] [CrossRef]
- Oh, B.S.; Oh, S.G.; Hwang, Y.Y.; Yu, H.W.; Kang, J.-W.; Kim, I.S. Formation of hazardous inorganic by-products during electrolysis of seawater as a disinfection process for desalination. Sci. Total Environ. 2010, 408, 5958–5965. [Google Scholar] [CrossRef]

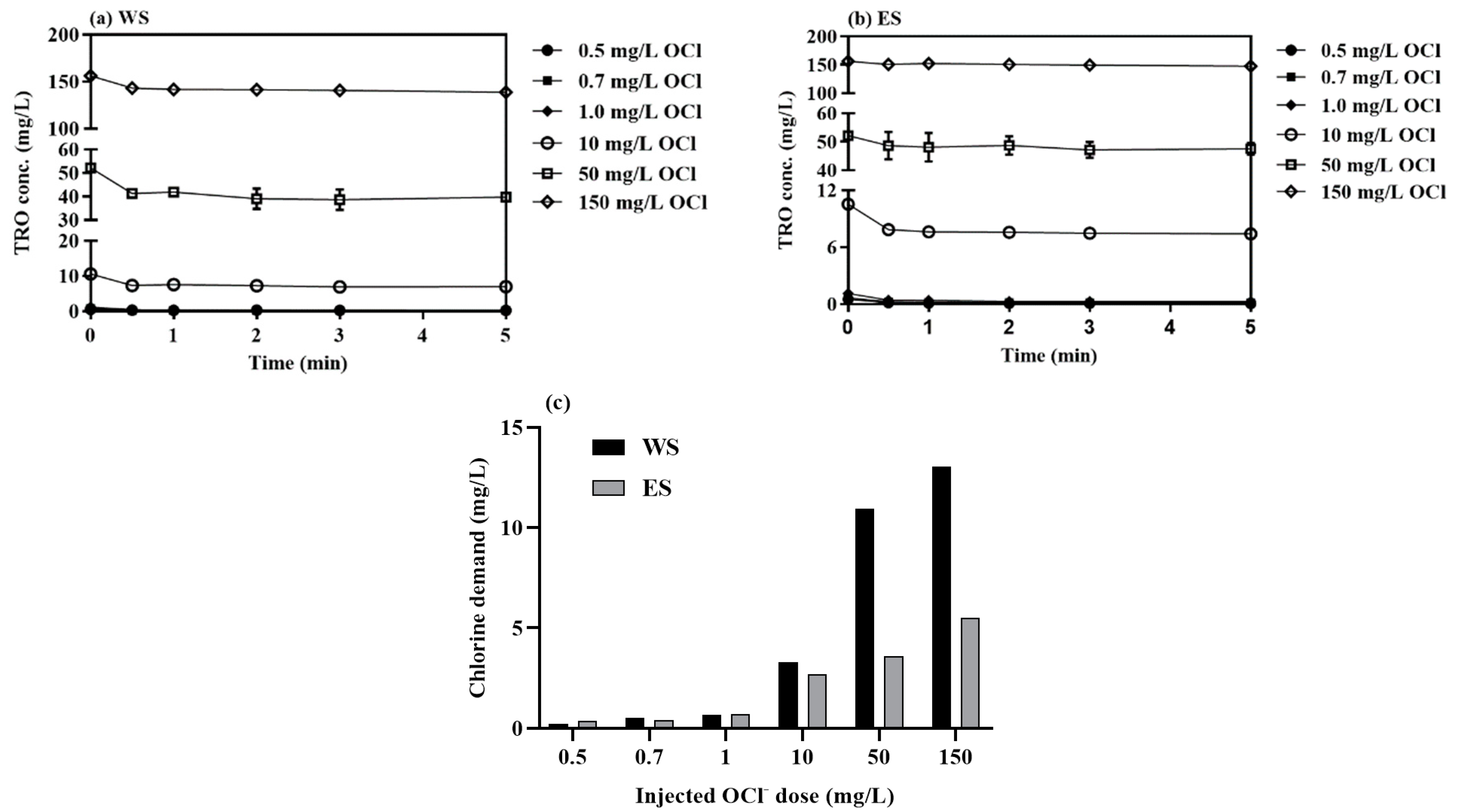
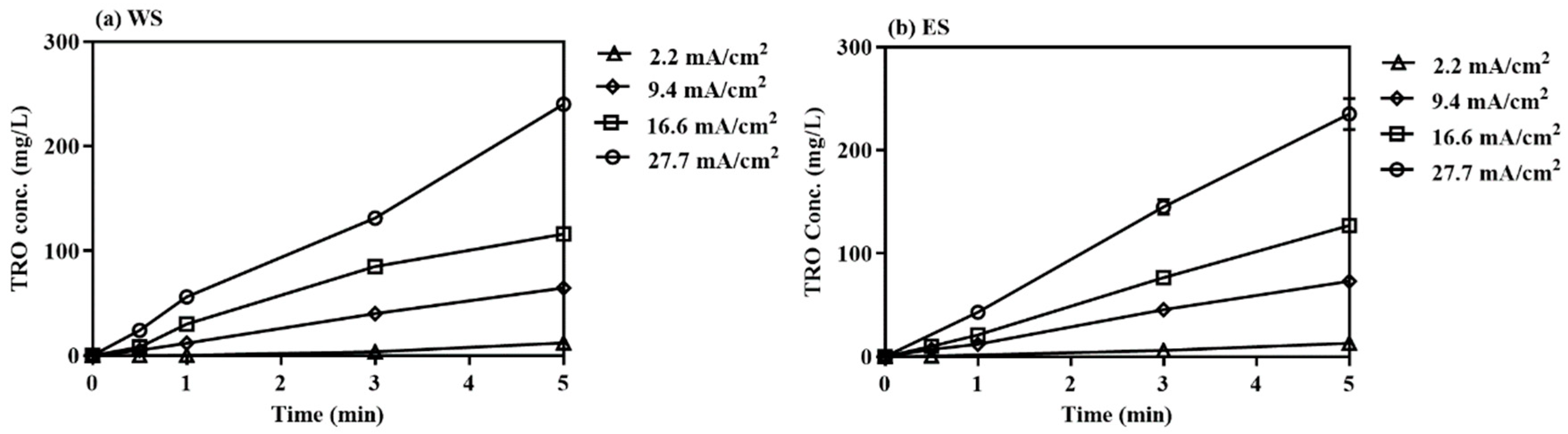


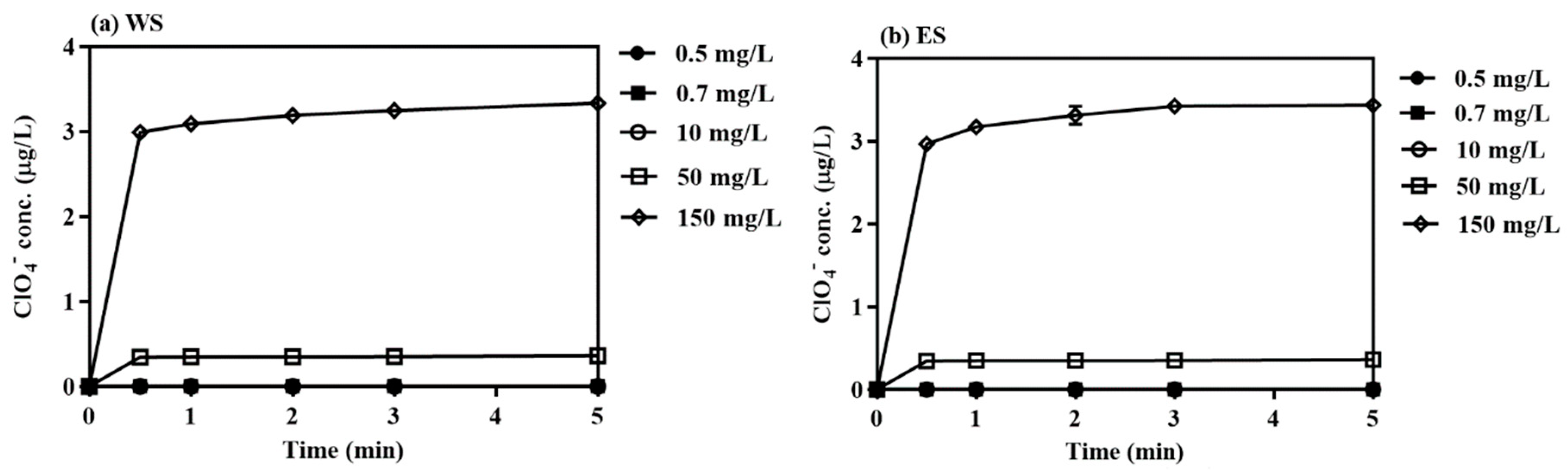
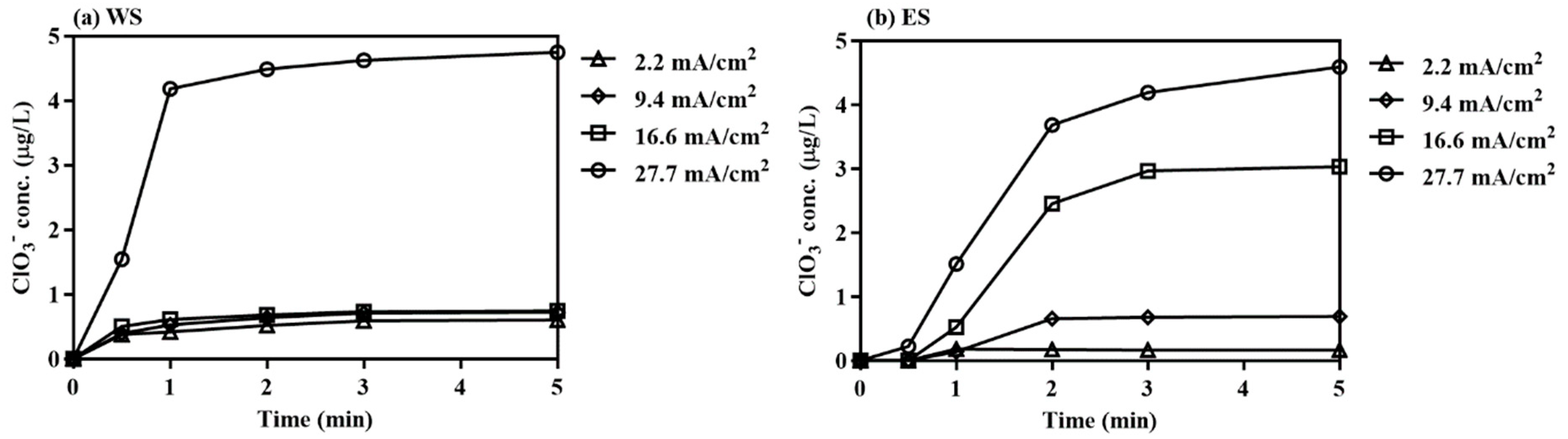
| Parameter | West Seawater (WS) | East Seawater (ES) |
|---|---|---|
| pH | 8.3 | 8.0 |
| Conductivity(ms/cm) | 44.37 | 44.67 |
| Salinity (PSU) | 28.66 | 28.88 |
| TOC (mg/L) | 2.2 | 1.9 |
| TN (mg/L) | 1.3 | 0.5 |
| TP (mg/L) | 0.1 | 0.1 |
| Equation | Equivalent | Equations No. |
|---|---|---|
| 6 OCl− + 3 H2O → 2 ClO3− + 4 Cl− + 6 H+ + 3/2 O2 + 6 e− | 0.46 V | (8) |
| 6 HOCl + 3 H2O → 2 ClO3− + 4 Cl− + 12 H+ + 3/2 O2 + 6 e− | 0.46 V | (9) |
| Cl− + 3 H2O → ClO3− + 6 H+ + 6 e− | −1.45 V | (10) |
| Cl− + 2 OH− → OCl− + H2O + 2 e− | −0.94 V | (11) |
| Cl− + 4 OH− → ClO2− + H2O + 4 e− | −0.76 V | (12) |
| OCl− + 2 OH− → ClO2− + H2O + 2 e− | −0.59 V | (13) |
| ClO2− + 2 OH− → ClO3− + H2O + 2 e− | −0.35 V | (14) |
| ClO2− + OCl− → Cl− + ClO3− | (15) | |
| ClO2− + HOCl → ClO3− + H+ + Cl− | (16) | |
| HClO2 + H2O → ClO3− + 3 H+ +2 e− | (17) | |
| ClO3− + H2O → ClO4− + 2 H+ + 2e− | 0.95 V | (18) |
| 2 ClO3− + 2 Cl− + 4 H+ → Cl2 + 2 ClO2 + 2 H2O | (19) | |
| ClO3− + Cl− + 2 H+ → HClO2 + HOCl | (20) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Ahn, Y.; Hong, M.; Kim, G.-H.; Shabnam, N.; Jeon, B.; Sang, B.-I.; Kim, H. Comparison between OCl−-Injection and In Situ Electrochlorination in the Formation of Chlorate and Perchlorate in Seawater. Appl. Sci. 2019, 9, 229. https://doi.org/10.3390/app9020229
Yi J, Ahn Y, Hong M, Kim G-H, Shabnam N, Jeon B, Sang B-I, Kim H. Comparison between OCl−-Injection and In Situ Electrochlorination in the Formation of Chlorate and Perchlorate in Seawater. Applied Sciences. 2019; 9(2):229. https://doi.org/10.3390/app9020229
Chicago/Turabian StyleYi, Jongchan, Yongtae Ahn, Moongi Hong, Gi-Hyeon Kim, Nisha Shabnam, Byongsueng Jeon, Byoung-In Sang, and Hyunook Kim. 2019. "Comparison between OCl−-Injection and In Situ Electrochlorination in the Formation of Chlorate and Perchlorate in Seawater" Applied Sciences 9, no. 2: 229. https://doi.org/10.3390/app9020229
APA StyleYi, J., Ahn, Y., Hong, M., Kim, G.-H., Shabnam, N., Jeon, B., Sang, B.-I., & Kim, H. (2019). Comparison between OCl−-Injection and In Situ Electrochlorination in the Formation of Chlorate and Perchlorate in Seawater. Applied Sciences, 9(2), 229. https://doi.org/10.3390/app9020229







