Simulation of a Radio-Frequency Wave Based Bacterial Biofilm Detection Method in Dairy Processing Facilities
Abstract
1. Introduction
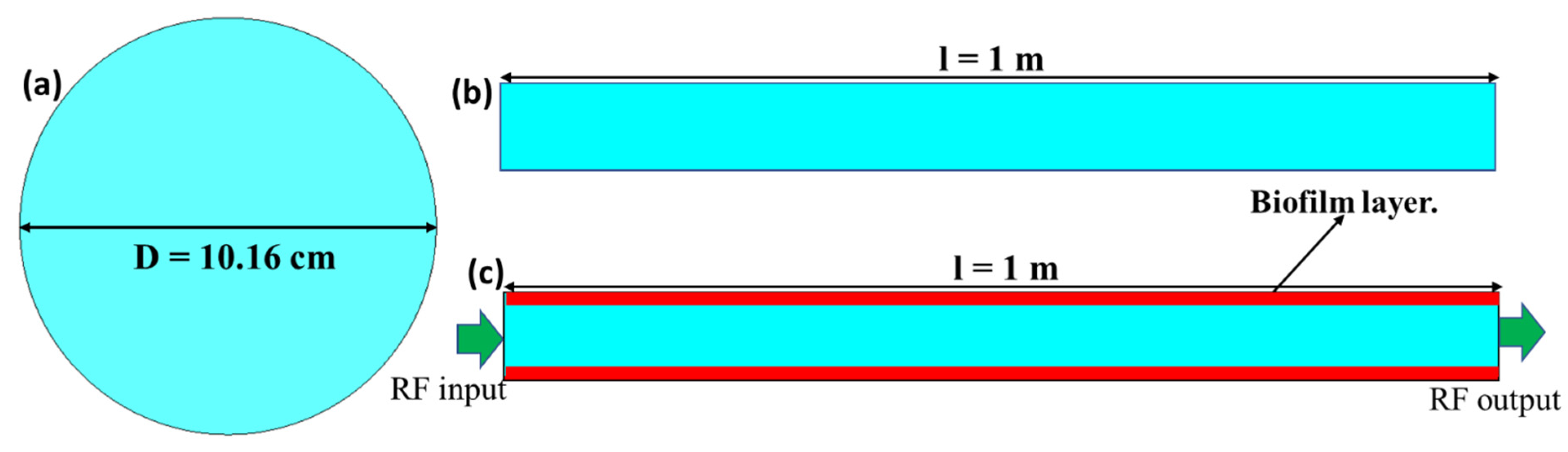
2. Materials and Methods: RF Waveguides and Cavities
3. Simulation Setup
4. Results and Discussion
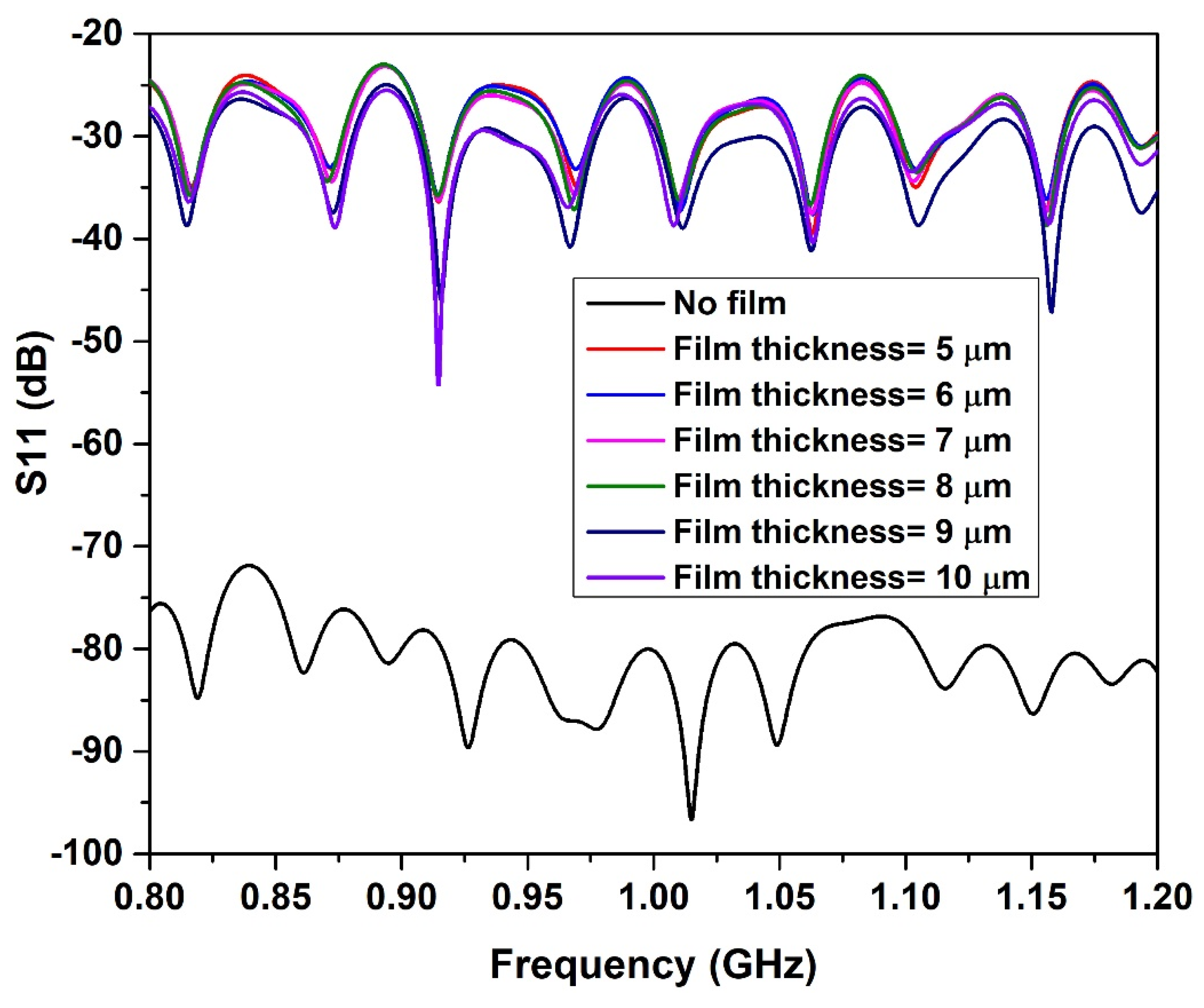
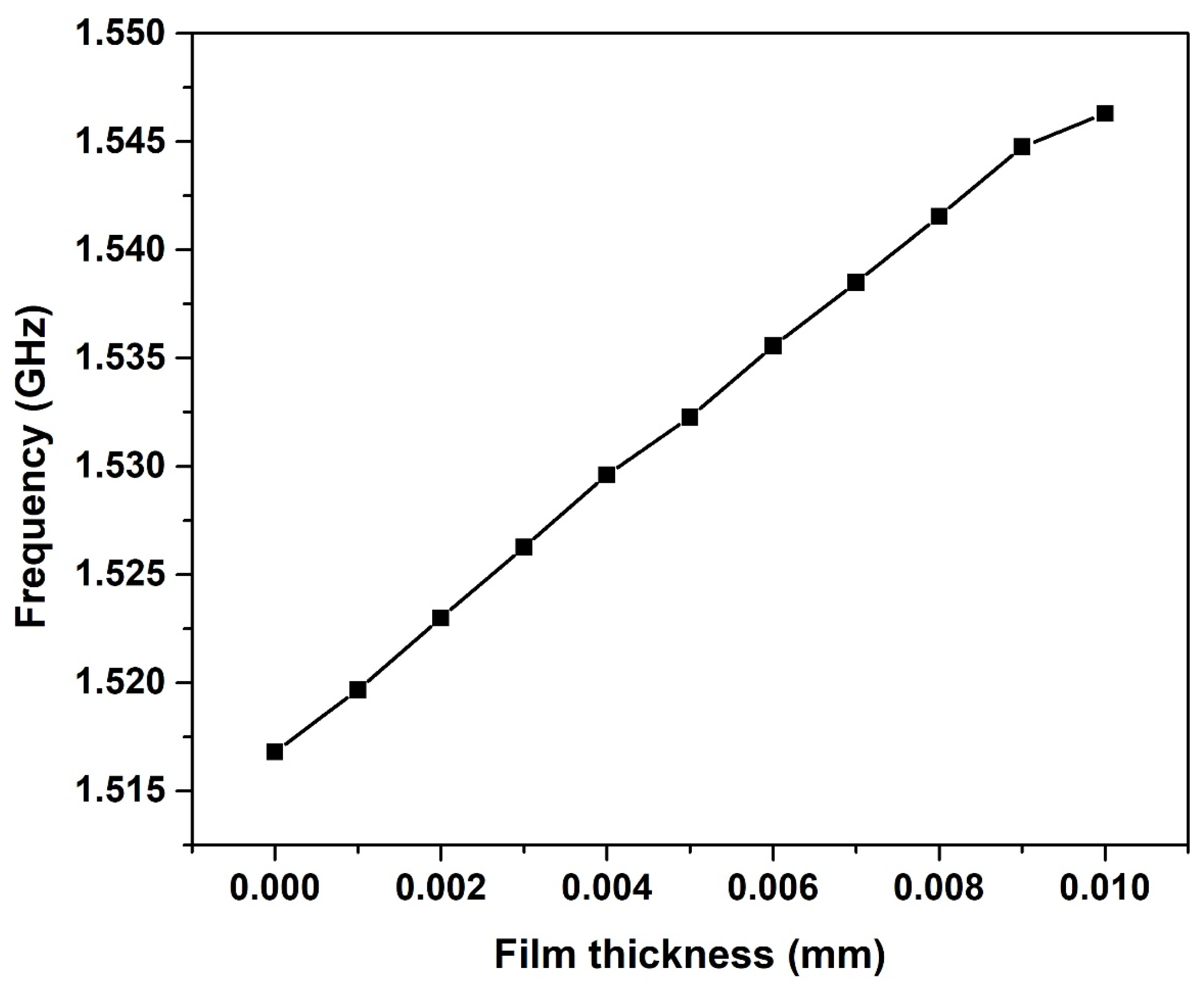
4.1. Milk-Filled Pipe
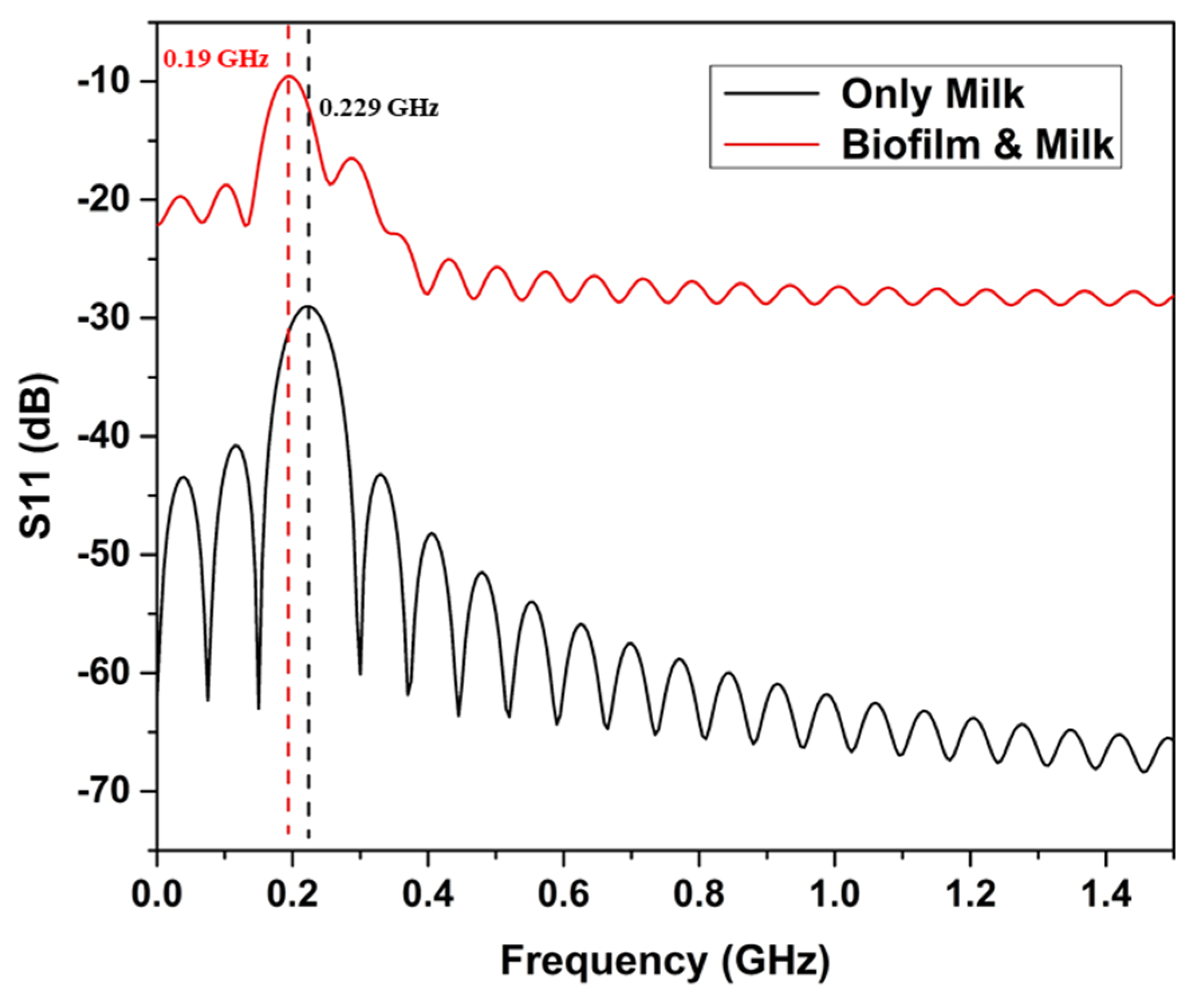
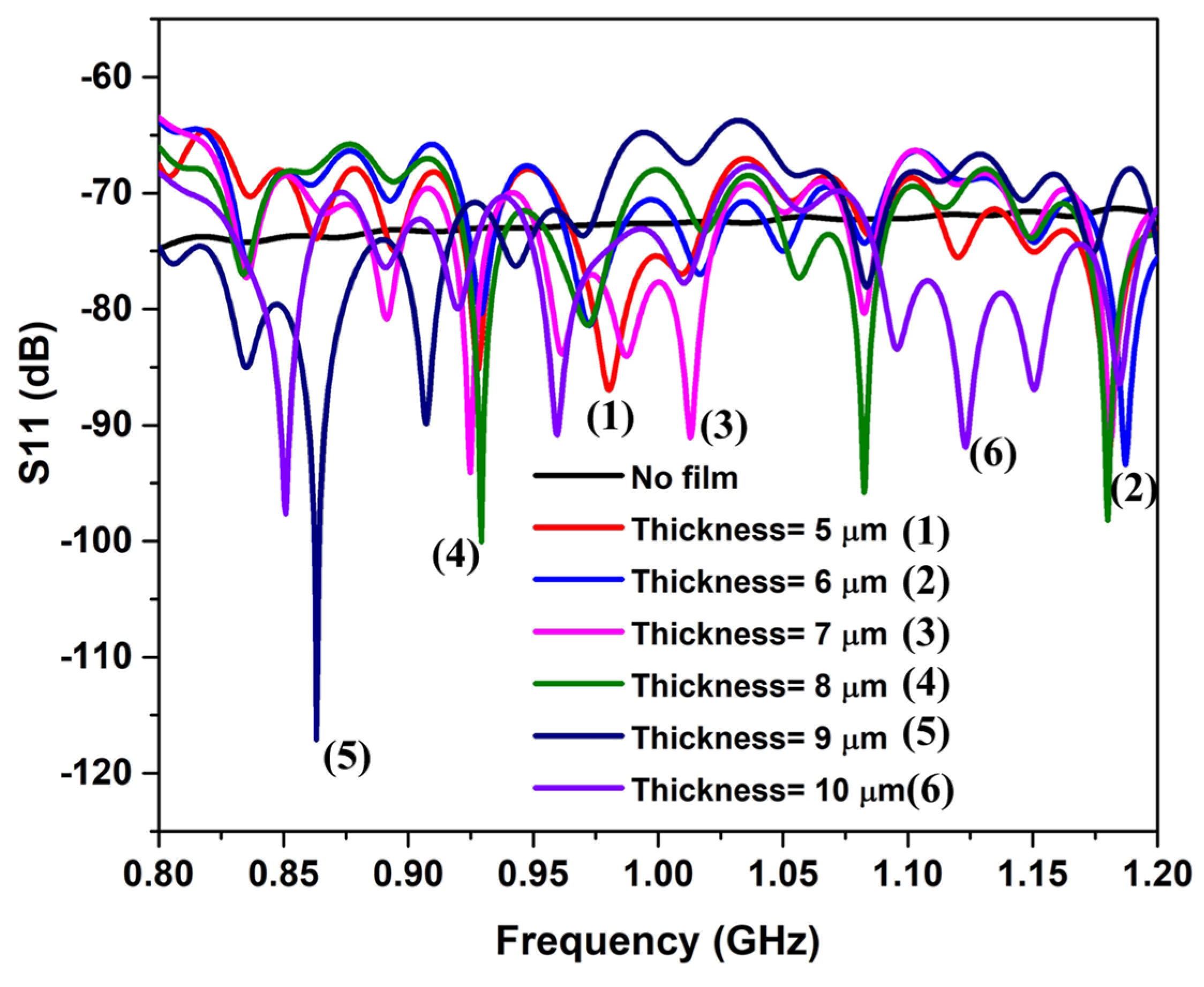
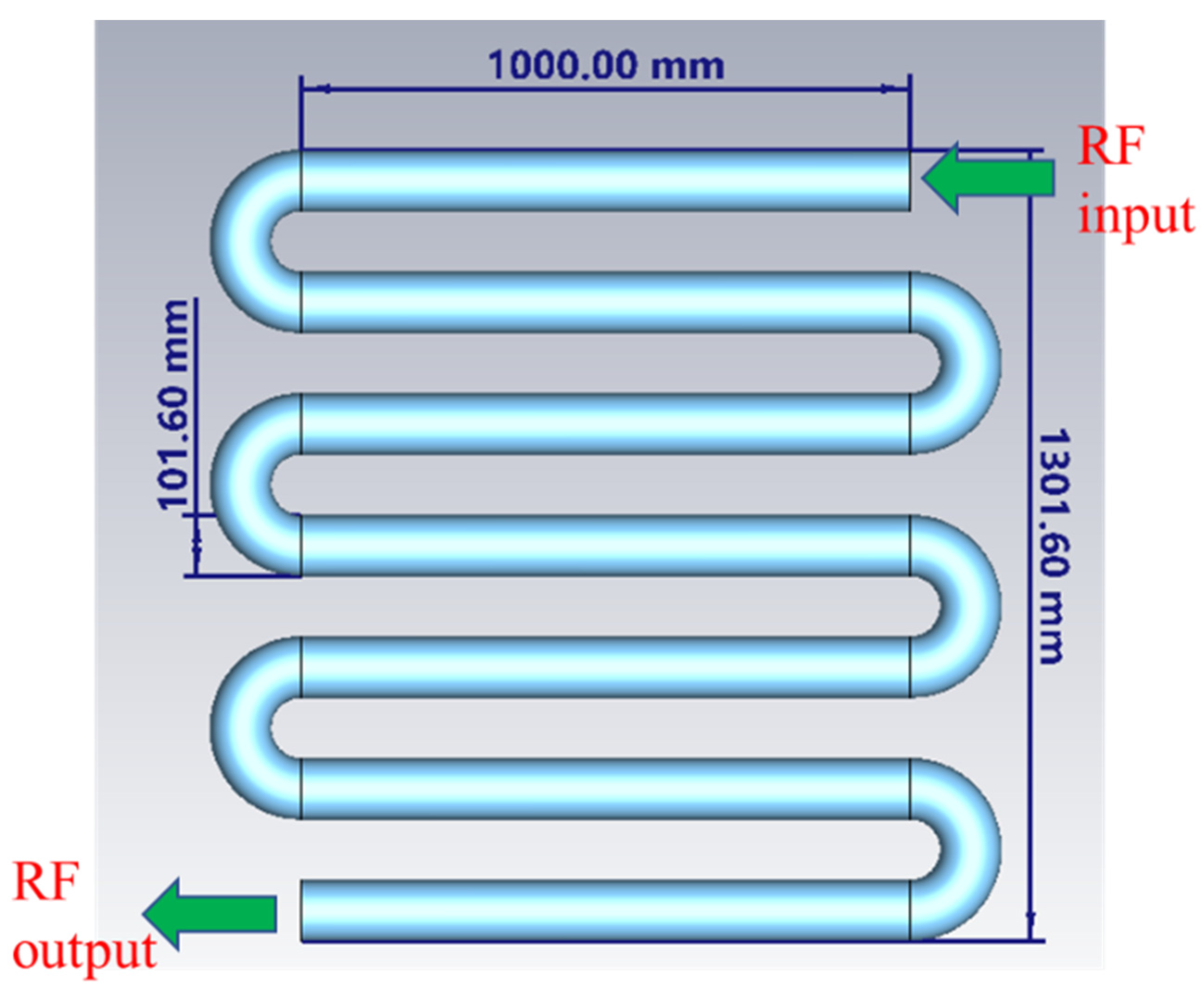
4.2. Milk-Filled Zigzag Holding Tube
4.3. Cavity Resonator
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Champagne, C.P.; Laing, R.R.; Roy, D.; Mafu, A.A.; Griffiths, M.W.; White, C. Psychrotrophs in Dairy Products: Their Effects and Their Control. Crit. Rev. Food Sci. Nutr. 1994, 34, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Bremer, P.; Brooks, J. Biofilms in Dairy Manufacturing Plant-description, Current Concerns and Methods of Control. Biofouling 1997, 11, 81–97. [Google Scholar] [CrossRef]
- Bremer, P.; Seale, B.; Flint, S.; Palmer, J. Biofilms in Dairy Processing. Biofilms Food Beverage Ind. 2009, 396–431. [Google Scholar] [CrossRef]
- Fabres-Klein, M.H.; Caizer Santos, M.J.; Contelli Klein, R.; Nunes de Souza, G.; de Oliveira Barros Ribon, A. An Association between Milk and Slime Increases Biofilm Production by Bovine Staphylococcus aureus. BMC Vet. Res. 2015, 11, 3. [Google Scholar] [CrossRef]
- Bhosale, S.; Brahmane, P.; Kubade, A.; Desale, R. Biofilm in the Dairy Industry: Detection and Common Process for Control Biofilms. Pharma Innov. J. 2021, SP-10, 809–817. [Google Scholar]
- Nyfors, E.; Vainikainen, P. Industrial Microwave Sensors; Artech House Microwave Library: Sacramento, CA, USA, 1989; ISBN 0-89006-397-4. [Google Scholar]
- Korostynska, O.; Mason, A.; Al-Shamma, A. Monitoring of Nitrates and Phosphates in Wastewater: Current Technologies and Further Challenges. Int. J. Smart Sens. Intell. Syst. 2012, 5, 149. [Google Scholar] [CrossRef]
- Boon, J.D.; Brubaker, J.M. Acoustic-Microwave Water Level Sensor Comparisons in an Estuarine Environment; IEEE: Quebec City, QC, Canada, 2008; pp. 1–5. [Google Scholar]
- Bernou, C.; Rebière, D.; Pistré, J. Microwave Sensors: A New Sensing Principle. Application to Humidity Detection. Sens. Actuators B Chem. 2000, 68, 88–93. [Google Scholar] [CrossRef]
- Nacke, T.; Barthel, A.; Pflieger, C.; Pliquett, U.; Beckmann, D.; Göller, A. Continuous Process Monitoring for Biogas Plants Using Microwave Sensors; IEEE: Tallinn, Estonia, 2010; pp. 239–242. [Google Scholar]
- Bej, A.K.; McCarty, S.C.; Atlas, R. Detection of Coliform Bacteria and Escherichia Coli by Multiplex Polymerase Chain Reaction: Comparison with Defined Substrate and Plating Methods for Water Quality Monitoring. Appl. Environ. Microbiol. 1991, 57, 2429–2432. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; Wylie, S.; Thomas, A.; Keele, H.; Shaw, A.; Al-Shammaa, A. Hepa Filter Material Load Detection Using a Microwave Cavity Sensor. Int. J. Smart Sens. Intell. Syst. 2010, 3, 322–337. [Google Scholar] [CrossRef]
- Hascakir, B.; Acar, C.; Akin, S. Microwave-Assisted Heavy Oil Production: An Experimental Approach. Energy Fuels 2009, 23, 6033–6039. [Google Scholar] [CrossRef]
- Saeed, K.; Pollard, R.D.; Hunter, I.C. Substrate Integrated Waveguide Cavity Resonators for Complex Permittivity Characterization of Materials. IEEE Trans. Microw. Theory Tech. 2008, 56, 2340–2347. [Google Scholar] [CrossRef]
- Bossavit, A. Solving Maxwell Equations in a Closed Cavity, and the Question of ‘spurious Modes’. IEEE Trans. Magn. 1990, 26, 702–705. [Google Scholar] [CrossRef]
- CST Studio Suite 2020. Available online: https://www.3ds.com/products/simulia/cst-studio-suite (accessed on 19 May 2024).
- Nunes, A.; Bohigas, X.; Tejada, J. Dielectric Study of Milk for Frequencies between 1 and 20 GHz. J. Food Eng. 2006, 76, 250–255. [Google Scholar] [CrossRef]
- Muñoz, I.; Gou, P.; Picouet, P.A.; Barlabé, A.; Felipe, X. Dielectric Properties of Milk during Ultra-Heat Treatment. J. Food Eng. 2018, 219, 137–146. [Google Scholar] [CrossRef]
- Nielen, M.; Deluyker, H.; Schukken, Y.; Brand, A. Electrical Conductivity of Milk: Measurement, Modifiers, and Meta Analysis of Mastitis Detection Performance. J. Dairy Sci. 1992, 75, 606–614. [Google Scholar] [CrossRef]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.; Friggens, N.; Sloth, K.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef]
- Cho, H.-M.; Yoo, W.; Yoo, B. Steady and Dynamic Rheological Properties of Thickened Beverages Used for Dysphagia Diets. Food Sci. Biotechnol. 2012, 21, 1775–1779. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, W.; Liang, Z. Determination of the Fat Content in Cow’s Milk Based on Dielectric Properties. Food Bioprocess Technol. 2015, 8, 1485–1494. [Google Scholar] [CrossRef]
- Dhar, B.R.; Sim, J.; Ryu, H.; Ren, H.; Santo Domingo, J.W.; Chae, J.; Lee, H.-S. Microbial Activity Influences Electrical Conductivity of Biofilm Anode. Water Res. 2017, 127, 230–238. [Google Scholar] [CrossRef]
- Checa, M.; Millan-Solsona, R.; Blanco, N.; Torrents, E.; Fabregas, R.; Gomila, G. Mapping the Dielectric Constant of a Single Bacterial Cell at the Nanoscale with Scanning Dielectric Force Volume Microscopy. Nanoscale 2019, 11, 20809–20819. [Google Scholar] [CrossRef]
- Boda, S.K.; Bajpai, I.; Basu, B. Inhibitory Effect of Direct Electric Field and HA-Z n O Composites on S. aureus Biofilm Formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1064–1075. [Google Scholar] [CrossRef]
- Jaeggi, M.; Gyr, S.; Astasov-Frauenhoffer, M.; Zitzmann, N.U.; Fischer, J.; Rohr, N. Influence of Different Zirconia Surface Treatments on Biofilm Formation in Vitro and in Situ. Clin. Oral Implant. Res. 2022, 33, 424–432. [Google Scholar] [CrossRef]
- Pilz, M.; Staats, K.; Tobudic, S.; Assadian, O.; Presterl, E.; Windhager, R.; Holinka, J. Zirconium Nitride Coating Reduced Staphylococcus epidermidis Biofilm Formation on Orthopaedic Implant Surfaces: An in Vitro Study. Clin. Orthop. Relat. Res. 2019, 477, 461. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, R.; Cornell, K.; Browning, J. Simulation of a Radio-Frequency Wave Based Bacterial Biofilm Detection Method in Dairy Processing Facilities. Appl. Sci. 2024, 14, 4342. https://doi.org/10.3390/app14114342
Bhattacharya R, Cornell K, Browning J. Simulation of a Radio-Frequency Wave Based Bacterial Biofilm Detection Method in Dairy Processing Facilities. Applied Sciences. 2024; 14(11):4342. https://doi.org/10.3390/app14114342
Chicago/Turabian StyleBhattacharya, Ranajoy, Ken Cornell, and Jim Browning. 2024. "Simulation of a Radio-Frequency Wave Based Bacterial Biofilm Detection Method in Dairy Processing Facilities" Applied Sciences 14, no. 11: 4342. https://doi.org/10.3390/app14114342
APA StyleBhattacharya, R., Cornell, K., & Browning, J. (2024). Simulation of a Radio-Frequency Wave Based Bacterial Biofilm Detection Method in Dairy Processing Facilities. Applied Sciences, 14(11), 4342. https://doi.org/10.3390/app14114342






