Review on Mercury Control during Co-Firing Coal and Biomass under O2/CO2 Atmosphere
Abstract
:1. Introduction
2. Mercury in BECCS System
2.1. BECCS System
2.2. Hazards of Mercury
3. Mercury Migration under Coal/Biomass Co-Firing Conditions
3.1. Impacts of Chlorine
3.2. Impacts of Particulate Matters (PM)
3.3. Impacts of Alkali Metals
4. Mercury Migration under O2/CO2 Atmosphere
4.1. Impacts of CO2
4.2. Impacts of Other Flue Gas Components
4.3. Hg Re-Emission from WFGD
5. Mercury Removal Methods
5.1. Adsorption
5.2. Catalytic Oxidation
6. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. CO2 Emissions in 2022. Available online: https://www.iea.org/reports/co2-emissions-in-2022 (accessed on 15 April 2024).
- Intergovernmental Panel on Climate Change. Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/download/#full (accessed on 15 April 2024).
- Yi, Q.; Zhao, Y.; Huang, Y.; Wei, G.; Hao, Y.; Feng, J.; Mohamed, U.; Pourkashanian, M.; Nimmo, W.; Li, W. Life cycle energy-economic-CO2 emissions evaluation of biomass/coal, with and without CO2 capture and storage, in a pulverized fuel combustion power plant in the United Kingdom. Appl. Energy 2018, 225, 258–272. [Google Scholar] [CrossRef]
- Lyu, Q.; Guan, Y.; Du, Y.; Liu, Y.; Che, D. Review and perspectives on mercury release and migration during chemical looping combustion of solid fuels. Energy Fuels 2024, 38, 2690–2707. [Google Scholar] [CrossRef]
- Li, H.L.; Zu, H.X.; Yang, Z.Q.; Yang, J.P.; Xu, H.; Qu, W.Q. The adsorption mechanisms of Hg0 on marcasite-type metal selenides: The influences of metal-terminated site. Chem. Eng. J. 2021, 406, 126723. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, Y.C.; Xiong, Z.; Xiao, R.H.; Gao, T.; Liu, P.F.; Liu, J.; Zhang, J.Y. Enhanced photocatalytic Hg0 oxidation activity of iodine doped bismuth molybdate (Bi2MoO6) under visible light. J. Colloid Interface Sci. 2022, 607, 1864–1875. [Google Scholar] [CrossRef]
- Lyu, Q.; Wang, C.; Liu, X.; Che, D. Numerical study on the homogeneous reactions of mercury in a 600 MW coal-fired utility boiler. Energies 2022, 15, 446. [Google Scholar] [CrossRef]
- GB 13223-2011; Emission Standard of Air Pollutants for Thermal Power Plants. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2011.
- Milford, J.; Pienciak, A. After the clean air mercury rule: Prospects for reducing mercury emissions from coal-fired power plants. Environ. Sci. Technol. 2009, 43, 2669–2673. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A. Mercury Emissions from Coal Fired Power Plants—The Case for Regulatory Action; NESCAUM: Boston, MA, USA, 2003. [Google Scholar]
- Carnell, P.J.H. A re-think of the mercury removal problem for LNG plants. In Proceedings of the 15th International Conference & Exhibition on Liquefied Natural Gas (LNG 15), Barcelona, Spain, 24–27 April 2007. [Google Scholar]
- Lv, Q.; Wang, C.; He, Y.; Cai, M.; Che, D. Elemental mercury removal over CeO2/TiO2 catalyst prepared by sol-gel method. Appl. Sci. 2020, 10, 2706. [Google Scholar] [CrossRef]
- Lv, Q.; Cai, M.; Wang, C.; Zhang, Z.; Che, D. Investigation on elemental mercury removal and antideactivation performance of modified SCR catalysts. Asia-Pac. J. Chem. Eng. 2018, 13, e2208. [Google Scholar] [CrossRef]
- Garcia, E.; Liu, H. Ilmenite as alternative bed material for the combustion of coal and biomass blends in a fluidised bed combustor to improve combustion performance and reduce agglomeration tendency. Energy 2022, 239, 121913. [Google Scholar] [CrossRef]
- Allgurén, T.; Viljanen, J.; Li, X.L.; Wang, Y.M.; Andersson, K.; Wendt, J.O.L. Alkali sulfation during combustion of coal in a pilot scale facility using additives to alter the global sulfur to potassium and chlorine to potassium ratios. Proc. Combust. Inst. 2021, 38, 4171–4178. [Google Scholar] [CrossRef]
- Sun, Y.L.; Lv, G.K.; Zhang, H.F.; Zhang, X.Q.; Bu, X.G.; Wang, X.J.; Zhang, W.; Tong, Y.D. Characteristics of speciated mercury emissions from coal combustion in air and oxygen-enriched environment. Bull. Environ. Contam. Toxicol. 2019, 102, 695–700. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Q.; Jin, X.; Deng, L. The influence of co-firing coal with biomass syngas on the thermodynamic parameters of a boiler. Appl. Sci. 2023, 13, 11477. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, C.; Liu, X.; Du, Y.; Li, D.; Che, D. Combustion and heat transfer characteristics of co-firing biomass and coal under oxy-fuel condition. Int. J. Energy Res. 2018, 42, 4170–4183. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Wu, Q. Research progress of carbon capture technology based on alcohol amine solution. Sep. Purif. Technol. 2024, 333, 125715. [Google Scholar] [CrossRef]
- Guo, J.; Guo, T.; Zhang, T.; Hu, F.; Li, P.; Liu, Z. Numerical investigation on NOxformation of staged oxy-fuel combustion in a 35 MW large pilot boiler. Fuel 2024, 358, 130177. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Global Mercury Assessment 2018; UNEP: Geneva, Switzerland, 2019. [Google Scholar]
- Zhou, P.Y.; Zhang, A.C.; Zhang, D.; Feng, C.X.; Su, S.; Zhang, X.M.; Xiang, J.; Chen, G.Y.; Wang, Y. Efficient removal of Hg0 from simulated flue gas by novel magnetic Ag2WO4/BiOI/CoFe2O4 photocatalysts. Chem. Eng. J. 2019, 373, 780–791. [Google Scholar] [CrossRef]
- Tuzen, M.; Uluozlu, O.D.; Karaman, I.; Soylak, M. Mercury(II) and methyl mercury speciation on Streptococcus pyrogenes loaded Dowex Optipore SD-2. J. Hazard. Mater. 2009, 169, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Monperrus, M.; Pécheyran, C.; Bolliet, V. Imaging differential mercury species bioaccumulation in glass eels using isotopic tracers and laser ablation inductively coupled plasma mass spectrometry. Appl. Sci. 2020, 10, 2463. [Google Scholar] [CrossRef]
- Mulenga, M.; Ouma, K.; Monde, C.; Syampungani, S. Aquatic mercury pollution from artisanal and small-scale gold mining in sub-Saharan Africa: Status, Impacts, and Interventions. Water 2024, 16, 756. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y. Spatial-temporal changes of land-use mercury emissions in China. Ecol. Indic. 2023, 146, 109430. [Google Scholar] [CrossRef]
- Bishop, K.; Li, C.; Osterwalder, S. Plant demethylation in global mercury cycling. Nature Food 2024, 5, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Chalkidis, A.; Jampaiah, D.; Hartley, P.G.; Sabri, Y.M.; Bhargava, S.K. Mercury in natural gas streams: A review of materials and processes for abatement and remediation. J. Hazard. Mater. 2020, 382, 121036. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.P. Failures of structures and components by metal-induced embrittlement. J. Fail. Anal. Prev. 2008, 8, 259–274. [Google Scholar] [CrossRef]
- Li, D.; Lv, Q.; Feng, Y.; Wang, C.; Liu, X.; Du, Y.; Zhong, J.; Che, D. Numerical study of co-firing biomass with lean coal under air-fuel and oxy-fuel conditions in a wall-fired utility boiler. Energy Fuels 2017, 31, 5344–5354. [Google Scholar] [CrossRef]
- Dziok, T.; Kołodziejska, E.K.; Kołodziejska, E.L. Mercury content in woody biomass and its removal in the torrefaction process. Biomass Bioenergy 2020, 143, 105832. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; de Matos Macchi, B.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef] [PubMed]
- Rokni, E.; Ren, X.; Panahi, A.; Levendis, Y.A. Emissions of SO2, NOx, CO2, and HCl from co-firing of coals with raw and torrefied biomass fuels. Fuel 2018, 211, 363–374. [Google Scholar] [CrossRef]
- Sliger, R.N.; Kramlich, J.C.; Marinov, N.M. Towards the development of a chemical kinetic model for the homogeneous oxidation of mercury by chlorine species. Fuel Process. Technol. 2000, 65, 423–438. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, X.; Shen, B.; Ren, K.; Wang, Y.; Luo, J. Enhanced elemental mercury removal via chlorine-based hierarchically porous biochar with CaCO3 as template. Chem. Eng. J. 2021, 406, 126828. [Google Scholar] [CrossRef]
- Pan, H.Y.; Minet, R.G.; Benson, S.W.; Tsotsis, T.T. Process for converting hydrogen-chloride to chlorine. Ind. Eng. Chem. Res. 1994, 33, 2996–3003. [Google Scholar] [CrossRef]
- Pilling, M.; Seakins, P.W. Reaction Kinetics; Oxford Science Publications: New York, NY, USA, 1995. [Google Scholar]
- Niksa, S.; Fujiwara, N. A predictive mechanism for mercury oxidation on selective catalytic reduction catalysts under coal-derived flue gas. J. Air Waste Manage. Assoc. 2005, 55, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.L.; Ganesh, N.; Rodilla, I.; Bahillo, A. Assess of biomass co-firing under oxy-fuel conditions on Hg speciation and ash deposit formation. Fuel 2018, 215, 395–405. [Google Scholar] [CrossRef]
- Hao, R.; Yang, F.; Mao, X.; Mao, Y.; Zhao, Y.; Lu, Y.J. Emission factors of mercury and particulate matters, and in situ control of mercury during the co-combustion of anthracite and dried sawdust sludge. Fuel 2018, 230, 202–210. [Google Scholar] [CrossRef]
- Wang, Q. Study on Emission Behavior of Particulate Matter during Co-Combustion of Biomass and Coal. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2009. [Google Scholar]
- Zheng, Y.J.; Jensen, P.A.; Jensen, A.D.; Sander, B.; Junker, H. Ash transformation during co-firing coal and straw. Fuel 2007, 86, 1008–1020. [Google Scholar] [CrossRef]
- Zevenhoven, M.; Backman, R.; Skrifvars, B.J.; Hupa, M. The ash chemistry in fluidised bed gasification of biomass fuels. Part I: Predicting the chemistry of melting ashes and ash-bed material interaction. Fuel 2001, 80, 1489–1502. [Google Scholar] [CrossRef]
- Obernberger, I.; Dahl, J.; Brunner, T. Formation, composition and particle size distribution of fly ashes from biomass combustion plants. In Proceedings of the 4th Biomass Conference of the Americas, Oakland, CA, USA, 29 August–2 September 1999. [Google Scholar]
- Bruner, T.; Joeller, M. Aerosol and fly ash formation in fixed bed biomass combustion systems using woody biofuels. In Proceedings of the 12th European Biomass Conference, Amsterdam, The Netherlands, 17–21 June 2002. [Google Scholar]
- Xue, Z.; Zhong, Z.; Lai, X. Investigation on gaseous pollutants emissions during co-combustion of coal and wheat straw in a fluidized bed combustor. Chemosphere 2020, 240, 124853. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Diao, Y.; Shen, H. Thermodynamics study of effects of alkali metals on mercury transformation during co-combustion of biomass with coal. Proc. CSEE 2012, 32, 96–102. [Google Scholar]
- Chen, L.; Duan, Y.; Zhuo, Y.; Yang, L.; Zhang, L.; Yang, X.; Yao, Q.; Jiang, Y.; Xu, X. Mercury transformation across particulate control devices in six power plants in China: The co-effect of chlorine and ash composition. Fuel 2007, 86, 603–610. [Google Scholar]
- Xu, J.; Bao, J.; Liu, H.; Mo, Z.; Du, M.; Sun, L. Effects of metal ions on Hg0 re-emission under air and oxy-fuel combustion atmospheres in a WFGD system. Fuel 2021, 299, 120881. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Wang, H.; Xue, Y.; Zhu, C.; Zhou, Q.; Zhang, J.; She, M.; Liu, M. Release characteristics of mercury in coal under O2/CO2 atmosphere. Chem Ind. Eng. Prog. 2017, 36, 372–377. [Google Scholar]
- Wu, H.; Liu, H.; Wang, Q.; Luo, G.; Yao, H.; Qiu, J. Experimental study of homogeneous mercury oxidation under O2/CO2 atmosphere. Proc. Combust. Inst. 2013, 34, 2847–2854. [Google Scholar] [CrossRef]
- Fernández-Miranda, N.; Lopez-Anton, M.A.; Díaz-Somoano, M.; Martínez-Tarazona, M.R. Effect of oxy-combustion flue gas on mercury oxidation. Environ. Sci. Technol. 2014, 48, 7164–7170. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Duan, Y.F.; Li, Y.N.; Xue, Y.; Ying, Z.F. Activated carbon for capturing Hg in flue gas under O2/CO2 combustion conditions. Part 1: Experimental and kinetic study. Energy Fuels 2018, 32, 1900–1906. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Chang, L.; Zhang, J.; Zheng, C. Mercury adsorption and oxidation over cobalt oxide loaded magnetospheres catalyst from fly ash in oxyfuel combustion flue gas. Environ. Sci. Technol. 2015, 49, 8210–8218. [Google Scholar] [CrossRef] [PubMed]
- Gharebaghi, M.; Goh, B.; Porter, R.T.J.; Pourkashanian, M.; Williams, A. Modelling methods for co-fired pulverized fuel furnaces. In Proceedings of the 1st Oxyfuel Combustion Conference, Cottbus, Germany, 8–11 September 2009. [Google Scholar]
- de las Obras-Loscertales, M.; Izquierdo, M.T.; Rufas, A.; de Diego, L.F.; García-Labiano, F.; Abad, A.; Gayán, P.; Adánez, J. The fate of mercury in fluidized beds under oxy-fuel combustion conditions. Fuel 2016, 167, 75–81. [Google Scholar] [CrossRef]
- Miller, S.J.; Dunham, G.E.; Olson, E.S.; Brown, T.D. Flue gas effects on a carbon-based mercury sorbent. Fuel Process. Technol. 2000, 65, 343–363. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Shen, C.; Wu, J.; Zhu, Y.; Shi, W.; Zhang, X.; Ying, Z.F. Effect of atmosphere of SO2 coexisted with oxidizing gas on mercury removal under oxy-fuel condition. Chemosphere 2020, 259, 127525. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Ham, S.-W.; Lee, J.-B. Oxidation of gaseous elemental mercury by hydrochloric acid over CuCl2/TiO2-based catalysts in SCR process. Appl. Catal. B-Environ. 2010, 99, 272–278. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, C.Y.; Li, Y.; Li, L.Q.; Zhao, Y.C.; Zhang, J.Y. Impact of SO2 on elemental mercury oxidation over CeO2-TiO2 catalyst. Chem. Eng. J. 2013, 219, 319–326. [Google Scholar] [CrossRef]
- Wang, F.; Shen, B.; Yang, J.; Singh, S. Review of mercury formation and capture from CO2-enriched oxyfuel combustion flue gas. Energy Fuels 2017, 31, 1053–1064. [Google Scholar] [CrossRef]
- Stam, A.; Ploumen, P.; Brem, G. Coal: World energy security. In Proceedings of the 34th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 31 May–4 June 2009. [Google Scholar]
- Yu, M.Y.; Luo, G.Q.; Sun, R.Z.; Zou, R.J.; Li, X.; Yao, H. A mechanism study on effects of bromide ion on mercury re-emission in WFGD slurry. Chem. Eng. J. 2021, 406, 127010. [Google Scholar] [CrossRef]
- Hsu, C.J.; Atkinson, J.D.; Chung, A.; Hsi, H.C. Gaseous mercury re-emission from wet flue gas desulfurization wastewater aeration basins: A review. J. Hazard. Mater. 2021, 420, 126546. [Google Scholar] [CrossRef]
- Ochoa-González, R.; Díaz-Somoano, M.; Martínez-Tarazona, M.R. A comprehensive evaluation of the influence of air combustion and oxy-fuel combustion flue gas constituents on Hg0 re-emission in WFGD systems. J. Hazard. Mater. 2014, 276, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bao, J.; Liu, H.; Yu, J.; Mo, Z.; Xiang, Z.; Sun, L. Characteristics of mercury re-emission and migration in a lab-scale wet flue gas desulfurization scrubber under simulated air and oxy-fuel combustion atmospheres. Energy Fuels 2020, 34, 16356–16365. [Google Scholar] [CrossRef]
- Cai, J.; Shen, B.; Li, Z.; Chen, J.; He, C. Removal of elemental mercury by clays impregnated with KI and KBr. Chem. Eng. J. 2014, 241, 19–27. [Google Scholar] [CrossRef]
- Tong, L.; Yue, T.; Zuo, P.; Zhang, X.; Wang, C.; Gao, J.; Wang, K. Effect of characteristics of KI-impregnated activated carbon and flue gas components on Hg0 removal. Fuel 2017, 197, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Chen, J.; Jiang, Y.; Wang, W. Adsorption performance and mechanism of bentonite modified by ammonium bromide for gas-phase elemental mercury removal. J. Fuel Chem. Technol. 2014, 42, 1266–1272. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Hu, C.X.; Zhou, J.S.; Cen, K.F. Stability of mercury on three activated carbon sorbents. Fuel Process. Technol. 2006, 87, 679–685. [Google Scholar] [CrossRef]
- Krishnan, S.V.; Gullett, B.K.; Jozewicz, W. Sorption of elemental mercury by activated carbons. Environ. Sci. Technol. 1994, 28, 1506–1512. [Google Scholar] [CrossRef]
- Ie, I.; Hung, C.; Jen, Y.; Yuan, C.; Chen, W. Adsorption of vapor-phase elemental mercury (Hg0) and mercury chloride (HgCl2) with innovative composite activated carbons impregnated with Na2S and S0 in different sequences. Chem. Eng. J. 2013, 229, 469–476. [Google Scholar] [CrossRef]
- Liu, W.; Vidic, R.D.; Brown, T.D. Impact of flue gas conditions on mercury uptake by sulfur-impregnated activated carbon. Environ. Sci. Technol. 2000, 34, 154–159. [Google Scholar] [CrossRef]
- Korpiel, J.A.; Vidic, R.D. Effect of sulfur impregnation method on activated carbon uptake of gas-phase mercury. Environ. Sci. Technol. 1997, 31, 2319–2325. [Google Scholar] [CrossRef]
- Liu, W.; Vidic, R.D.; Brown, T.D. Optimization of sulfur impregnation protocol for fixed bed application of activated carbon-based sorbents for gas-phase mercury removal. Environ. Sci. Technol. 1998, 32, 531–538. [Google Scholar] [CrossRef]
- Liu, W.; Vidic, R.D.; Brown, T.D. Optimization of high temperature sulfur impregnation on activated carbon for permanent sequestration of elemental mercury vapors. Environ. Sci. Technol. 2000, 34, 483–488. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Prabhu, A.; Al Shoaibi, A.; Srinivasakannan, C. Application of sulfonated carbons for mercury removal in gas processing. Energy Fuels 2016, 30, 3227–3232. [Google Scholar] [CrossRef]
- His, H.C.; Chen, C.T. Influences of acidic/oxidizing gases on elemental mercury adsorption equilibrium and kinetics of sulfur-impregnated activated carbon. Fuel 2012, 98, 229–235. [Google Scholar]
- Mullett, M.; Pendleton, P.; Badalyan, A. Removal of elemental mercury from Bayer stack gases using sulfur-impregnated activated carbons. Chem. Eng. J. 2012, 211, 133–142. [Google Scholar] [CrossRef]
- Morimoto, T.; Wu, S.J.; Uddin, M.A.; Sasaoka, E. Characteristics of the mercury vapor removal from coal combustion flue gas by activated carbon using H2S. Fuel 2005, 84, 1968–1974. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, B.; Zeng, X.; Luo, G.; Li, X.; Yao, H.; Zheng, C. Deep study on effects of activated carbon’s oxygen functional groups for elemental mercury adsorption using temperature programmed desorption method. Fuel 2017, 200, 100–106. [Google Scholar] [CrossRef]
- Lopez-Anton, M.A.; Gil, R.R.; Fuente, E.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Ruiz, B. Activated carbons from biocollagenic wastes of the leather industry for mercury capture in oxy-combustion. Fuel 2015, 142, 227–234. [Google Scholar] [CrossRef]
- Li, D.; Han, J.; Han, L.; Wang, J.; Chang, L. Pd/activated carbon sorbents for mid-temperature capture of mercury from coal-derived fuel gas. J. Environ. Sci. 2014, 26, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, C.; Li, Y.; Zhang, J. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environ. Sci. Technol. 2011, 45, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, Y.; Zhou, Q.; Zhu, C.; She, M.; Ding, W. Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem. Eng. J. 2016, 294, 281–289. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Liu, Y. Zeolite-Supported Silver Nanoparticles for Coal-Fired Power Plant Mercury Emission Control. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2009. [Google Scholar]
- Chiu, C.H.; His, H.C.; Lin, C.C. Control of mercury emissions from coal-combustion flue gases using CuCl2-modified zeolite and evaluating the cobenefit effects on SO2 and NO removal. Fuel Process. Technol. 2014, 126, 138–144. [Google Scholar] [CrossRef]
- Morency, J.R. Zeolite sorbent that effectively removes mercury from flue gases. Filtr. Sep. 2002, 39, 24–26. [Google Scholar] [CrossRef]
- Kwon, S.; Vidic, R.D. Evaluation of two sulfur impregnation methods on activated carbon and bentonite for the production of elemental mercury sorbents. Environ. Eng. Sci. 2000, 17, 303–313. [Google Scholar] [CrossRef]
- Xin, F.; Xiao, R.H.; Zhao, Y.C.; Zhang, J.Y. Surface sulfidation modification of magnetospheres from fly ash for elemental mercury removal from coal combustion flue gas. Chem. Eng. J. 2022, 436, 135212. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Yang, J.P.; Dong, L.C.; Gao, T.; Li, Z.H.; Ji, Y.S.; Zhao, Y.C.; Pan, S.W.; Zhang, J.Y.; Zheng, C.G. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres from fly ash: Part 5. Industrial scale studies at a 50 MWth coal-fired power plant. Fuel 2020, 266, 117052. [Google Scholar] [CrossRef]
- Hower, J.; Senior, C.; Suuberg, E.; Hurt, R.; Wilcox, J.; Olson, E. Mercury capture by native fly ash carbons in coal-fired power plants. Prog. Energy Combust. Sci. 2010, 36, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Zhu, T.; Kuang, J.; Jing, P. Mercury removal from coal combustion flue gas by modified fly ash. J. Environ. Sci. 2013, 25, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.F.; Meng, J.; Wang, H. Experimental study on Hg0 removal by filter material loaded with fly ash-CaO adsorbent. J. Donghua Univ. (Nat. Sci.) 2013, 39, 223–229. [Google Scholar]
- Hou, W.; Zhou, J.; Yu, C.; You, S.; Gao, X.; Luo, Z. Pd/Al2O3 sorbents for elemental mercury capture at high temperatures in syngas. Ind. Eng. Chem. Res. 2014, 53, 9909–9914. [Google Scholar] [CrossRef]
- Song, Y.C.; Lee, T.G. Preparation of gold- and chlorine-impregnated bead-type activated carbon for a mercury sorbent trap. Chemosphere 2016, 165, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanloo, H.; Osanloo, M.; Ghazaghi, M.; Hassani, H. Validation of a new and cost-effective method for mercury vapor removal based on silver nanoparticles coating on micro glassy balls. Atmos. Pollut. Res. 2017, 8, 359–365. [Google Scholar] [CrossRef]
- Dunham, G.E.; DeWall, R.A.; Senior, C.L. Fixed-bed studies of the interactions between mercury and coal combustion fly ash. Fuel Process. Technol. 2003, 82, 197–213. [Google Scholar] [CrossRef]
- Xiao, Y.; Pudasainee, D.; Gupta, R.; Xu, Z.; Diao, Y. Elemental mercury reaction chemistry on brominated petroleum cokes. Carbon 2017, 124, 89–96. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Wu, F.; Huang, Y.; Yin, Z. Experimental and theoretical studies for the mechanism of mercury oxidation over chlorine and cupric impregnated activated carbon. Appl. Surf. Sci. 2018, 458, 790–799. [Google Scholar] [CrossRef]
- Ghorishi, S.; Lee, C.; Jozewicz, W.; Kilgroe, J. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol. 2006, 40, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shen, Z.; Yuan, T.; Wang, W.; Han, H. Removal of vapor-phase elemental mercury by N-doped CuCoO4 loaded on activated carbon. Fuel Process. Technol. 2007, 88, 623–629. [Google Scholar] [CrossRef]
- Kong, F.; Qiu, J.; Liu, H.; Zhao, R.; Ai, Z. Catalytic oxidation of gas-phase elemental mercury by nano-Fe2O3. J. Environ. Sci. 2011, 23, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Y.; Yan, N.; Qu, Z.; Xie, J.; Yang, C.; Jia, J. Capture of gaseous elemental mercury from flue gas using a magnetic and sulfur poisoning resistant sorbent Mn/γ-Fe2O3 at lower temperatures. J. Hazard. Mater. 2011, 186, 508–515. [Google Scholar] [CrossRef]
- Scala, F.; Cimino, S. Elemental mercury capture and oxidation by a regenerable manganese-based sorbent: The effect of gas composition. Chem. Eng. J. 2015, 278, 134–139. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, B.; Liu, Y.; Zhao, W.; Guan, B. Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by sol-gel method. J. Hazard. Mater. 2007, 145, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Ke, R.; Luo, C.; Hao, J. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts. Catal. Commun. 2007, 8, 1896–1900. [Google Scholar] [CrossRef]
- Qi, G.S.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B-Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Li, G.; Wu, Y.; Hao, J.; Pirrone, N.; Sprovieri, F.; Ancora, M. Mercury emission and speciation of coal-fired power plants in China. Atmos. Chem. Phys. 2010, 10, 1183–1192. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y. Effect of manganese ions on the structure of Ca(OH)2 and mercury adsorption performance of Mnx+/Ca(OH)2 composites. Energy Fuels 2011, 25, 1553–1558. [Google Scholar] [CrossRef]
- Qiao, S.; Chen, J.; Li, J.; Qu, Z.; Liu, P.; Yan, N.; Jia, J. Adsorption and catalytic oxidation of gaseous elemental mercury in flue gas over MnOx/alumina. Ind. Eng. Chem. Res. 2009, 48, 3317–3322. [Google Scholar] [CrossRef]
- Li, J.; Yan, N.; Qu, Z.; Qiao, S.; Yang, S.; Guo, Y.; Liu, P.; Jia, J. Catalytic oxidation of elemental mercury over the modified catalyst Mn/alpha-Al2O3 at lower temperatures. Environ. Sci. Technol. 2010, 44, 426–431. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, Q.; Liu, X. Elemental mercury oxidation and adsorption on magnesite powder modified by Mn at low temperature. J. Hazard. Mater. 2015, 283, 252–259. [Google Scholar] [CrossRef]
- Ji, L.; Sreekanth, P.; Smirniotis, P.; Thiel, S.; Pinto, N. Manganese oxide/titania materials for removal of NOx and elemental mercury from flue gas. Energy Fuels 2008, 22, 2299–2306. [Google Scholar] [CrossRef]
- Tsai, C.; His, H.; Kuo, T.; Chang, Y.; Liou, J. Preparation of Cu-doped TiO2 photocatalyst with thermal plasma torch for low-concentration mercury removal. Aerosol Air Qual. Res. 2013, 13, 639–648. [Google Scholar] [CrossRef]
- Kim, B.J.; Bae, K.M.; Park, S.J. Elemental mercury vapor adsorption of copper-coated porous carbonaceous materials. Microporous Mesoporous Mat. 2012, 163, 270–275. [Google Scholar] [CrossRef]
- Du, W.; Yin, L.; Zhuo, Y.; Xu, Q.; Zhang, L.; Chen, C. Performance of CuOx-neutral Al2O3 sorbents on mercury removal from simulated coal combustion flue gas. Fuel Process. Technol. 2015, 131, 403–408. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, H.; Tang, X.; Li, Q.; Liu, D.; Gao, F. Copper modified activated coke for mercury removal from coal-fired flue gas. Chem. Eng. J. 2016, 286, 585–593. [Google Scholar] [CrossRef]
- Mei, Z.; Shen, Z.; Zhao, Q.; Wang, W.; Zhang, Y. Removal and recovery of gas-phase element mercury by metal oxide-loaded activated carbon. J. Hazard. Mater. 2008, 152, 721–729. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, H.; Wu, Z. Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol-gel method. Catal. Commun. 2011, 12, 1291–1294. [Google Scholar] [CrossRef]
- Mei, Z.; Shen, Z.; Zhao, Q.; Yuan, T.; Zhang, Y.; Xiang, F.; Wang, W. Removing and recovering gas-phase elemental mercury by CuxCO3-xO4 (0.75 ≤ x ≤ 2.25) in the presence of sulphur compounds. Chemosphere 2008, 70, 1399–1404. [Google Scholar] [CrossRef] [PubMed]

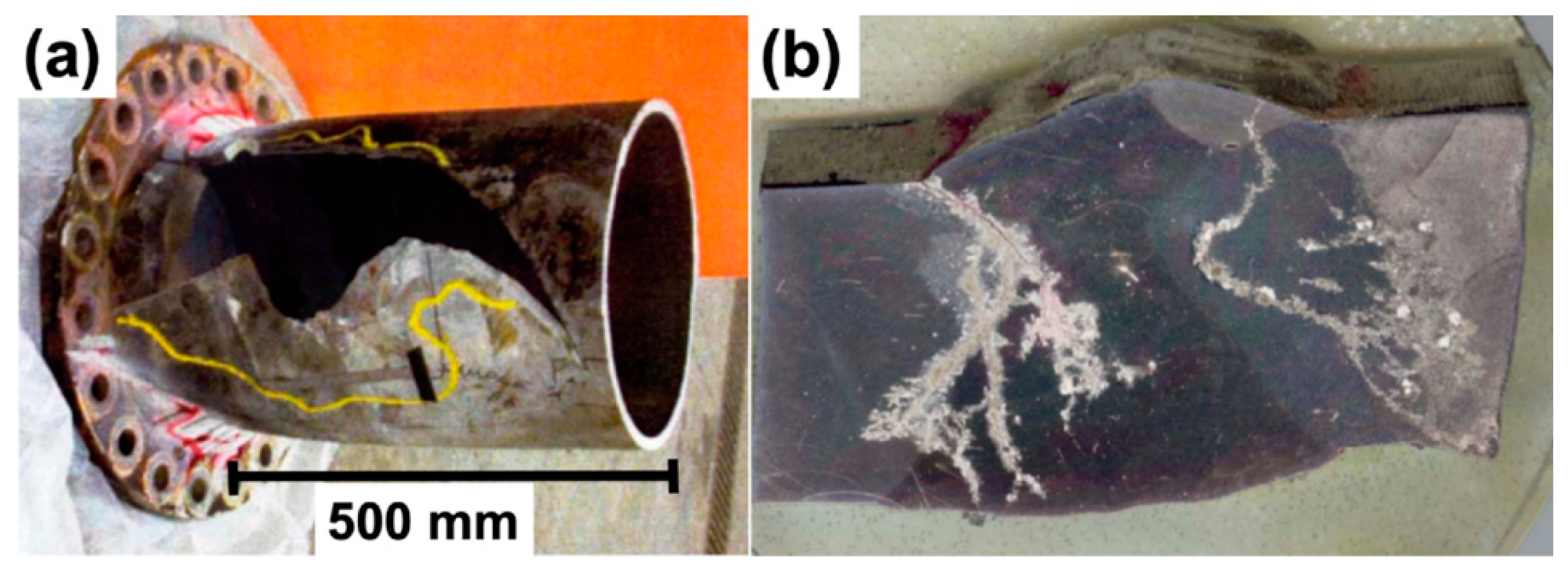
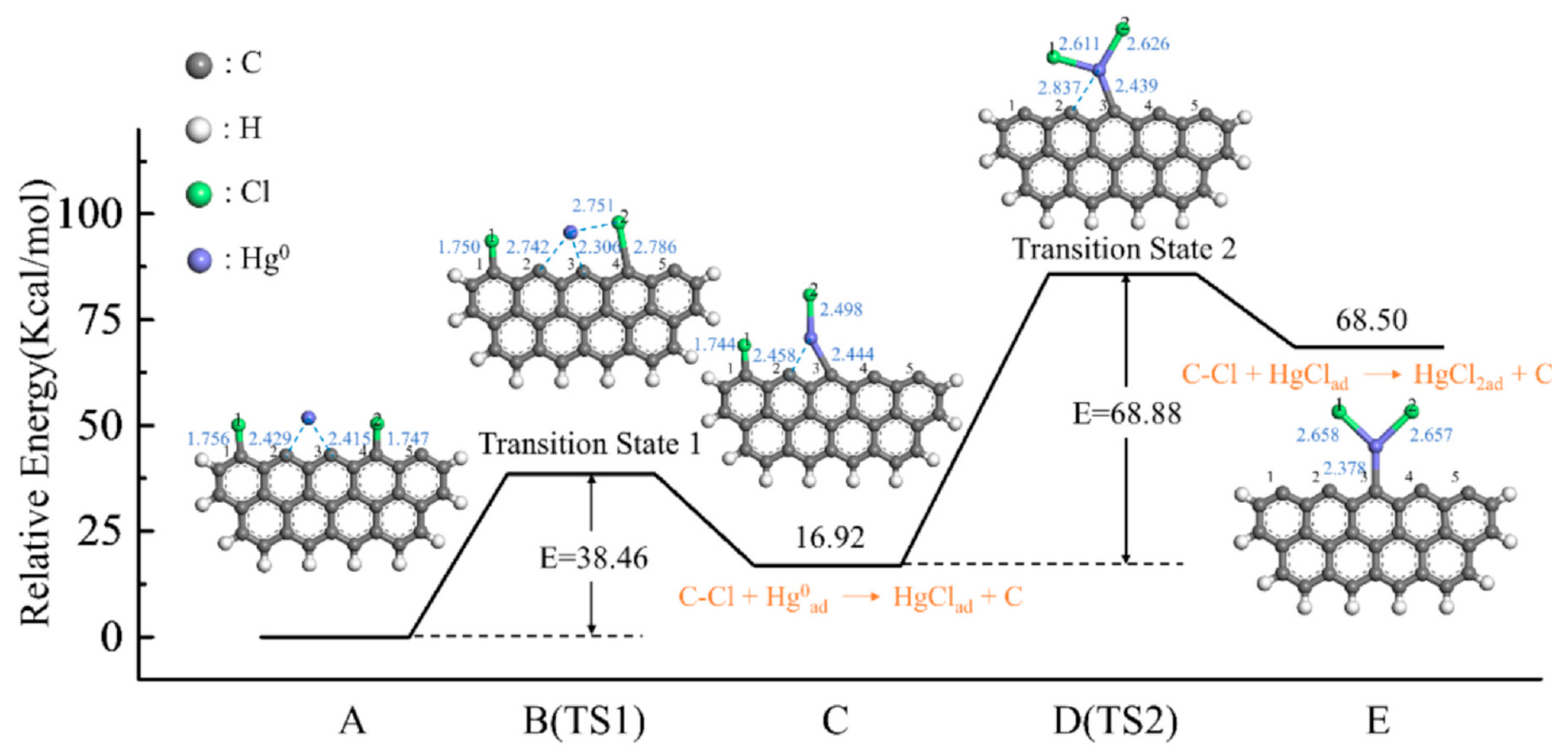



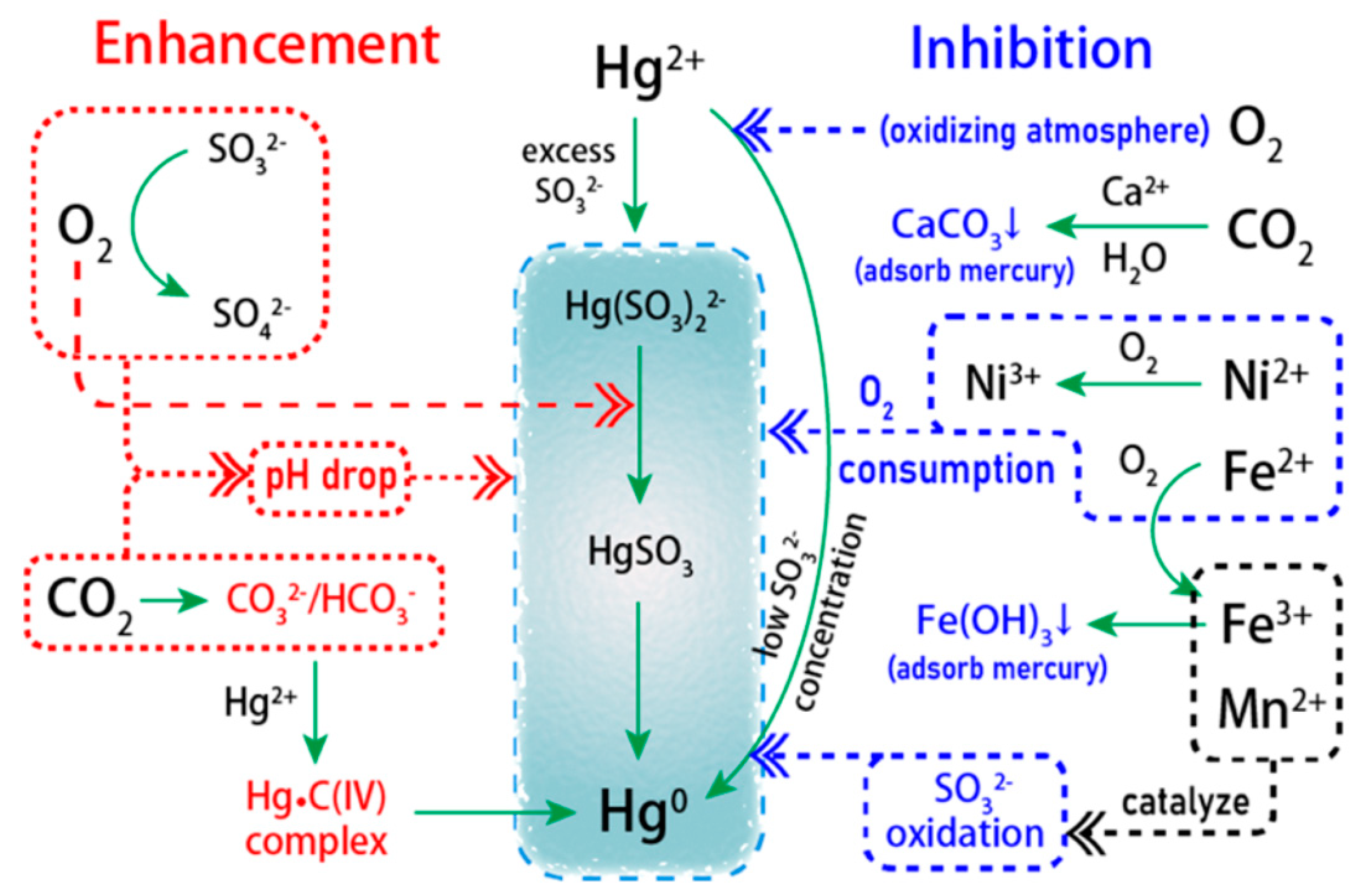


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.; Xin, F. Review on Mercury Control during Co-Firing Coal and Biomass under O2/CO2 Atmosphere. Appl. Sci. 2024, 14, 4209. https://doi.org/10.3390/app14104209
Lyu Q, Xin F. Review on Mercury Control during Co-Firing Coal and Biomass under O2/CO2 Atmosphere. Applied Sciences. 2024; 14(10):4209. https://doi.org/10.3390/app14104209
Chicago/Turabian StyleLyu, Qiang, and Fei Xin. 2024. "Review on Mercury Control during Co-Firing Coal and Biomass under O2/CO2 Atmosphere" Applied Sciences 14, no. 10: 4209. https://doi.org/10.3390/app14104209



