The Spatial Niche and Influencing Factors of Desert Rodents
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
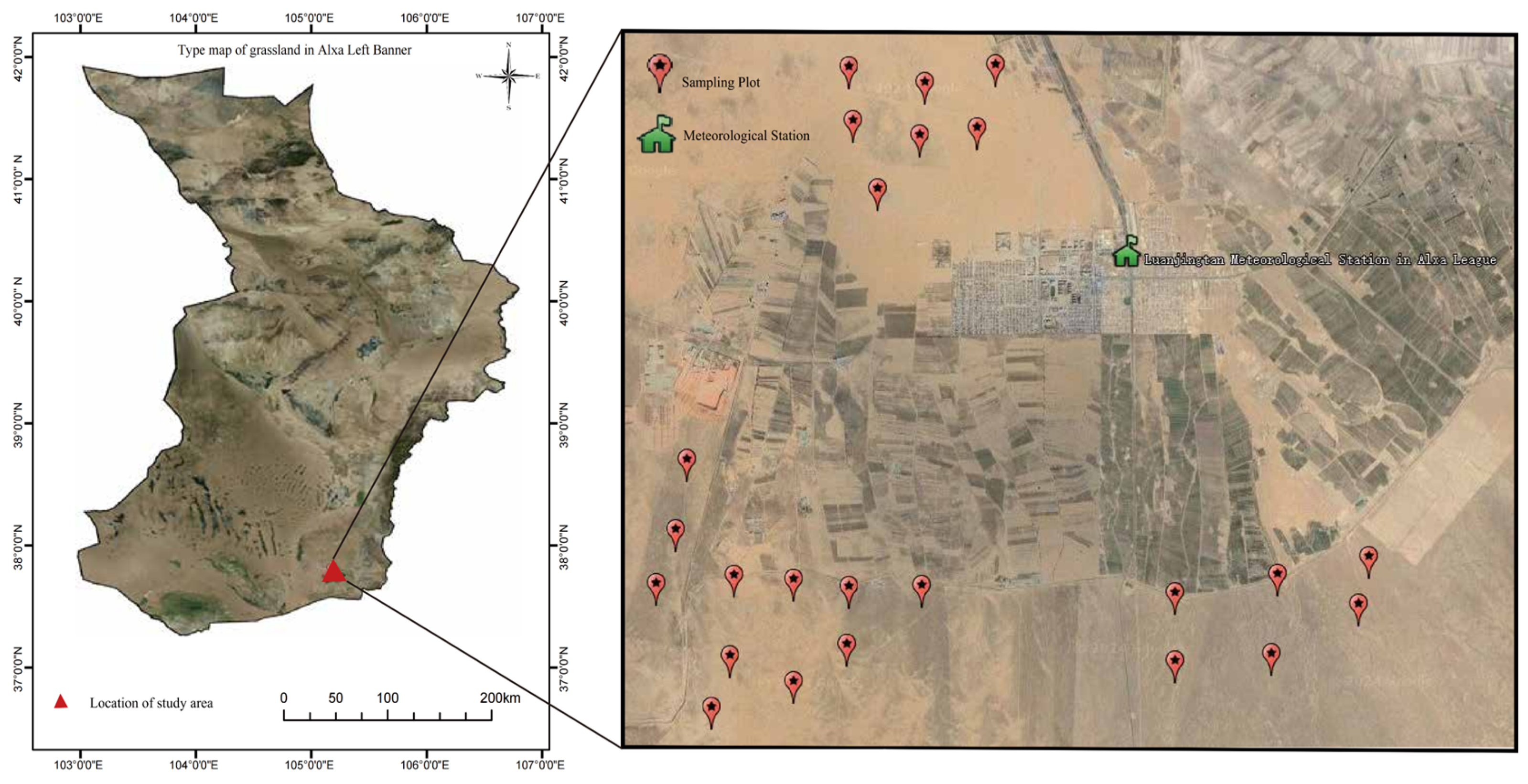
2.1. Study Area
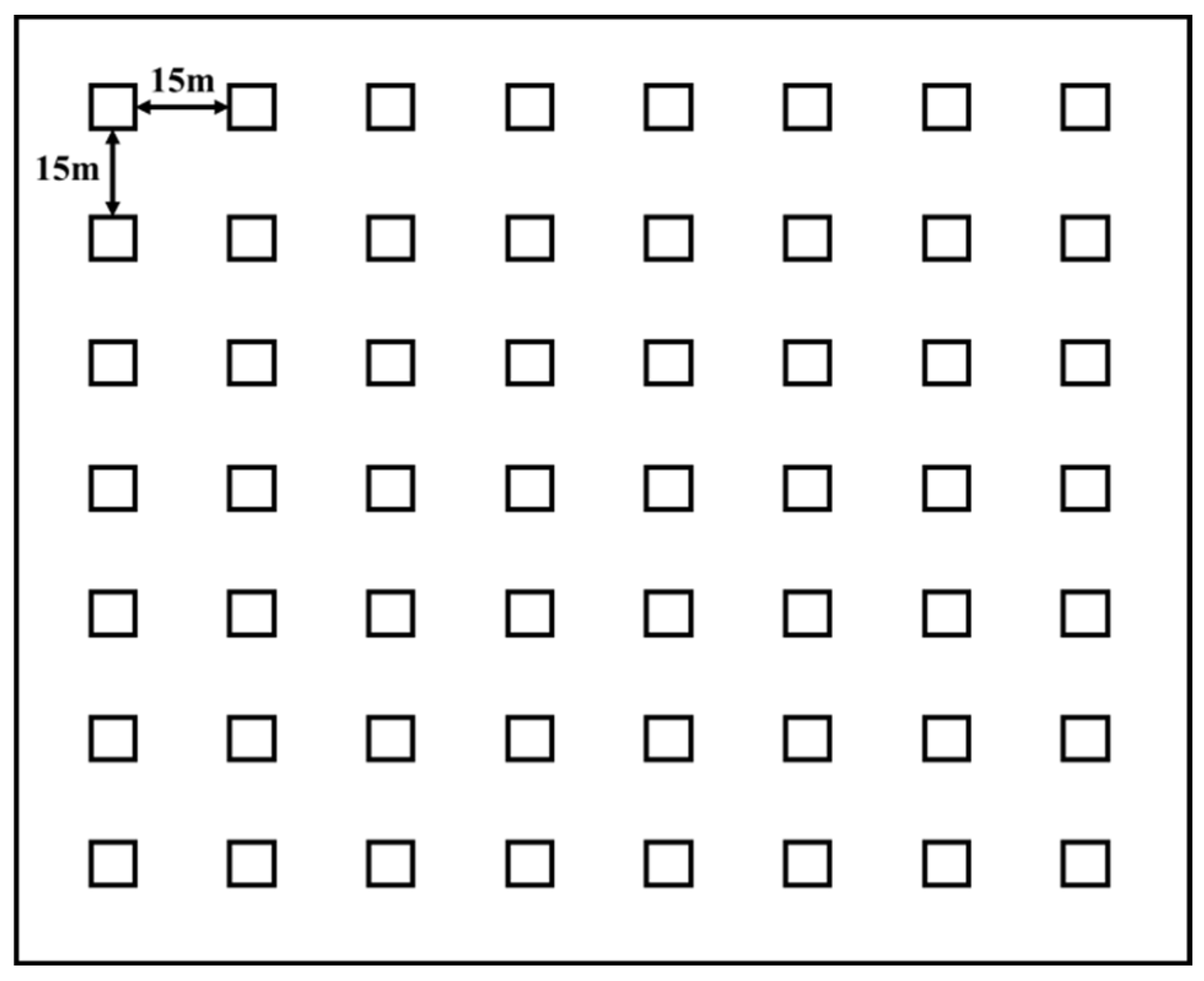
2.2. Data Collection
2.2.1. Rodent Data Collection
2.2.2. Environmental Factor Data Collection
2.3. Statistical Analyses
3. Results
3.1. Population Density of Rodents in Desert Areas
3.2. Spatial Niche Characteristics of Rodents in Desert Areas
3.2.1. Spatial Niche Breadth of Rodents in Desert Areas
3.2.2. Spatial Niche overlap of Rodents in Desert Areas
3.3. Screening of Key Factors Affecting the Spatial Niche of Rodents
3.4. Response of Spatial Niche of Rodents to Environmental Changes in Desert Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhajeri, B.H.; Porto, L.M.V.; Maestri, R. Habitat Productivity Is a Poor Predictor of Body Size in Rodents. Curr. Zool. 2020, 66, 135–143. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Araújo, M.S.; Persson, L. Comparative Support for the Niche Variation Hypothesis That More Generalized Populations Also Are More Heterogeneous. Proc. Natl. Acad. Sci. USA 2007, 104, 10075–10079. [Google Scholar] [CrossRef]
- Brown, J.H.; Bartholomew, G.A. Periodicity and Energetics of Torpor in the Kangaroo Mouse, Microdipodops pallidus. Ecology 1969, 50, 705–709. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource Partitioning in Ecological Communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Farris, Z.J.; Gerber, B.D.; Karpanty, S.; Murphy, A.; Wampole, E.; Ratelolahy, F.; Kelly, M.J. Exploring and Interpreting Spatiotemporal Interactions between Native and Invasive Carnivores across a Gradient of Rainforest Degradation. Biol. Invasions 2020, 22, 2033–2047. [Google Scholar] [CrossRef]
- Daly, B.G.; Dickman, C.R.; Crowther, M.S. Selection of Habitat Components by Two Species of Agamid Lizards in Sandridge Desert, Central Australia. Austral Ecol. 2007, 32, 825–833. [Google Scholar] [CrossRef]
- Downes, S.; Bauwens, D. An Experimental Demonstration of Direct Behavioural Interference in Two Mediterranean Lacertid Lizard Species. Anim. Behav. 2002, 63, 1037–1046. [Google Scholar] [CrossRef]
- Kumstátová, T.; Brinke, T.; Tomková, S.; Fuchs, R.; Petrusek, A. Habitat Preferences of Tree Pipit (Anthus Trivialis) and Meadow Pipit (A. pratensis) at Sympatric and Allopatric Localities. J. Ornithol. 2004, 145, 334–342. [Google Scholar] [CrossRef]
- Merkle, J.A.; Stahler, D.R.; Smith, D.W. Interference Competition between Gray Wolves and Coyotes in Yellowstone National Park. Can. J. Zool. 2009, 87, 56–63. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Yao, B.; Xin, Z.; Bao, Y.; Tuo, D.; Yi, F.; Duan, R.; Li, X.; Wang, J.; et al. Niche of the Dominant Species in Wetland Ecosystem Enclosed by Extremely Dry Desert Region in Dunhuang Xihu. Acta Ecol. Sin. 2020, 40, 6841–6849. [Google Scholar] [CrossRef]
- Dangremond, E.M.; Pardini, E.A.; Knight, T.M. Apparent Competition with an Invasive Plant Hastens the Extinction of an Endangered Lupine. Ecology 2010, 91, 2261–2271. [Google Scholar] [CrossRef]
- Hunt, J.J.F.G.; Bonsall, M.B. The Effects of Colonization, Extinction and Competition on Co-Existence in Metacommunities. J. Anim. Ecol. 2009, 78, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Price, M.V. The Role of Microhabitat in Structuring Desert Rodent Communities. Ecology 1978, 59, 910–921. [Google Scholar] [CrossRef]
- Davis, C.L.; Rich, L.N.; Farris, Z.J.; Kelly, M.J.; Di Bitetti, M.S.; Blanco, Y.D.; Albanesi, S.; Farhadinia, M.S.; Gholikhani, N.; Hamel, S.; et al. Ecological Correlates of the Spatial Co-Occurrence of Sympatric Mammalian Carnivores Worldwide. Ecol. Lett. 2018, 21, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, G.; Zhou, Q.; Ma, L.; Wan, X.; Liu, W. Spatial Niche Partitioning of Coexisting Small Mammals in Sand Dunes. Ital. J. Zool. 2016, 83, 248–254. [Google Scholar] [CrossRef]
- Karanth, K.U.; Srivathsa, A.; Vasudev, D.; Puri, M.; Parameshwaran, R.; Kumar, N.S. Spatio-Temporal Interactions Facilitate Large Carnivore Sympatry across a Resource Gradient. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161860. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.; Qureshi, Q. Response of Leopards to Re-Introduced Tigers in Sariska Tiger Reserve, Western India. Int. J. Biodivers. Conserv. 2012, 4, 228–236. [Google Scholar] [CrossRef]
- Kelt, D.A. Comparative Ecology of Desert Small Mammals: A Selective Review of the Past 30 Years. J. Mammal. 2011, 92, 1158–1178. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Fu, H.; Cha, M.; Han, Y. The Variation Characteristics of the Beta Diversity of Rodent Communities in Fragmented Landscape in the Alxa Desert, China. Acta Theriol. Sin. 2013, 33, 133–143. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Hu, J. Mountain Frog Species Losing out to Climate Change around the Sichuan Basin. Sci. Total Environ. 2022, 806, 150605. [Google Scholar] [CrossRef]
- Schirmer, A.; Herde, A.; Eccard, J.A.; Dammhahn, M. Individuals in Space: Personality-Dependent Space Use, Movement and Microhabitat Use Facilitate Individual Spatial Niche Specialization. Oecologia 2019, 189, 647–660. [Google Scholar] [CrossRef]
- Monterroso, P.; Díaz-Ruiz, F.; Lukacs, P.M.; Alves, P.C.; Ferreras, P. Ecological Traits and the Spatial Structure of Competitive Coexistence among Carnivores. Ecology 2020, 101, e03059. [Google Scholar] [CrossRef]
- De Satgé, J.; Teichman, K.; Cristescu, B. Competition and Coexistence in a Small Carnivore Guild. Oecologia 2017, 184, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Sommers, P.; Chesson, P. Effects of Predator Avoidance Behavior on the Coexistence of Competing Prey. Am. Nat. 2019, 193, E132–E148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Microhabitat Selection and Spatial Niche Segregation of Three Sympatric Mammals During Summer in Altun Mountain National Nature Reserve. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2015. [Google Scholar]
- Li, X.; Wang, T. An Analysis of Relationship between Ecological Factors and Number of Species of Rodents in Shaanxi District. Acta Theriol. Sin. 1996, 16, 129–135. [Google Scholar] [CrossRef]
- Fragata, I.; Costa-Pereira, R.; Kozak, M.; Majer, A.; Godoy, O.; Magalhães, S. Specific sequence of arrival promotes coexistence via spatial niche pre-emption by the weak competitor. Ecol. Lett. 2022, 25, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Age Dvision of Dominant Rodent Species and Analysis of Age Structure under Different Disturbance in Desert Area. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Wang, L.; Zhou, S.; Sun, G. Circadian rhythms of activity, metabolic rate and body temperature in desert hamsters (Phodopus roborovskii). Acta Ecol. Sin. 2012, 32, 3182–3188. [Google Scholar] [CrossRef]
- Shuai, L.; Song, Y.; Kotler, B.P.; Embar, K.; Zeng, Z. Foraging Behaviour in East Asian Desert Rodents and Its Implications on Coexistence. Isr. J. Ecol. Evol. 2016, 62, 171–177. [Google Scholar] [CrossRef]
- Shuai, L.; Song, Y.-L. Foraging Behavior of the Midday Gerbil (Meriones meridianus): Combined Effects of Distance and Microhabitat. Behav. Process. 2011, 86, 143–148. [Google Scholar] [CrossRef]
- Zhao, K. The Ecology of Northern Three-Toed Jerboa (Dipus sagitta Pallas). Chin. J. Zool. 1964, 2, 59–62. [Google Scholar] [CrossRef]
- Song, K.; Liu, R. The ecology of midday gerbil (Meriones meridianus Pallas). Acta Theriol. Sin. 1984, 4, 291–300. [Google Scholar] [CrossRef]
- Kotler, B.P.; Brown, J.; Mukherjee, S.; Berger-Tal, O.; Bouskila, A. Moonlight Avoidance in Gerbils Reveals a Sophisticated Interplay among Time Allocation, Vigilance and State-Dependent Foraging. Proc. R. Soc. B Biol. Sci. 2010, 277, 1469–1474. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, S.; Wu, X.; Zhang, R.; Yue, X.; Ji, Y.; Li, L.; Li, X.; Fu, H. The Effect of Grazing on Winter Survival of Midday Gerbil (Meriones meridianus) of Different Genders. Ecol. Evol. 2020, 10, 12395–12406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fu, H.; Wu, X.; Yang, S.; Malqin, X.; Yue, X. Effects of Grazing on the Northern Three-toed Jerboa Pre- and Post-hibernation. J. Wildl. Manag. 2018, 82, 1588–1597. [Google Scholar] [CrossRef]
- Krebs, C.J. Demographic Changes in Fluctuating Populations of Microtus californicus. Ecol. Monogr. 1966, 36, 239–273. [Google Scholar] [CrossRef]
- Yuan, S. Response Mechanism of Desert Rodent Communities and Dominant Species to Different Disturbances. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2013. [Google Scholar]
- Sun, R. Principles of Animal Ecology, 3rd ed.; Beijing Normal University Publishing Group: Beijing, China, 2005; ISBN 978-7-303-01389-0. [Google Scholar]
- Colwell, R.K.; Futuyma, D.J. On the Measurement of Niche Breadth and Overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Response Mechanism of Rodent Metacommunity in Desert Area to Disturbance and Climate Change. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2021. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.2-0; R Foundation: Vienna, Austria, 2018. [Google Scholar]
- Shang, Y. Animal Behaviour, 2nd ed.; Peking University Press: Beijing, China, 2005; ISBN 978-7-301-24847-8. [Google Scholar]
- Fu, H.; Wu, X.; Yang, Z. Niche Characteristics of Rodents by Diverse Disturbance in Alashan Desert, Inner Mongolia, China. Front. Biol. China 2007, 2, 456–462. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, S.; Fu, H.; Zhang, X.; Zhang, F.; Gao, Q. Responses of Dominant Rodent Species to Climate Change in Different Disturbed Habitats in the Alashan Desert. Acta Ecol. Sin. 2016, 36, 1765–1773. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yang, Y.; Dong, W. Niche Characteristics of Rodents in Different Habitats in Kubuqi Sandy Land. Chin. J. Grassl. 2020, 42, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Spatial Niche of the Rodents in Summer in Tangjiahe Nature Reserve. Acta Theriol. Sin. 2005, 23, 39–44. [Google Scholar] [CrossRef]
- Brown, J.S. Desert Rodent Community Structure: A Test of Four Mechanisms of Coexistence. Ecol. Monogr. 1989, 59, 1–20. [Google Scholar] [CrossRef]
- Howard, W.E. Relation between Low Temperature and Available Food to Survival of Small Rodents. J. Mammal. 1951, 32, 300–312. [Google Scholar] [CrossRef]
- Kenagy, G.J. Daily and Seasonal Patterns of Activity and Energetics in a Heteromyid Rodent Community. Ecology 1973, 54, 1201–1219. [Google Scholar] [CrossRef]
- Reichman, O.J.; Brown, J.H. The Use of Torpor by Perognathus amplus in Relation to Resource Distribution. J. Mammal. 1979, 60, 550–555. [Google Scholar] [CrossRef]
- Calder, W.A. Size, Function, and Life History; Dover Publications: Mineola, NY, USA, 1996; ISBN 978-0-486-69191-6. [Google Scholar]
- Bowers, M.A.; Brown, J.H. Body Size and Coexistnce in Desert Rodents: Chance or Community Structure? Ecology 1982, 63, 391–400. [Google Scholar] [CrossRef]
- Cha, M.; Wu, X.; Fu, H.; Yuan, S.; Wu, Y.; Zhang, X. An Empirical Research of Rodent Metacommunities in Alashan Desert. Acta Ecol. Sin. 2015, 35, 5612–5622. [Google Scholar] [CrossRef]
- Yuan, S.; Fu, H.; Wu, X.; Xing, A.; Gan, H.; Yue, X. Response of Dominant Desert Rodent Species to Grazing Disturbances. Acta Ecol. Sin. 2017, 37, 4795–4806. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Bao, W. Evaluation on the Roles of Rodents in Erdos Sandland. Grassl. China 2000, 2, 35–41. [Google Scholar] [CrossRef]
- Dong, W.; Hou, X.; Yang, Y. A Study on the Population Dynamics of Allactage sibirica Jerboa in the Central and Western Region of Inner Mongoia. Chin. J. Vector Biol. Control 2006, 17, 444–446. [Google Scholar] [CrossRef]
- Ji, Y. Affecting Factors of the Population Density of Rodents in Different Grassland Habitat and the Causes of Coexistence in the Same Area. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2021. [Google Scholar]
- Zhu, C. The Niche Ecostate-Ecorole Theory and Expansion Hypothesis. Acta Ecol. Sin. 1997, 17, 324–332. [Google Scholar]
- Roughgarden, J. Niche Width: Biogeographic Patterns Among Anolis Lizard Populations. Am. Nat. 1974, 108, 429–442. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Frantz, A.C.; Cowan, D.P.; White, P.C.L.; Searle, J.B. Tapering Bias Inherent in Minimum Number Alive (MNA) Population Indices. J. Mammal. 2004, 85, 959–962. [Google Scholar] [CrossRef]

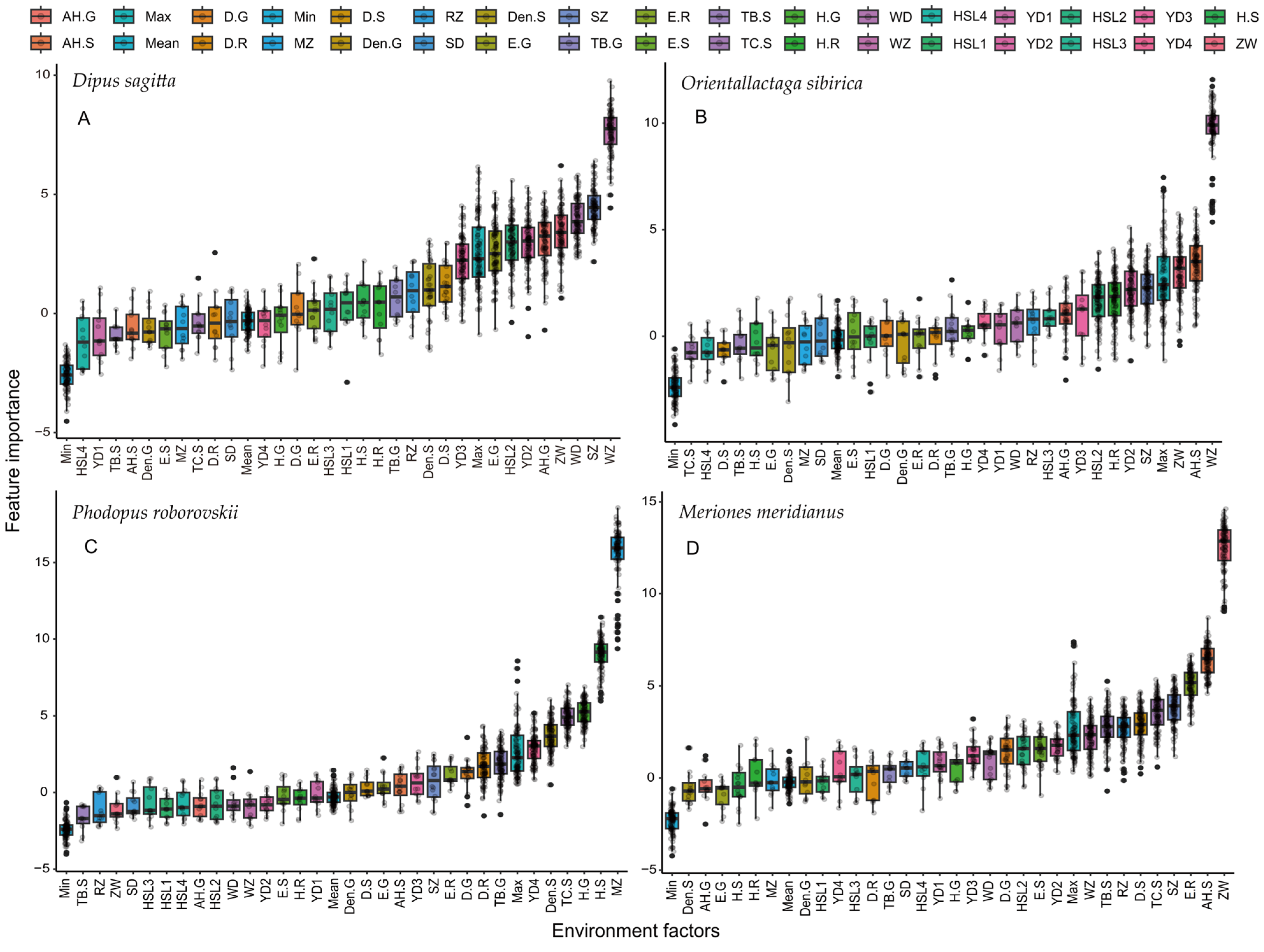

| Environmental Factor | Acronym | Environmental Factor | Acronym |
|---|---|---|---|
| Simpson diversity index of rodents | D.R | Shannon-Wiener index of shrub | H.S |
| Pielou diversity index of rodents | E.R | Soil water content of 0–5 cm | HSL1 |
| Shannon–Wiener index of rodents | H.S | Soil water content of 5–10 cm | HSL2 |
| Population of M. meridianus | ZW | Soil water content of 10–15 cm | HSL3 |
| Population of D. sagitta | SZ | Soil water content of 15–20 cm | HSL4 |
| Population of P. roborovskii | MZ | Soil hardness of 0–5 cm | YD1 |
| Population of O. sibirica | WZ | Soil hardness of 5–10 cm | YD2 |
| Average height of grasses | AH.G | Soil hardness of 10–15 cm | YD3 |
| Average height of a shrub | AH.S | Soil hardness of 15–20 cm | YD4 |
| Simpson diversity index of grasses | D.G | Biomass of grasses | TB.G |
| Simpson diversity index of shrub | D.S | Biomass of shrub | TB.S |
| Density of grasses | Den.G | Coverage of shrub | TC.S |
| Density of shrub | Den.S | Monthly mean relative humidity | SD |
| Pielou diversity index of grasses | E.G | Monthly mean relative temperature | WD |
| Pielou diversity index of shrub | E.S | Monthly mean sunshine duration | RZ |
| Shannon–Wiener index of grasses | H.G |
| Method | Species | Spring (Mean ± se) | Summer (Mean ± se) | Autumn (Mean ± se) | Season |
|---|---|---|---|---|---|
| Simple Effects | D. sagitta | 0.70 ± 0.06 Aa | 0.74 ± 0.03 Aa | 0.65 ± 0.01 Aa | F(2,12) = 1.473, p > 0.05, η2 = 0.160 |
| O. sibirica | 0.71 ± 0.05 Aa | 0.75 ± 0.02 Aa | 0.67 ± 0.0 Aa | F(2,12) = 1.199, p = 0.309, η2 = 0.145 | |
| P. roborovskii | 0.42 ± 0.07 Bab | 0.51 ± 0.06 Ba | 0.32 ± 0.09 Bb | F(2,12) = 6.060, p < 0.05, η2 = 0.447 | |
| M. meridianus | 0.85 ± 0.03 Aa | 0.85 ± 0.05 Aa | 0.76 ± 0.06 Aa | F(2,12) = 1.024, p = 0.383, η2 = 0.120 | |
| Species | F(3,16) = 10.288, p < 0.01, η2 = 0.659 | F(3,16) = 13.536, p < 0.001, η2 = 0.717 | F(3,16) = 11.622, p < 0.001, η2 = 0.685 | — | |
| Repeated Measures | Species | F = 29.866, p < 0.001, η2 = 0.848 | |||
| Season | F = 5.141, p < 0.05, η2 = 0.243 | ||||
| Species × Season | F = 0.314, p > 0.05, η2 = 0.056 | ||||
| Season | Species | D. sagitta | O. sibirica | P. roborovskii | M. meridianus |
|---|---|---|---|---|---|
| Spring | D. sagitta | 1 | |||
| O. sibirica | 0.18 ± 0.02 | 1 | |||
| P. roborovskii | 0.33 ± 0.10 | 0.14 ± 0.07 | 1 | ||
| M. meridianus | 0.45 ± 0.05 | 0.14 ± 0.07 | 0.17 ± 0.07 | 1 | |
| Summer | D. sagitta | 1 | |||
| O. sibirica | 0.16 ± 0.01 | 1 | |||
| P. roborovskii | 0.55 ± 0.04 | 0.07 ± 0.03 | 1 | ||
| M. meridianus | 0.45 ± 0.03 | 0.07 ± 0.03 | 0.28 ± 0.06 | 1 | |
| Autumn | D. sagitta | 1 | |||
| O. sibirica | 0.11 ± 0.05 | 1 | |||
| P. roborovskii | 0.35 ± 0.09 | 0.04 ± 0.04 | 1 | ||
| M. meridianus | 0.26 ± 0.043 | 0.04 ± 0.04 | 0.16 ± 0.05 | 1 |
| Environment Factor | Full Models | Forward Selection | |||||
|---|---|---|---|---|---|---|---|
| RDA1 | RDA2 | r2 | p | r2 | F | p | |
| WD | −0.836 | −0.549 | 0.020 | 0.739 | |||
| RZ | 0.119 | 0.993 | 0.024 | 0.715 | |||
| AH.G | −0.315 | 0.949 | 0.054 | 0.483 | |||
| TB.G | 0.889 | 0.458 | 0.157 | 0.101 | |||
| E.G | −0.563 | −0.826 | 0.009 | 0.889 | |||
| AH.S | 0.562 | 0.827 | 0.377 | 0.003 ** | 0.173 | 10.323 | 0.005 |
| Den.S | −1.000 | −0.032 | 0.228 | 0.03 * | |||
| TB.S | 0.225 | 0.974 | 0.055 | 0.476 | |||
| TC.S | −0.961 | −0.278 | 0.136 | 0.117 | |||
| D.S | −0.691 | −0.723 | 0.085 | 0.298 | 0.059 | 4.535 | 0.030 |
| HSL | 0.452 | −0.892 | 0.061 | 0.457 | |||
| YD | −1.000 | 0.029 | 0.057 | 0.450 | |||
| SZ | −0.994 | 0.114 | 0.040 | 0.591 | |||
| WZ | −0.173 | −0.985 | 0.088 | 0.294 | |||
| MZ | −0.996 | −0.093 | 0.308 | 0.007 ** | 0.268 | 10.266 | 0.001 |
| ZW | −0.188 | 0.982 | 0.222 | 0.037 * | 0.067 | 4.571 | 0.023 |
| E.R | 0.626 | −0.780 | 0.374 | 0.003 ** | 0.123 | 5.471 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, N.; Ming, M.; Li, L.-L.; Bu, F.; Wu, X.-D.; Yuan, S.; Fu, H.-P. The Spatial Niche and Influencing Factors of Desert Rodents. Animals 2024, 14, 734. https://doi.org/10.3390/ani14050734
Li X, Zhu N, Ming M, Li L-L, Bu F, Wu X-D, Yuan S, Fu H-P. The Spatial Niche and Influencing Factors of Desert Rodents. Animals. 2024; 14(5):734. https://doi.org/10.3390/ani14050734
Chicago/Turabian StyleLi, Xin, Na Zhu, Ming Ming, Lin-Lin Li, Fan Bu, Xiao-Dong Wu, Shuai Yuan, and He-Ping Fu. 2024. "The Spatial Niche and Influencing Factors of Desert Rodents" Animals 14, no. 5: 734. https://doi.org/10.3390/ani14050734






