Fine-Scale Interactions between Leopard Cats and Their Potential Prey with Contrasting Diel Activities in a Livestock-Dominated Nature Reserve
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Camera Trap Survey
2.3. Covariates
2.4. Spatial Analysis
2.5. Temporal Activity Patterns
3. Results
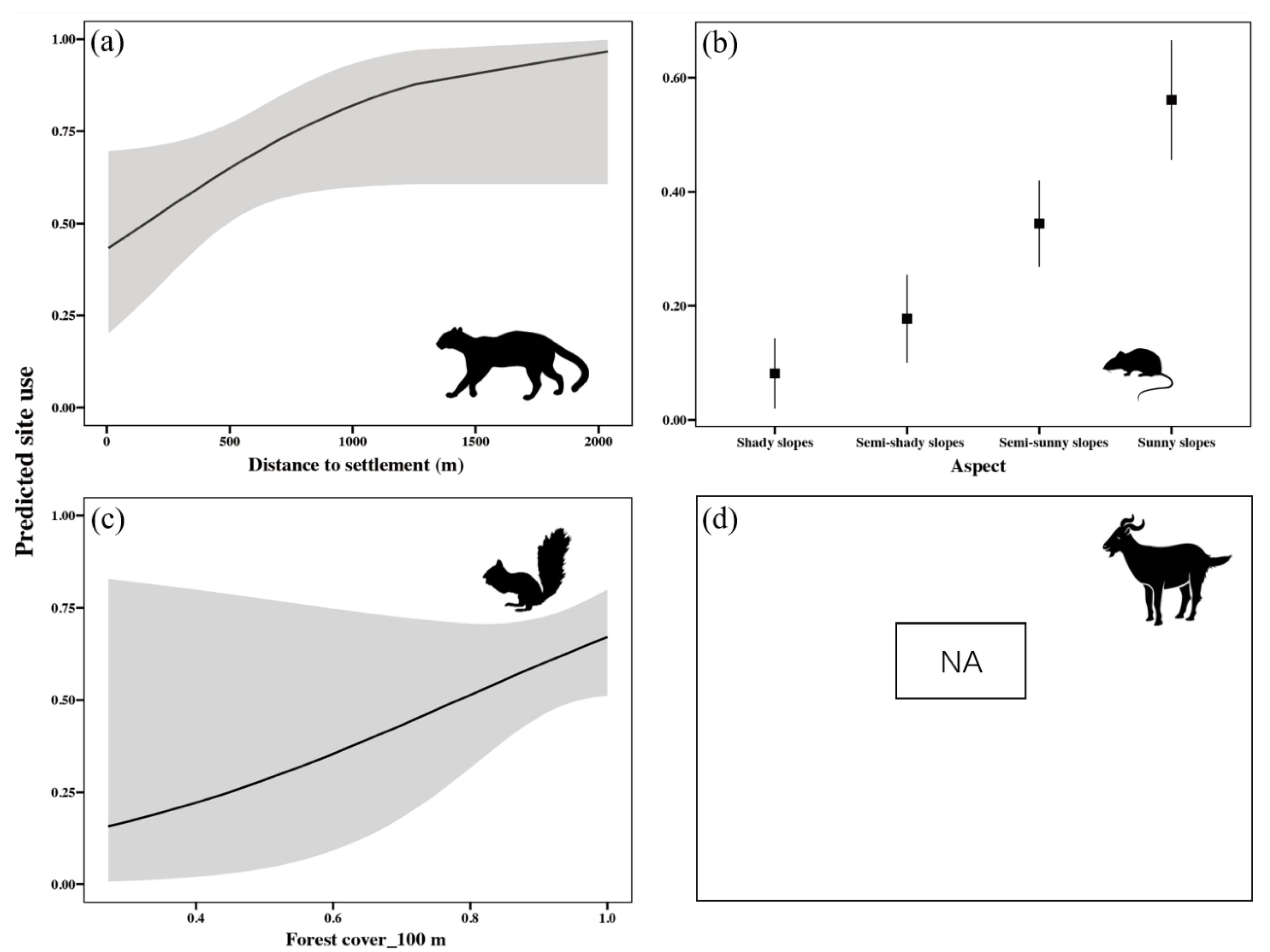
3.1. Site Use and Driving Factors for the Leopard Cat and Its Prey
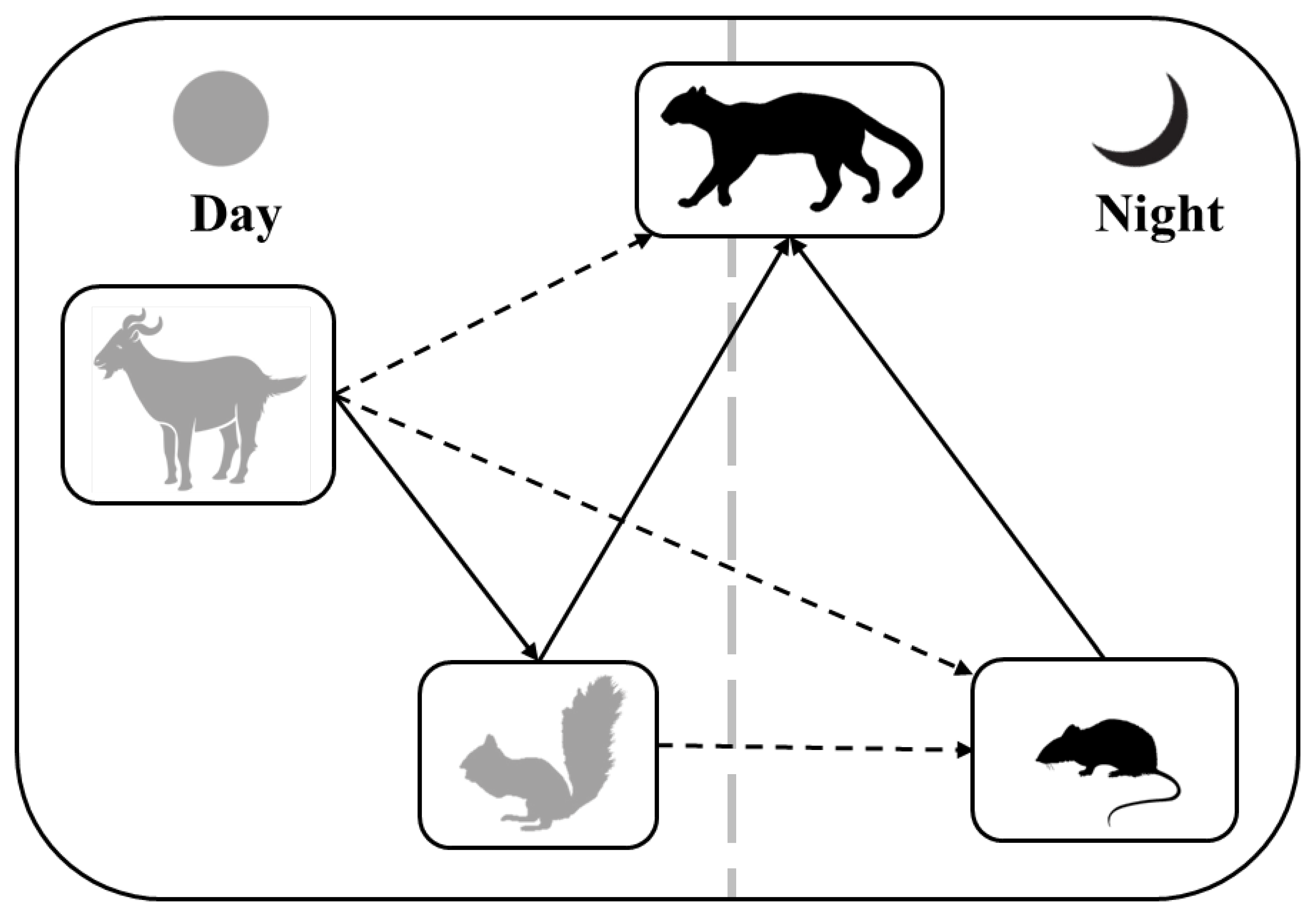
3.2. Direct and Indirect Effects on the Interactions between the Leopard Cat and Its Prey
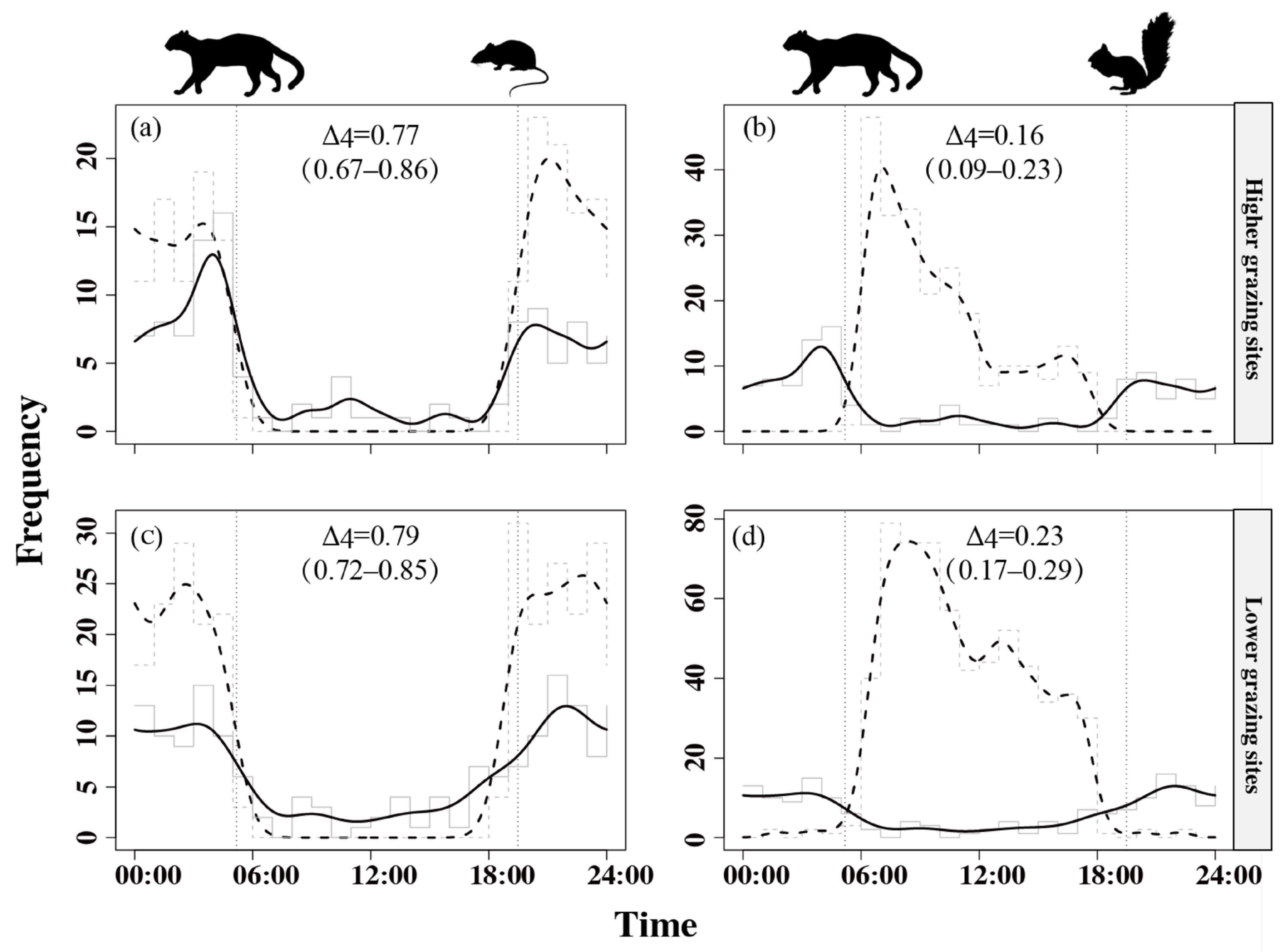
3.3. Temporal Activity Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trainor, A.M.; Schmitz, O.J.; Ivan, J.S.; Shenk, T.M. Enhancing species distribution modeling by characterizing predator-prey interactions. Ecol. Appl. 2014, 24, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Carbone, C.; Wearn, O.R.; Rowcliffe, J.M.; Espinosa, S.; Lima, M.G.M.; Ahumada, J.A.; Goncalves, A.L.S.; Trevelin, L.C.; Alvarez-Loayza, P.; et al. Prey availability and temporal partitioning modulate felid coexistence in Neotropical forests. PLoS ONE 2019, 14, e0213671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilella, M.; Ferrandiz-Rovira, M.; Sayol, F. Coexistence of predators in time: Effects of season and prey availability on species activity within a Mediterranean carnivore guild. Ecol. Evol. 2020, 10, 11408–11422. [Google Scholar] [CrossRef]
- Hood, A.S.C.; Aryawan, A.A.K.; Advento, A.D.; Purnomo, D.; Wahyuningsih, R.; Luke, S.H.; Ps, S.; Snaddon, J.L.; Foster, W.A.; Caliman, J.P.; et al. Understory vegetation in oil palm plantations promotes leopard cat activity, but does not affect rats or rat damage. Front. For. Glob. Chang. 2019, 2, 51. [Google Scholar] [CrossRef]
- Ferreiro-Arias, I.; Isla, J.; Jordano, P.; Benitez-Lopez, A. Fine-scale coexistence between Mediterranean mesocarnivores is mediated by spatial, temporal, and trophic resource partitioning. Ecol. Evol. 2021, 11, 15520–15533. [Google Scholar] [CrossRef]
- Wang, Y.W.; Allen, M.L.; Wilmers, C.C. Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol. Conserv. 2015, 190, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.J.; Yang, H.T.; Xie, B.; Gong, Y.N.; Ge, J.P.; Feng, L.M. Spatio-temporal coexistence of sympatric mesocarnivores with a single apex carnivore in a fine-scale landscape. Glob. Ecol. Conserv. 2020, 21, e00897. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Pudyatmoko, S. Free-ranging livestock influence species richness, occupancy, and daily behaviour of wild mammalian species in Baluran National Park, Indonesia. Mamm. Biol. 2017, 86, 33–41. [Google Scholar] [CrossRef]
- Li, B.B.V.; Jiang, B.K. Responses of forest structure, functions, and biodiversity to livestock disturbances: A global meta-analysis. Glob. Chang. Biol. 2021, 27, 4745–4757. [Google Scholar] [CrossRef]
- Zhang, J.D.; Hull, V.; Ouyang, Z.Y.; Li, R.G.; Connor, T.; Yang, H.B.; Zhang, Z.J.; Silet, B.; Zhang, H.M.; Liu, J.G. Divergent responses of sympatric species to livestock encroachment at fine spatiotemporal scales. Biol. Conserv. 2017, 209, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The influence of human disturbance on wildlife nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [Green Version]
- Prugh, L.R.; Stoner, C.J.; Epps, C.W.; Bean, W.T.; Ripple, W.J.; Laliberte, A.S.; Brashares, J.S. The Rise of the Mesopredator. Bioscience 2009, 59, 779–791. [Google Scholar] [CrossRef]
- Shao, X.N.; Lu, Q.; Liu, M.Z.; Xiong, M.Y.; Bu, H.L.; Wang, D.J.; Liu, S.Y.; Zhao, J.D.; Li, S.; Yao, M. Generalist carnivores can be effective biodiversity samplers of terrestrial vertebrates. Front. Ecol. Environ. 2021, 19, 557–563. [Google Scholar] [CrossRef]
- Horncastle, V.J.; Chambers, C.L.; Dickson, B.G. Grazing and wildfire effects on small mammals inhabiting montane meadows. J. Wildl. Manag. 2019, 83, 534–543. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Ripple, W.J.; Chapron, G.; Lopez-Bao, J.V.; Durant, S.M.; Macdonald, D.W.; Lindsey, P.A.; Bennett, E.L.; Beschta, R.L.; Bruskotter, J.T.; Campos-Arceiz, A.; et al. Saving the World’s Terrestrial Megafauna. Bioscience 2016, 66, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Wang, J.; Zhu, Y.J.; Bu, X.L.; Xiang, R.W.; Lu, Q.B.; Cui, S.P.; Hao, Y.H.; Sheng, Y.; Meng, X.X. Summer habitat selection and impacts of human disturbance on leopard cats (Prionailurus bengalensis). Ecosyst. Health Sustain. 2020, 6, 1856630. [Google Scholar] [CrossRef]
- Marneweck, C.J.; Allen, B.L.; Butler, A.R.; San, E.D.; Harris, S.N.; Jensen, A.J.; Saldo, E.A.; Somers, M.J.; Titus, K.; Muthersbaugh, M.; et al. Middle-out ecology: Small carnivores as sentinels of global change. Mammal Rev. 2022, 52, 471–479. [Google Scholar] [CrossRef]
- Vitekere, K.; Wang, J.; Karanja, H.; Consolee, K.T.; Jiang, G.S.; Hua, Y. Dynamic in Species Estimates of Carnivores (Leopard Cat, Red Fox, and North Chinese Leopard): A Multi-Year Assessment of Occupancy and Coexistence in the Tieqiaoshan Nature Reserve, Shanxi Province, China. Animals 2020, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, Y.; Liu, S.; Jiang, X.; Zhou, K.; Hu, H.; Ding, C. China’s Red List of Biodiversity: Vertebrates, Volume I, Mammals; Science Press: Beijing, China, 2021. [Google Scholar]
- Bandyopadhyay, M.; Burton, A.C.; Gupta, S.K.; Krishnamurthy, R. Understanding the distribution and fine-scale habitat selection of mesocarnivores along a habitat quality gradient in western Himalaya. PeerJ 2022, 10, e13993. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.A.H.; Sivasothi, N.; Meier, R. Population density, spatiotemporal use and diet of the leopard cat (Prionailurus bengalensis) in a human-modified succession forest landscape of Singapore. Mammal Res. 2016, 61, 99–108. [Google Scholar] [CrossRef]
- Silmi, M.; Putra, K.; Amran, A.; Huda, M.; Fanani, A.F.; Galdikas, B.M.; Anggara, S.P.; Traeholt, C. Activity and Ranging Behavior of Leopard Cats (Prionailurus bengalensis) in an Oil Palm Landscape. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Kamler, J.F.; Inthapanya, X.; Rasphone, A.; Bousa, A.; Vongkhamheng, C.; Johnson, A.; Macdonald, D.W. Diet, prey selection, and activity of Asian golden cats and leopard cats in northern Laos. J. Mammal. 2020, 101, 1267–1278. [Google Scholar] [CrossRef]
- Grassman, L.I.; Tewes, M.E.; Silvy, N.J.; Kreetiyutanont, K. Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J. Zool. 2005, 266, 45–54. [Google Scholar] [CrossRef]
- Chen, M.-T.; Liang, Y.-J.; Kuo, C.-C.; Pei, K.J.-C. Home Ranges, Movements and Activity Patterns of Leopard Cats (Prionailurus bengalensis) and Threats to Them in Taiwan. Mammal Study 2016, 41, 77–86. [Google Scholar] [CrossRef]
- Xiong, M.Y.; Wang, D.J.; Bu, H.L.; Shao, X.N.; Zhang, D.; Li, S.; Wang, R.J.; Yao, M. Molecular dietary analysis of two sympatric felids in the Mountains of Southwest China biodiversity hotspot and conservation implications. Sci. Rep. 2017, 7, 41909. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, A. Notes on the Behavior and Movements of Leopard Cats, Felis-Bengalensis, in a Dry Tropical Forest Mosaic in Thailand. Biotropica 1990, 22, 397–403. [Google Scholar] [CrossRef]
- Johnston, A.N.; Anthony, R.G. Small-Mammal Microhabitat Associations and Response to Grazing in Oregon. J. Wildl. Manag. 2008, 72, 1736–1746. [Google Scholar] [CrossRef]
- Randler, C.; Kalb, J. Predator avoidance behavior of nocturnal and diurnal rodents. Behav. Process. 2020, 179, 104214. [Google Scholar] [CrossRef]
- Schieltz, J.M.; Rubenstein, D.I. Evidence based review: Positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ. Res. Lett. 2016, 11, 113003. [Google Scholar] [CrossRef] [Green Version]
- Vitekere, K.; Lango, L.M.; Wang, J.; Zhu, M.Y.; Jiang, G.S.; Hua, Y. Threats to Site Occupation of Carnivores: A Spatiotemporal Encroachment of Non-native Species on the Native Carnivore Community in A Human-dominated Protected Area. Zool. Stud. 2021, 60, e52. [Google Scholar] [CrossRef]
- Powers, B.; Johnson, M.D.; LaManna, J.A.; Rich, A. The Influence of Cattle Grazing on Pocket Gophers in the Central Sierra Nevada Mountains, California: Potential Implications for Great Gray Owls. Northwest. Nat. 2011, 92, 13–18. [Google Scholar] [CrossRef]
- Widodo, F.A.; Imron, M.A.; Sunarto, S.; Giordano, A.J. Carnivores and their prey in Sumatra: Occupancy and activity in human-dominated forests. PLoS ONE 2022, 17, e0265440. [Google Scholar] [CrossRef]
- Tian, J.P.; Zou, Q.X.; Zhang, M.M.; Hu, C.S.; Khattak, R.H.; Su, H.J. Spatial and temporal differentiation are not distinct but are covariant for facilitating coexistence of small and medium-sized carnivores in Southwestern China. Glob. Ecol. Conserv. 2022, 34, e02017. [Google Scholar] [CrossRef]
- Feng, J.W.; Sun, Y.F.; Li, H.L.; Xiao, Y.Q.; Zhang, D.D.; Smith, J.L.D.; Ge, J.P.; Wang, T.M. Assessing mammal species richness and occupancy in a Northeast Asian temperate forest shared by cattle. Divers. Distrib. 2021, 27, 857–872. [Google Scholar] [CrossRef]
- Reher, S.; Dausmann, K.H.; Warnecke, L.; Turner, J.M. Food availability affects habitat use of Eurasian red squirrels (Sciurus vulgaris) in a semi-urban environment. J. Mammal. 2016, 97, 1543–1554. [Google Scholar] [CrossRef] [Green Version]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Marquez, J.R.G.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R.; Miller, D.; Royle, A.; Kery, M.; Hostetler, J.; Hutchinson, R.; Royle, M.A. Package ‘unmarked’. In R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- MacKenzie, D.I.; Bailey, L.L.; Nichols, J.D. Investigating species co-occurrence patterns when species are detected imperfectly. J. Anim. Ecol. 2004, 73, 546–555. [Google Scholar] [CrossRef]
- Mazerolle, M.J.; Mazerolle, M.M.J. Package ‘AICcmodavg’. In R Package; R Foundation for Statistical Computing: Vienna, Austria, 2017; Volume 281. [Google Scholar]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- West, S.G.; Taylor, A.B.; Wu, W. Model fit and model selection in structural equation modeling. Handb. Struct. Equ. Model. 2012, 1, 209–231. [Google Scholar]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns From Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Mohamed, A.; Sollmann, R.; Bernard, H.; Ambu, L.N.; Lagan, P.; Mannan, S.; Hofer, H.; Wilting, A. Density and habitat use of the leopard cat (Prionailurus bengalensis) in three commercial forest reserves in Sabah, Malaysian Borneo. J. Mammal. 2013, 94, 82–89. [Google Scholar] [CrossRef]
- Young, J.K.; Olson, K.A.; Reading, R.P.; Amgalanbaatar, S.; Berger, J. Is Wildlife Going to the Dogs? Impacts of Feral and Free-roaming Dogs on Wildlife Populations. BioScience 2011, 61, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; McShea, W.; Diao, Y.X.; Yang, H.B.; Zhang, X.F.; Gu, B.J.; Bu, H.L.; Wang, F. The incursion of free-ranging dogs into protected areas: A spatio-temporal analysis in a network of giant panda reserves. Biol. Conserv. 2022, 265, 109423. [Google Scholar] [CrossRef]
- Yen, S.C.; Ju, Y.T.; Shaner, P.L.; Chen, H.L. Spatial and temporal relationship between native mammals and free-roaming dogs in a protected area surrounded by a metropolis. Sci. Rep. 2019, 9, 8161. [Google Scholar] [CrossRef] [Green Version]
- Filazzola, A.; Brown, C.; Dettlaff, M.A.; Batbaatar, A.; Grenke, J.; Bao, T.; Peetoom Heida, I.; Cahill, J.F., Jr. The effects of livestock grazing on biodiversity are multi-trophic: A meta-analysis. Ecol. Lett. 2020, 23, 1298–1309. [Google Scholar] [CrossRef]
- Yamamura, N.; Tsuji, N. Optimal Patch Time under Exploitative Competition. Am. Nat. 1987, 129, 553–567. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Cunningham, S.A. Livestock grazing exclusion and microhabitat variation affect invertebrates and litter decomposition rates in woodland remnants. For. Ecol. Manag. 2009, 258, 178–187. [Google Scholar] [CrossRef]
- Roos, S.; Smart, J.; Gibbons, D.W.; Wilson, J.D. A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: A case study of the UK. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1915–1937. [Google Scholar] [CrossRef]
- Smith, J.A.; Wang, Y.; Wilmers, C.C. Top carnivores increase their kill rates on prey as a response to human-induced fear. Proc. Biol. Sci. 2015, 282, 20142711. [Google Scholar] [CrossRef]
- Pyke, G.H.; Pulliam, H.R.; Charnov, E.L. Optimal Foraging: A Selective Review of Theory and Tests. Q. Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.S.; Spaulding, S.; Swanson, H.; Keeley, W.; Gramza, A.R.; VandeWoude, S.; Crooks, K.R. Human activity influences wildlife populations and activity patterns: Implications for spatial and temporal refuges. Ecosphere 2021, 12, e03487. [Google Scholar] [CrossRef]
- Bennie, J.J.; Duffy, J.P.; Inger, R.; Gaston, K.J. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 13727–13732. [Google Scholar] [CrossRef] [Green Version]
- Puls, S.; Teichman, K.J.; Jansen, C.; O’Riain, M.J.; Cristescu, B. Activity patterns of leopards (Panthera pardus) and temporal overlap with their prey in an arid depredation hotspot of southern Africa. J. Arid Environ. 2021, 187, 104430. [Google Scholar] [CrossRef]



| Species | Activity Pattern | Number of Independent Photos | Camera Sites with Detections | Naïve Occupancy | RAI |
|---|---|---|---|---|---|
| Leopard cats | nocturnal | 259 | 36 | 0.64 | 3.10 |
| Nocturnal rats | nocturnal | 410 | 18 | 0.32 | 4.90 |
| Diurnal squirrels | diurnal | 855 | 35 | 0.59 | 10.23 |
| Livestock | diurnal | 1817 | 38 | 0.46 | 21.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Li, H.-D.; Xiao, W.; Xu, K.; Ren, Y.; Li, H.; Wang, P.; Fan, M.; Huang, X.; Xiao, Z. Fine-Scale Interactions between Leopard Cats and Their Potential Prey with Contrasting Diel Activities in a Livestock-Dominated Nature Reserve. Animals 2023, 13, 1296. https://doi.org/10.3390/ani13081296
Ji C, Li H-D, Xiao W, Xu K, Ren Y, Li H, Wang P, Fan M, Huang X, Xiao Z. Fine-Scale Interactions between Leopard Cats and Their Potential Prey with Contrasting Diel Activities in a Livestock-Dominated Nature Reserve. Animals. 2023; 13(8):1296. https://doi.org/10.3390/ani13081296
Chicago/Turabian StyleJi, Chengpeng, Hai-Dong Li, Wenhong Xiao, Kai Xu, Yingfeng Ren, Hongyun Li, Pengcheng Wang, Mingliang Fan, Xiaoqun Huang, and Zhishu Xiao. 2023. "Fine-Scale Interactions between Leopard Cats and Their Potential Prey with Contrasting Diel Activities in a Livestock-Dominated Nature Reserve" Animals 13, no. 8: 1296. https://doi.org/10.3390/ani13081296




