Simple Summary
The concentration of larval black flies in well-defined aquatic habitats makes knowledge of the breeding sites critical for the efficient management of pest species. Black flies can achieve pest status either as generalists by developing in many types of streams and rivers or as specialists by developing in one or a few types of flowing water, such as large rivers. These two developmental strategies are evident in pest and vector species of the Simulium (Gomphostilbia) varicorne species group in Thailand. Simulium chumpornense is a habitat generalist, whereas S. khelangense is a habitat specialist, developing in the large Mekong River, where we discovered its immature stages. The first descriptions of the larva and pupa of S. khelangense, along with mitochondrial and nuclear genetic markers, allow accurate identification and comparisons with structurally similar species in the S. varicorne species group, thus aiding the ability to monitor the pest and vector status of black flies in Southeast Asia.
Abstract
Two species of black flies (Simuliidae) in Thailand, Simulium chumpornense Takaoka and Kuvangkadilok, 2000, and S. khelangense Takaoka, Srisuka & Saeung, 2022, are potent vectors of avian blood protozoa of the genera Leucocytozoon and Trypanosoma and are pests of domestic avian species. Although the adults are abundant throughout Thailand, information on their breeding habitats is limited, and the immature stages of S. khelangense are unknown. We collected the larvae and pupae of S. khelangense from the Mekong River, the first-ever record of Simuliidae from this large continental river. Mitochondrial cytochrome c oxidase I and internal transcribed spacer 2 were used to associate the larvae and pupae with known adults. Both genetic markers strongly supported their identity as S. khelangense. The larvae and pupa of S. khelangense are described. The pupal gill filaments, larval abdominal protuberances, and setae distinguish this species from other members of the S. varicorne species group. The immature stages of S. chumpornense inhabit a wide variety of flowing waters, from small streams (3 m wide) to enormous continental rivers (400 m wide); thus, S. chumpornense is a habitat generalist. In contrast, S. khelangense was found only in the large Mekong River and is, therefore, a habitat specialist. Both species can exploit their principal habitats and produce abundant adult populations.
1. Introduction
Black flies (Diptera: Simuliidae) are significant hematophagous insects that act as pests and vectors of pathogens to humans and other animals [1]. The most significant disease associated with black flies is human onchocerciasis, caused by the filarial nematode Onchocerca volvulus. Black flies can also transmit viruses, bacteria, and protozoa, including those causing economically significant diseases such as leucocytozoonosis in domestic chickens [1]. Even without transmitting pathogens, black flies are significant pests of humans and other animals [1]. In some cases, biting can kill animals as a result of the toxicity of the salivary constituents, a syndrome referred to as “simuliotoxicosis” [2].
In Thailand, 145 black fly species have been recorded and are assigned to six subgenera in the genus Simulium: Asiosimulium Takaoka and Choochote; Daviesellum Takaoka and Adler; Gomphostilbia Enderlein; Montisimulium Rubstsov; Nevermannia Enderlein; and Simulium Latreille [3]. Females of seven species bite humans, and those of two species (S. nigrogilvum Summers, 1911, and S. nodosum Puri, 1933) are nuisance pests of humans [4]. These species, plus the S. asakoae Takaoka & Davies, 1995 complex, are possible vectors of filarial nematodes, including those of the genus Onchocerca [5,6,7,8]. Three species of the subgenus Gomphostilbia (S. asakoae complex, S. chumpornense Takaoka & Kuvangkadilok, 2000, and S. khelangense Takaoka, Srisuka & Saeung, 2022) are possible vectors of blood protozoa belonging to the genera Leucocytozoon and Trypanosoma [9,10,11]. These black flies are abundant around animal shelters and are potential pests of domestic birds [12].
Knowledge of the biology of insect vectors is crucial for understanding factors related to disease epidemiology and can be used for the development of effective management programs [13]. For example, the application of the highly effective larvicidal agent Bacillus thuringiensis var. israelensis (Bti) to suppress black flies requires knowledge of the breeding habitats of the target species and the physical and chemical conditions of these habitats [14,15]. Information on breeding habitats can also be used to design other management strategies, such as flow regulation [16].
During the past 15 years, several studies have examined habitat factors associated with species distributions of black flies in Thailand [17,18,19,20,21]. However, little is known about the breeding habitats of S. chumpornense. The immature stages of this species have been reported from only four stream sites, two from the southern and two from the western regions of Thailand [19], although adults are abundant throughout the country [12,22,23,24]. Another species, S. khelangense, is known only from the adult female [25]. Like S. chumpornense, this species is abundant in several areas in Thailand [11], but the breeding habitats and, therefore, the immature stages are unknown.
We collected larvae, pupae, and data on environmental factors of the associated stream habitats. The larvae and pupae of S. khelangense were also collected and described for the first time. The immature stages of this species were found in the Mekong River, the first record of black flies from this enormous continental river. The characteristics of the breeding habitats of S. chumpornense and S. khelangense that are related to adult abundance are evaluated, and mitochondrial and nuclear DNA markers are assessed as aids for identification.
2. Materials and Methods
2.1. Collection and Identification
Larvae and pupae were obtained from four locations (Table 1 and Figure 1). Information on stream variables reported previously [18] is included in Table 1. Larvae and pupae were collected by hand, using fine forceps, from submerged grasses and wood. Specimens were fixed in 80% (v/v) ethanol. Some pupae were reared to adults in plastic bottles. Adults were fixed in 80% ethanol for morphological study.

Table 1.
Ecological conditions of the breeding sites of Simulium chumpornense and S. khelangense in Thailand.
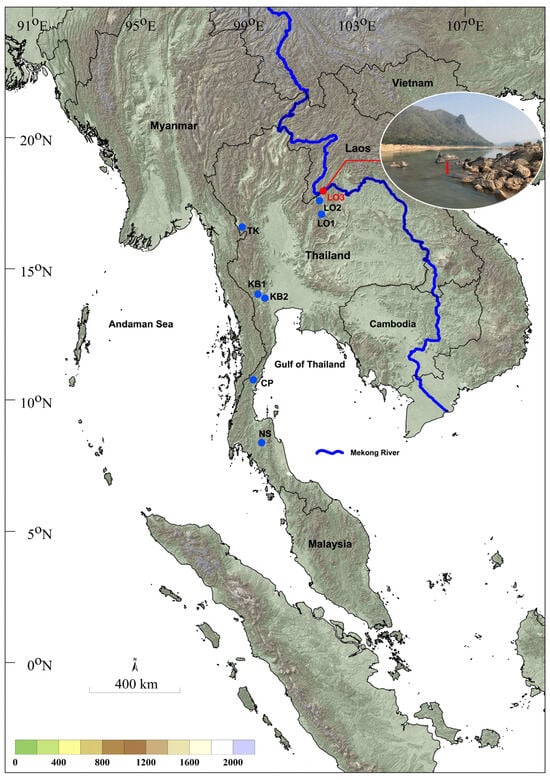
Figure 1.
Sampling locations of larvae and pupae of Simulium chumpornense (blue) and S. khelangense (red) in Thailand. Details of sampling sites are given in Table 1. Inset shows the sampling location for larvae and pupae of Simulium khelangense in the Mekong River. Arrow indicates submerged wood from which larvae and pupae were collected.
The following stream variables associated with black fly distributions were measured: width, depth, velocity, conductivity, dominant streambed particle size, canopy cover, and riparian vegetation. Classification of streambed particle size, canopy cover, and riparian vegetation, plus the calculation of current velocity, followed the procedures of [26]. Larvae and pupae of the S. varicorne species group were identified using available keys and descriptions of black flies in Thailand [27,28,29,30,31,32,33,34,35,36,37,38,39,40].
2.2. Morphological Descriptions
The morphological characters of larvae and pupae of S. khelangense were examined under a stereomicroscope and compound microscope. Measurements were made using an ocular micrometer. Photographs were taken using a stereomicroscope (Zeiss Stemi 508 equipped with an Axiomcam 208 camera) and compound microscope (Zeiss PrimoStar 3 light microscope, Carl Zeiss, Germany). Descriptions of morphological characters followed the terminology of Takaoka and Suzuki [41] and Adler et al. [42]. Representative specimens were deposited in the Department of Biology, Faculty of Science, Mahasarakham University, Mahasarakham Province, Thailand.
2.3. DNA Extraction, Amplification and Sequencing
DNA was extracted from the whole body of six larvae, one pupa, and one reared adult female of S. khelangense using the GF-1 Nucleic Acid Extraction Kit (Vivantis Technologies Sdn. Bhn, Malaysia). We also molecularly examined the closely related species S. chumpornense (four larvae) from a nearby location (14 km). A fragment of approximately 650 bp of the mitochondrial cytochrome c oxidase barcoding region was amplified using the primers LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’) and HCO2198 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’) [43]. The PCR reaction conditions followed those of [44]. In addition to the COI gene, the nuclear internal transcribed spacer 2 (ITS2) was also examined, as this genetic marker can differentiate closely related species of the S. varicorne species group [11]. An approximately 300-bp fragment of ITS2 was amplified using primers CP17 (5’-GCGCCGCGGTGTGAACTGCAGGACACATG-3’) and CP16 (5’-GCGGGTACCATGCTTAAATTTAGGGGGTA-3’) [45], with PCR reaction conditions as described by Thanwisai et al. [46]. The PCR products of COI and ITS2 were checked using 1% agarose gel electrophoresis staining with 1X Novel Juice Loading Dye (GenDirex®, Taiwan, China). Successful amplifications were purified using the PureDirex PCR CleanUp & Gel Extraction Kit (Bio-Helix, Taiwan, China). Purified PCR products were sequenced at the ATCG Company Limited (Thailand Science Park, Pathumthani, Thailand) using the same primers as for PCR.
2.4. DNA Sequence Analysis
A total of 12 COI (accession nos. PP564920-PP564931) and 12 ITS2 (accession nos. PP574877-PP574888) sequences (8 from S. khelangense and 4 from S. chumpornense for each gene) with a sequence length of 611 bp and 234 bp, respectively, were obtained. COI sequences (n = 45) of members of the S. varicorne species group were retrieved from GenBank and included in data analyses: S. piroonae Takaoka and Srisuka, 2014 (n = 3), S. kuvangkadilokae Pramual and Tangkawanit, 2008 (n = 16), S. chumpornense Takaoka and Kuvangkadilok, 2000 (n = 13), S. khelangense (n = 7), and S. novemarticulatum Takaoka and Davies, 1995 (n = 6). ITS2 sequences (n = 33) of S. khelangense (n = 18), S. chumpornense (n = 4), S. kuvangkadilokae (n = 8), and S. novemarticulatum (n = 3) were retrieved from GenBank and included for genetic distance and phylogenetic analyses. Intraspecific and interspecific genetic divergences were calculated based on a p-distance model using TaxonDNA [47]. Phylogenetic relationships were inferred using neighbor-joining (NJ), maximum likelihood (ML), and Bayesian analysis (BA) methods. NJ and ML were analyzed in MEGA X [48]. Branch support was estimated using 1000 bootstrapping replications. Bayesian analysis was performed in MrBayes ver. 3.2.7a [49] with 2,000,000 generations and a sampling frequency of 100 generations. In all phylogenetic analyses of COI and ITS2 sequences, S. novemarticulatum, a member of the S. novemarticulatum subgroup of the S. varicorne group [50], was treated as the outgroup.
3. Results
3.1. Genetic Variation, Species Differentiation, and Phylogenetic Relationships
The COI sequence divergence of larvae, pupae, and reared females of S. khelangense varied from 0.33% to 1.31%. Comparisons with other species of the S. varicorne species group revealed that these specimens were closest to S. khelangense, with sequence divergence ranging from 0.33% to 1.80%. This species is genetically close to S. chumpornense, S. kuvangkadilokae, and S. piroonae, with a minimum sequence divergence of 1.96%, 1.15%, and 2.78%, respectively. Simulium novemarticulatum showed a high level of genetic differentiation from the other species, with a minimum interspecific genetic divergence of 10.31% (Table 2). Genetic divergence based on ITS2 sequences showed similar patterns. The intraspecific genetic divergence of S. khelangense varied from 0.00% to 4.70%. Comparisons with other species revealed a considerable level of genetic differentiation, with minimum genetic divergences of 5.13% to 17.09 (Table 3).

Table 2.
Intraspecific and interspecific genetic divergences (%) between species of the Simulium varicorne species group and the immature stages of Simulium khelangense, based on mitochondrial COI sequences.

Table 3.
Intraspecific and interspecific genetic divergences (%) between species of the Simulium varicorne species group and the immature stages of Simulium khelangense, based on ITS2 sequences.
Phylogenetic relationships based on the COI gene sequences revealed similar tree topologies for NJ, ML, and BA methods; therefore, only the ML tree is shown (Figure 2). There were two main clades among the species. Simulium khelangense, S. chumpornense, S. piroonae, and S. kuvangkadilokae formed one clade. Simulium khelangense was monophyletic, although with low (<50%) support. All larvae, pupae, and reared females in our study belonged to this clade. Three specimens of S. piroonae were monophyletic, with >83% bootstrap support. The clade with S. chumpornense had strong bootstrap support (>96%) but was paraphyletic because all specimens of S. piroonae and 6 of 16 of S. kuvangkadilokae were included in this clade. Another clade comprised the remaining specimens of S. kuvangkadilokae with moderate bootstrap support (70%).
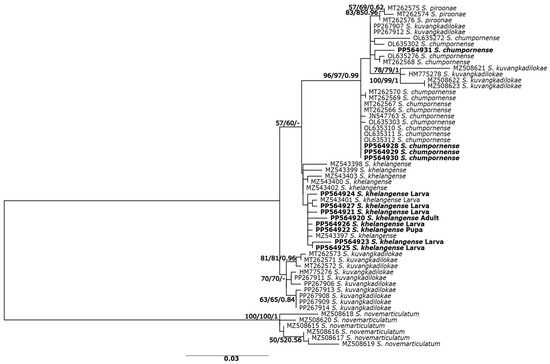
Figure 2.
Maximum likelihood tree inferred from COI sequences of five species of the Simulium varicorne species group in Thailand and larvae, pupae, and reared adults of S. khelangense. Bootstrap values for ML and NJ analyses and posterior probability for BA trees are shown above or near branches. Bold characters indicate specimens obtained in the present study.
The phylogenetic analyses based on ITS2 sequences revealed similar tree topologies for all three methods; thus, only the ML tree is presented (Figure 3). The ML tree based on ITS2 sequences revealed two clades. Simulium khelangense formed one clade, and S. chumpornense and S. kuvangkadilokae formed another clade. All species were monophyletic with high (>85%) support, except for S. kuvangkadilokae, which was low (63%) for the ML tree but high (84%) for the NJ and BA (0.9) trees. All the larvae, pupae, and reared females of S. khelangense clustered together.
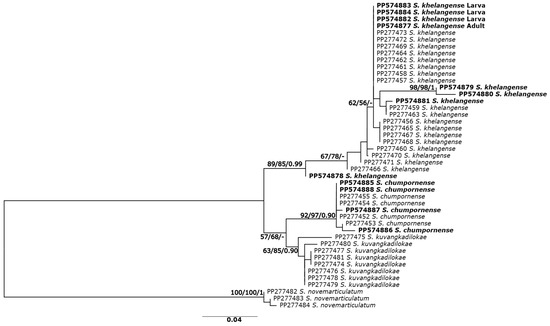
Figure 3.
Maximum likelihood tree inferred from ITS2 sequences of five species of the Simulium varicorne species group in Thailand and larvae, pupae, and reared adults of S. khelangense. Bootstrap values for ML and NJ analyses and posterior probability for BA trees are shown above or near branches. Bold characters indicate specimens obtained in the present study.
3.2. Descriptions of Pupa and Larva of Simulium khelangense Takaoka, Srisuka & Saeung, 2022
Morphological comparisons of an adult female reared from a pupa agree well with the morphological characteristics of S. khelangense [25]. Genetic data based on COI and ITS2 sequences also indicated that the larvae and pupae collected from the Mekong River are those of S. khelangense. Therefore, descriptions of the mature larvae and pupa are provided here.
Pupa (Figure 4). Body length (excluding gill filaments): 2.5–3.1 mm (n = 4) (Figure 4A,B). Head. Integument yellowish, moderately covered with round tubercles mostly medially and at the base of the frons (Figure 4C); antennal sheath without tubercles; frons with three pairs of unbranched long trichomes with uncoiled apices; face with a pair of unbranched long trichomes with uncoiled apices. Thorax. The integument is yellowish brown, moderately covered with round tubercles, with three pairs of unbranched long mediodorsal trichomes, two long unbranched anterolateral trichomes, one long unbranched posterolateral trichome, and three unbranched ventrolateral trichomes. Gills (Figure 4D). Each is composed of eight slender filaments arranged as 3 + (1 + 2) + 2 from dorsal to ventral; the middle triplet partially overlaps the upper triplets and lower pair; all stalks are short, although the secondary stalk of the pair of middle triplets is relatively longer than others; all filaments are light brown, subequal in thickness except for the lower filaments of the middle and lower pairs, which are slightly thicker than others; all filaments are subequal in length (ca. 1.1–1.5 mm), although the middle filament is slightly longer than others, with distinct annular ridges forming a reticulate pattern (Figure 4E), densely covered with minute tubercles. Abdomen. Dorsally, segment I with one simple, slender seta on each side; segment II with one simple, slender seta and five short, spinous setae submedially on each side; segments III and IV each with four hooked spines and one short spinous seta; segment V lacking spine-combs; segments VI–IX, each with a distinct spine-comb in a transverse row and with comb-like groups of minute spines on each side; segment IX with a pair of conical terminal hooks (Figure 4F). Ventrally, segment IV has one unbranched hook and a few short setae on each side; segment V has a pair of bifid hooks submedially and a few unbranched, slender short setae on each side; segments VI and VII with one inner bifid and one outer simple hook noticeably separated from each other and a few simple, slender short setae on each side; segments IV–VIII with comb-like groups of minute spines. Cocoon. Wall-pocket-shaped, moderately woven but more tightly woven at the anterior margin, ventrolaterally extended to varying extents, 2.5–3.2 mm long by 1.8–2.0 mm wide.
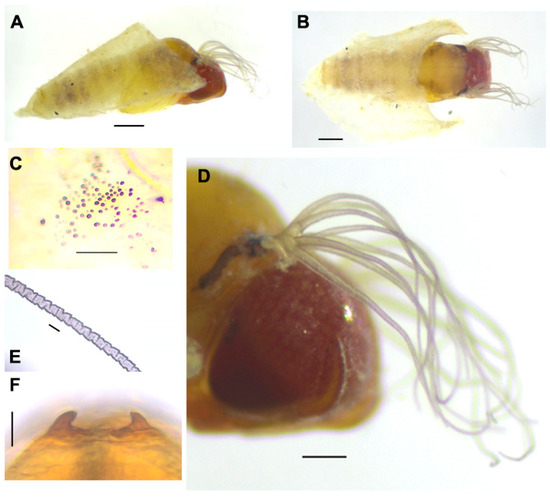
Figure 4.
Pupa of Simulium khelangense. (A) and (B), pupae and cocoons ((A), lateral view; (B), dorsal view). (C), tubercles on the median portion of the frons. (D), gills (right outer side). (E) middle portion of the upper gill filament of the upper triplet. (F) terminal hooks on the dorsum of abdominal segment IX. Scale bar: 0.5 mm for (A) and (B), 0.2 mm for (D), 0.05 mm for (C) and (E), and 0.02 for (F).
Mature Larva (Figure 5). Body length: 4.1–4.6 mm (n = 10). The body is characterized by a pair of dorsolateral transparent conical protuberances on thoracic segment III and abdominal segments I–V, with those of segments III–V more prominent. Body (Figure 5A): creamy with the following markings: thoracic segment I is surrounded by a wide grayish-brown band; the anterior surface of thoracic proleg gray; thoracic segment II is whitish yellow; thoracic segment III is whitish with a dark gray spot on the ventral surface; abdominal segments I and II are each surrounded by a wide grayish brown band; abdominal segments III–VIII, each surrounded by a wide brownish band, although disconnected on abdominal segments V–VIII. The head capsule is whitish yellow except near the posterior margin of the cephalic apotome, and the upper portion above eye spots is darker (Figure 5B,C), covered with minute, simple colorless setae. The cephalic apotome (Figure 5B) is whitish-yellow with indistinct head spots, although some larvae have moderately positive patterns. The ventral surface of the head capsule is whitish yellow. Antenna unpigmented, composed of three articles and apical sensillum, slightly longer than the stem of the labral fan; proportional length of proximal, medial, and distal articles is 1.00:0.80:1.00. Labral fan with 42–44 primary rays. Mandible (Figure 5D) with three comb-teeth decreasing in length from first to third; mandibular serrations composed of two teeth, one large and one small, large tooth at a parallel angle with mandible on the apical side. Hypostoma (Figure 5E) with nine teeth, median tooth, and corner teeth prominent and subequal in length; lateral margins serrated apically; three hypostomal bristles in a row, parallel to the lateral margin on each side. The postgenal cleft is wide and deep, reaching the postgenal margin of the hypostoma (Figure 5F). Cervical sclerites are composed of two small, light brown rod-like pieces that are not fused to the occiput and are widely separated from each other. The histoblast of the pharate pupal gill has eight slender filaments (Figure 5G). Thoracic segment III and abdominal segments I–V each have a pair of conical, transparent protuberances (Figure 5H). The thoracic cuticle is bare, while the abdominal cuticle of segments I and II is sparely covered with unbranched, bifid, and trifid colorless minute setae; segments III–IV are moderately covered with minute setae; segments V–IX are densely covered with minute setae, of which unbranched setae are relatively larger with basal half or two-thirds darkened and flattened, becoming colorless and tapered apically; bifid setae are similar in length, unbranched, darker on the basal half, and colorless apically; trifid setae are shorter than unbranched and bifid setae and almost colorless excepted at base; quadrifid setae are rare, colorless, and shorter than other seta types (Figure 5I–K). Rectal papillae (Figure 5L) compound each has 7–9 finger-like secondary lobules. Anal sclerite (Figure 5M) is X-shaped, with anterior arms 0.58–0.63 times the length of the posterior arms; accessory sclerites are absent. Ventral tubercles are well developed and conical. The posterior circlet has 12–14 hooklets in 70–75 rows.
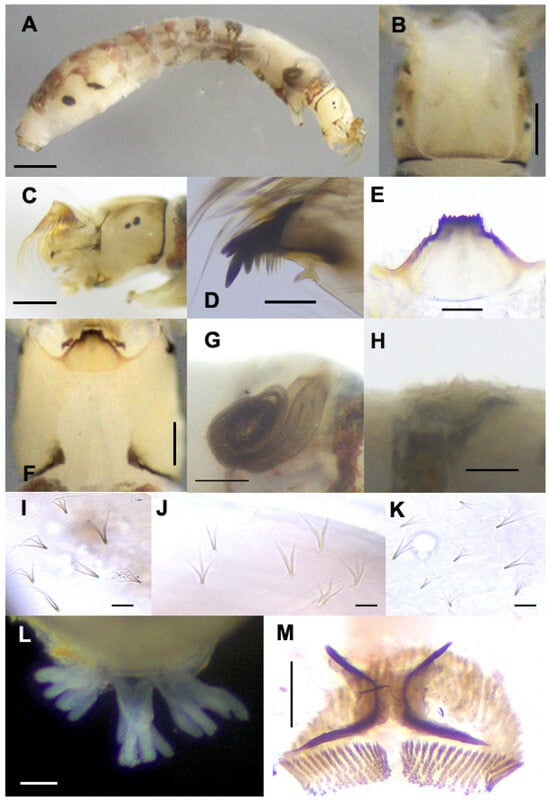
Figure 5.
Mature larva of Simulium khelangense. (A) whole body (lateral view); (B,C) head capsule ((B) dorsal view; (C) left lateral view); (D) mandible; (E) hypostoma; (F) postgenal cleft; (G) gill histoblast; (H) protuberance on abdominal segment IV (lateral view); (I–K) simple, bifid, trifid, and quadrifid setae on the dorsal surface of the abdomen (dorsal view); (L) rectal papillae (posterodorsal view); (M) anal sclerite and posterior circlet. Scale bar: 0.5 mm for (A); 0.2 for (B,C,F); 0.1 for (D); 0.05 mm for (E,G,H,L,M); and 0.02 for (I–K).
3.3. Diagnosis
The gill of S. khelangense comprises eight slender filaments in three groups (upper and middle triplets and a ventral pair) with a short common stalk (Table 4). The larva of S. khelangense has a pair of transparent dorsal protuberances on thoracic segment III and abdominal segments I–V and abdominal cuticle moderately covered with minute unbranched, bifid, trifid, and quadrifid setae. These morphological characteristics distinguish S. khelangense from all other members of the S. varicorne species group (Table 4).

Table 4.
Diagnostic morphological characters of pupae and mature larvae of all 15 species in the Simulium varicorne species group.
3.4. Breeding Habitats of S. khelangense and S. chumpornense
The pupae and larvae of S. khelangense were collected from aquatic vegetation and submerged wood in the open-canopy, large (approximately 400 m wide) Mekong River (location LO3) at an elevation of 210 m above sea level. The river depth at the collection site was 0.82 m, the current velocity was 0.44 m/s, the water conductivity was 234 µS/cm2, and the water temperature was 23.9 °C. Larvae and pupae of S. chumpornense were collected from nine sampling sites; four of these were reported previously [18]. The stream size varied from 3 m to 400 m wide, with depths of 0.02 m to 0.82 m and current velocities of 0.24 m/s to 0.68 m/s. Water conductivity was generally high, with values ranging from 234 µS/cm2 to 495 µS/cm2, and the water temperature was 22.0 °C to 32.5 °C. The dominant streambed particle size varied from sand and rubble to boulders and bedrock. Stream habitats of S. chumpornense were mostly without canopy cover (i.e., open), although one sampling site had a closed canopy. Riparian vegetation varied from grassland (open) to a continuous border of trees (forest) (Table 1).
4. Discussion
Molecular genetic analyses based on COI and ITS2 sequences confirmed that the larvae and pupae collected from the Mekong River are those of S. khelangense. Phylogenetic analyses based on these genetic markers further corroborated the identification of S. khelangense. Species of the S. varicorne species group are morphologically similar to adults, particularly those of the S. chumpornense subgroup (S. chumpornense, S. kuvangkadilokae, S. khelangense, S. tomae Takaoka, 2003, and S. varicorne Edwards, 1929).
Morphological differentiation among the members of the subgroup is based mainly on the shape, number, and arrangement of the pupal gill filaments [34]. The pupal stage of S. khelangense can be distinguished from that of other members of the S. varicorne species group by the number (eight filaments), arrangement (three groups: upper and middle triplets plus a lower pair), and length (short) of the secondary stalk of the gill filaments. Among the 15 species of the S. varicorne species group, 10 (S. sumbaense, S. chumpornense, S. varicorne, S. tomae, S. charlesi, S. novemarticulatum, S. huangi, S. shogakii, S. synanceium, and S. breviflagellum) have eight gill filaments like S. khelangense. However, the gill filaments of S. sumbaense, S. chumpornense, S. varicorne, and S. tomae are arranged in two groups (six upper filaments plus a lower pair) and, therefore, differ from those of S. khelangense. The gill filaments of S. charlesi, S. novemarticulatum, S. huangi, S. shogakii, S. synanceium, and S. breviflagellum are arranged in three groups similar to those of S. khelangense. However, these species can be differentiated from S. khelangense by the long stalk of the ventral pair or a long secondary stalk of the upper and dorsal triplets [28,29,30,31,32,33,34,35,36,37,38,39,40].
The larvae of S. khelangense can be distinguished from those of other species in the S. varicorne group by the presence of a pair of transparent dorsal protuberances on thoracic segment III and abdominal segments I–V. Larvae of only five species (S. chumpornense, S. piroonae, S. kuvangkadilokae, S. charlesi, and S. novemarticulatum) of the members of the S. varicorne species group have dorsal protuberances like those of S. khelangense. Larvae of S. khelangense, which have one pair of protuberances on each of abdominal segments I–V, can be distinguished from the larvae of these species because S. chumpornense has two pairs [31], S. kuvangkadilokae has four pairs [33], and S. piroonae has three pairs [34] per abdominal segment. Simulium novemarticulatum has one pair each on abdominal segments I and II but two pairs each on abdominal segments III–V [40]. Based on the number of protuberances on the dorsal surface of the abdomen, S. khelangense is most similar to S. charlesi, with both species having a pair of dorsal protuberances on thoracic segment III and abdominal segments I–V. These species also have a similar shape to the postgenal cleft, which is wide and deep, reaching the posterior margin of the hypostoma. However, S. khelangense can be differentiated from S. charlesi by the setae on the abdominal cuticle. The abdominal cuticle of S. khelangense is moderately covered with minute unbranched, bifid, trifid, and quadrifid setae, whereas that of S. charlesi is covered with minute flat, unbranched setae [30].
We have discovered the first record of black flies inhabiting the Mekong River, the third-longest river in Asia. Three black fly species, S. khelangense, S. chumpornense, and S. nakhonense, were found in this river. Among these species, only S. khelangense and S. chumpornense were also found abundantly in the adult stage. The immatures of S. chumpornense inhabit diverse streams and rivers, ranging from small flows (3 m wide) to huge (400 m wide) continental rivers (e.g., Mekong). They can also inhabit streams with particle sizes of the streambed varying from sand to bedrock and with a canopy varying from open to completely covered. We also collected larvae of S. chumpornense from a highly calcareous stream (Nang Kruan waterfall, Tak Province) in the northwestern region of Thailand. Thus, this species inhabits a wide variety of streams. Therefore, as a eurytopic species occupying many habitats, S. chumpornense is able to produce large adult populations, one of the two major pathways to the pest status of black flies [2]. The immature stages of S. khelangense, on the other hand, are known only from the large Mekong River. This species, therefore, is stenotopic, able to produce large adult populations by using fewer but larger breeding habitats [2].
In conclusion, we have provided descriptions of the pupa and larva of S. khelangense. Unlike the adult female, which is difficult to distinguish from closely related species, the morphological characteristics of the pupa, particularly of the gill filaments, and of the larva, such as abdominal protuberances and setae, can be used to identify this species. The wide range of breeding habitats of S. chumpornense indicates that this species is a generalist. In contrast, the closely related S. khelangense, known only from the huge Mekong River, is a habitat specialist. Both the generalist and specialist scenarios can facilitate the production of large adult populations [2], such as those observed in several areas of Thailand. Whether these large adult populations present pest problems for avian species, such as chickens, remains to be explored.
Author Contributions
Conceptualization, I.T., P.H.A. and P.P.; investigation, B.G., W.J., W.W., C.J., I.T. and S.N.; formal analysis, P.P. and P.H.A.; resources, W.J., W.W. and S.N.; data curation, C.J., W.J. and I.T.; writing—original draft preparation, P.P. and I.T.; writing—review and editing, I.T., S.N. and P.H.A.; visualization, B.G., C.J. and P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was financially supported by Mahasarakham University, grant number 6601001/2566.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee, Mahasarakham University (approval number: IACUC-MSU-34/2023) for studies involving animals.
Data Availability Statement
The sequences have been deposited into the NCBI GenBank under the accession numbers PP564920-PP564931 and PP574877-PP574888. All other data and materials supporting this article are available from the corresponding author, P. P., upon request.
Acknowledgments
We would like to thank Adrian Plant, Mahasarakham University, for valuable comments on a previous version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crosskey, R.W. The Natural History of Blackflies; John Wiley & Sons Ltd.: Chichester, UK, 1990. [Google Scholar]
- Adler, P.H.; Kúdelová, T.; Kúdela, M.; Seitz, G.; Ignjatović-Ćupina, A. Cryptic biodiversity and the origins of pest status revealed in the macrogenome of Simulium colombaschense (Diptera: Simuliidae), history’s most destructive black fly. PLoS ONE 2016, 11, e0147673. [Google Scholar] [CrossRef] [PubMed]
- Aupalee, K.; Srisuka, W.; Taai, K.; Takaoka, H.; Saeung, A. A new species of Simulium (Asiosimulium) (Diptera: Simuliidae) from northeastern Thailand, with its phylogenetic relationships with related species in the subgenus Asiosimulium. J. Med. Entomol. 2023, 60, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P. Black fly diversity and impacts on human welfare in Southeast Asia. In Biodiversity of Southeast Asian Parasites and Vectors Causing Human Disease; Petney, T.N., Saijuntha, W., Mehlhorn, H., Eds.; Parasitology Research Monographs; Springer: Cham, Switzerland, 2021; Volume 14, pp. 143–164. [Google Scholar]
- Fukuda, M.; Choochote, W.; Bain, O.; Aoki, C.; Takaoka, H. Natural infections with filarial larvae in two species of black flies (Diptera: Simuliidae) in northern Thailand. J. Trop. Med. Hyg. 2003, 31, 99–102. [Google Scholar] [CrossRef][Green Version]
- Takaoka, H.; Choochote, W.; Aoki, C.; Fukuda, M.; Bain, O. Black flies (Diptera: Simuliidae) attracted to humans and water buffalos and natural infections with filarial larvae, probably Onchocerca sp., in northern Thailand. Parasite 2003, 10, 3–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishii, Y.; Choochote, W.; Bain, O.; Fukuda, M.; Otsuka, Y.; Takaoka, H. Seasonal and diurnal biting activities and zoonotic filarial infections of two Simulium species (Diptera: Simuliidae) in northern Thailand. Parasite 2008, 15, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Saeung, A.; Srisuka, W.; Aupalee, K.; Fukuda, M.; Otsuka, Y.; Taai, K.; Maleewong, W.; Takaoka, H. Natural infections with larvae of Onchocerca species type I in the human-biting black fly, Simulium nigrogilvum (Diptera: Simuliidae), in western Thailand. Acta Trop. 2020, 204, 105344. [Google Scholar] [CrossRef]
- Jumpato, W.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Leucocytozoon (Apicomplexa: Haemosporida) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 190, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Thaijarern, J.; Tangkawanit, U.; Wongpakam, K.; Pramual, P. Molecular detection of Trypanosoma (Kinetoplastida: Trypanosomatidae) in black flies (Diptera: Simuliidae) from Thailand. Acta Trop. 2019, 200, 105196. [Google Scholar] [CrossRef]
- Stangarm, J.; Mintara, R.; Jumpato, W.; Gomontean, B.; Thanee, I.; Wongpakam, K.; Adler, P.H.; Saijuntha, W.; Pramual, P. Molecular detection of blood protozoa and identification of black flies of the Simulium varicorne species group (Diptera: Simuliidae) in Thailand. Acta Trop. 2024, 254, 107207. [Google Scholar] [CrossRef] [PubMed]
- Gomontean, B.; Jumpato, W.; Wongpakam, K.; Tangkawanit, U.; Wannasingha, W.; Thanee, I.; Ya’cob, Z.; Pramual, P. Diversity, distribution and host blood meal analysis of adult black flies (Diptera: Simuliidae) from Thailand. Insects 2024, 15, 74. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, R.; Finney, J.R. A review of Bacillus thuringiensis var. israelensis (serotype 14) as a biological control agent of black flies (Simuliidae). Misc. Publ. Entomol. Soc. Am. 1982, 12, 1–17. [Google Scholar]
- Gray, E.W.; Adler, P.H.; Coscaron-Arias, C.; Coscarón, S.; Noblet, R. Development of the first black fly (Diptera: Simuliidae) management program in Argentina and comparison with other programs. J. Am. Mosq. Control Assoc. 1999, 15, 400–406. [Google Scholar] [PubMed]
- Rivers-Moore, N.A.; Hughes, D.A.; De Moor, F.C. A model to predict outbreak periods of the pest blackfly Simulium chutteri Lewis (Simuliidae, Diptera) in the Great Fish River, Eastern Cape Province, South Africa. River Res. Appl. 2008, 24, 132–147. [Google Scholar] [CrossRef]
- Pramual, P.; Kuvangkadilok, C. Agricultural land use and black fly (Diptera, Simuliidae) species richness and species assemblages in tropical streams, Northeastern Thailand. Hydrobiologia 2009, 625, 173–184. [Google Scholar] [CrossRef]
- Pramual, P.; Wongpakam, K. Seasonal variation of black fly (Diptera: Simuliidae) species diversity and community structure in tropical streams of Thailand. Entomol. Sci. 2010, 13, 17–28. [Google Scholar] [CrossRef]
- Pramual, P.; Kuvangkadilok, C.; Jitklang, S.; Tangkawanit, U.; Adler, P.H. Geographical versus ecological isolation of closely related black flies (Diptera: Simuliidae) inferred from phylogeny, geography, and ecology. Org. Divers. Evol. 2012, 12, 183–195. [Google Scholar] [CrossRef]
- Srisuka, W.; Takaoka, H.; Otsuka, Y.; Fukuda, M.; Thongsahuan, S.; Taai, K.; Choochote, W.; Saeung, A. Seasonal biodiversity of black flies (Diptera: Simuliidae) and evaluation of ecological factors influencing species distribution at Doi Pha Hom Pok National Park, Thailand. Acta Trop. 2015, 149, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Jitklang, S.; Sawangproh, W.; Kuvangkadilok, C.; Baimai, V.; Adler, P.H. Ecology of black flies (Diptera: Simuliidae) in streams of northern and southern Thailand: Factors associated with larval and pupal distributions. Acta Trop. 2020, 204, 105357. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Tangkawanit, U.; Kunprom, C.; Vaisusuk, K.; Chatan, W.; Wongpakam, K.; Thongboonma, S. Seasonal population dynamics and a role as natural vector of Leucocytozoon of black fly, Simulium chumpornense Takaoka & Kuvangkadilok. Acta Trop. 2020, 211, 105617. [Google Scholar]
- Pramual, P.; Jomkumsing, P.; Wathasith, P.; Wongpakam, K. Population structure and population history of the black fly Simulium chumpornense (Diptera: Simuliidae) from Thailand. Acta Trop. 2022, 227, 106301. [Google Scholar] [CrossRef] [PubMed]
- Srisuka, W.; Sulin, C.; Aupalee, K.; Phankaen, T.; Taai, K.; Thongsahuan, S.; Saeung, A.; Takaoka, H. Community structure, biodiversity and spatiotemporal distribution of the black flies (Diptera: Simuliidae) using Malaise traps on the highest mountain in Thailand. Insects 2021, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Srisuka, W.; Aupalee, K.; Otsuka, Y.; Fukuda, M.; Takaoka, H.; Saeung, A. A new species of Simulium (Gomphostilbia) (Diptera, Simuliidae) from Thailand, with a key to identify females of 14 species of the Simulium varicorne species-group. ZooKeys 2022, 1083, 1. [Google Scholar] [CrossRef] [PubMed]
- McCreadie, J.W.; Adler, P.H.; Grillet, M.E.; Hamada, N. Sampling statistics in understanding distributions of black fly larvae (Diptera: Simuliidae). Acta Entomol. Serbica 2006, 11, 89–96. [Google Scholar]
- Takaoka, H.; Srisuka, W.; Saeung, A. Checklist and keys for the black flies (Diptera: Simuliidae) of Thailand. Med. Entomol. Zool. 2019, 70, 53–77. [Google Scholar] [CrossRef]
- Takaoka, H.; Sofian-Azirun, M.; Ya’cob, Z.; Chen, C.D.; Lau, K.W.; Pham, X.D. The black flies (Diptera: Simuliidae) from Thua Thien Hue and Lam Dong Provinces, Vietnam. Zootaxa 2015, 3961, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Davies, D.M. The Black Flies (Diptera: Simuliidae) of West Malaysia; Kyushu University Press: Fukuoka, Japan, 1995. [Google Scholar]
- Takaoka, H. Four new species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Sarawak, Malaysia. Med. Entomol. Zool. 2008, 59, 181–211. [Google Scholar] [CrossRef]
- Kuvangkadilok, C.; Takaoka, H. Taxonomic notes on Simuliidae (Diptera) from Thailand: Description of a new species and new distributional records of nine known species. Jpn. J. Trop. Med. Hyg. 2000, 28, 167–175. [Google Scholar] [CrossRef]
- Takaoka, H.; Huang, Y.T. A new black fly species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Taiwan, with keys to all 13 species of the Simulium varicorne species-group. Zootaxa 2017, 4312, 438–448. [Google Scholar] [CrossRef]
- Pramual, P.; Tangkawanit, U. A new species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from northeastern Thailand. Med. Entomol. Zool. 2008, 59, 297–303. [Google Scholar] [CrossRef][Green Version]
- Takaoka, H.; Srisuka, W.; Saeung, A.; Choochote, W. A new species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Thailand, with keys to 11 species of the Simulium varicorne species-group. J. Med. Entomol. 2014, 51, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Revised descriptions of the larvae and pupae of the subgenera Gomphostilbia and Nevermannia (Diptera: Simuliidae) in Korea. Entomol. Res. 2015, 45, 193–208. [Google Scholar] [CrossRef]
- Takaoka, H.; Sofian-Azirun, M.; Chen, C.D.; Lau, K.W.; Halim, M.R.A.; Low, V.L.; Suana, I.W. Three new species of black flies (Diptera: Simuliidae) from the Lesser Sunda Archipelago, Indonesia. Trop. Biomed. 2018, 35, 951–974. [Google Scholar] [PubMed]
- Chen, J.Y.; Cao, Y.C. New species and new records of Eusimulium from China (Diptera: Simuliidae). Acta Entomol. Sin. 1983, 26, 229–232. [Google Scholar]
- Takaoka, H. The Black Flies (Diptera: Simuliidae) of Sulawesi, Maluku and Irian Jaya; Kyushu University Press: Fukuoka, Japan, 2003. [Google Scholar]
- Davies, D.M.; Győrkős, H. The Simuliidae (Diptera) of Sri Lanka. Description of a new species of Simulium (Morops). Can. J. Zool. 1988, 66, 605–610. [Google Scholar] [CrossRef]
- Takaoka, H.; Otsuka, Y.; Choochote, W.; Aoki, C.; Hayakawa, H.; Thongsahuan, S. Descriptions of the male, pupa and larva of Simulium (Gomphostilbia) novemarticulatum (Diptera: Simuliidae) from Peninsular Malaysia and Thailand. Med. Entomol. Zool. 2010, 61, 59–67. [Google Scholar] [CrossRef][Green Version]
- Takaoka, H.; Suzuki, H. The blackflies (Diptera: Simuliidae) from Thailand. Med. Entomol. Zool. 1984, 35, 7–45. [Google Scholar] [CrossRef][Green Version]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The Black Flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Marine Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tangkawanit, U.; Wongpakam, K.; Pramual, P. A new black fly (Diptera: Simuliidae) species of the subgenus Asiosimulium Takaoka Choochote from Thailand. Zootaxa 2018, 4388, 111–122. [Google Scholar] [CrossRef]
- Porter, C.H.; Collins, F.H. Species-diagnostic differences in a ribosomal DNA internal transcribed spacer from the sibling species Anopheles freeborni and Anopheles hermsi (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 1991, 45, 271–279. [Google Scholar] [CrossRef]
- Thanwisai, A.; Kuvangkadilok, C.; Baimai, V. Molecular phylogeny of black flies (Diptera: Simuliidae) from Thailand, using ITS2 rDNA. Genetica 2006, 128, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Takaoka, H. Morphotaxonomic revision of Simulium (Gomphostilbia) (Diptera: Simuliidae) in the Oriental Region. Zootaxa 2012, 3577, 1–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).