Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fly Strains
2.3. Generation of Knock-In Flies
2.4. Insecticide Bioassays
2.5. Molecular Docking
3. Results
3.1. Generation and Confirmation of Rdl Mutants
3.2. Differential Susceptibility of Rdl Mutants to Meta-Diamides and Isoxazolines
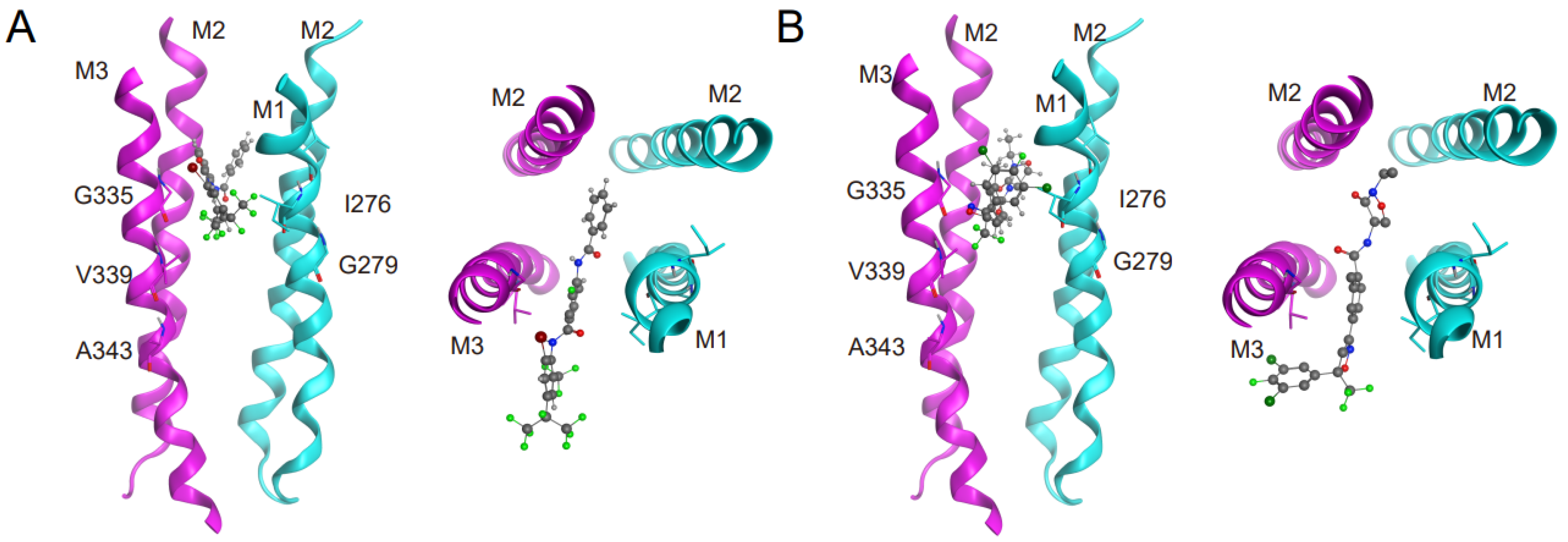
3.3. Interactions of Insecticides with RDL Homology Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ffrench-Constant, R.H.; Rocheleau, T.A.; Steichen, J.C.; Chalmers, A.E. A Point Mutation in a Drosophila GABA Receptor Confers Insecticide Resistance. Nature 1993, 363, 449–451. [Google Scholar] [CrossRef]
- Nakao, T.; Banba, S. Important Amino Acids for Function of the Insect Rdl GABA Receptor. Pest. Manag. Sci. 2021, 77, 3753–3762. [Google Scholar] [CrossRef]
- McGonigle, I.; Lummis, S.C.R. RDL Receptors. Biochem. Soc. Trans. 2009, 37, 1404–1406. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Novel GABA Receptor Pesticide Targets. Pestic. Biochem. Physiol. 2015, 121, 22–30. [Google Scholar] [CrossRef]
- Garrood, W.T.; Zimmer, C.T.; Gutbrod, O.; Lüke, B.; Williamson, M.S.; Bass, C.; Nauen, R.; Emyr Davies, T.G. Influence of the RDL A301S Mutation in the Brown Planthopper Nilaparvata Lugens on the Activity of Phenylpyrazole Insecticides. Pestic. Biochem. Physiol. 2017, 142, 1–8. [Google Scholar] [CrossRef]
- Gomard, Y.; Alout, H.; Lebon, C.; Latreille, A.; Benlali, A.; Mavingui, P.; Tortosa, P.; Atyame, C. Fitness Costs Associated with a GABA Receptor Mutation Conferring Dieldrin Resistance in Aedes Albopictus. Heredity 2022, 129, 273–280. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Z.; Li, M.; Feng, X.; Qiu, X. RDL Mutations Predict Multiple Insecticide Resistance in Anopheles Sinensis in Guangxi, China. Malar. J. 2017, 16, 482. [Google Scholar] [CrossRef]
- Ozoe, Y.; Asahi, M.; Ozoe, F.; Nakahira, K.; Mita, T. The Antiparasitic Isoxazoline A1443 Is a Potent Blocker of Insect Ligand-Gated Chloride Channels. Biochem. Biophys. Res. Commun. 2010, 391, 744–749. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Biggin, P.C.; Mark, B.; Laurence, S.; Brown, A.; Sattelle, D.B.; Sattelle, D.B. Insect GABA Receptors: Splicing, Editing, and Targeting by Antiparasitics and Insecticides. Mol. Pharmacol. Fast Forward. Publ. July 2005, 18, 942–951. [Google Scholar] [CrossRef]
- Cassayre, J.; Smejkal, T.; Blythe, J.; Hoegger, P.; Renold, P.; Pitterna, T.; Prasanna, C.S.; Smits, H.; Godineau, E.; Luksch, T.; et al. The Discovery of Isocycloseram: A Novel Isoxazoline Insecticide. Recent Highlights Discov. Optim. Crop Prot. Prod. 2021, 165–212. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Kagami, T.; Nakahira, K.; Furukawa, Y.; Ozoe, Y. Fluxametamide: A Novel Isoxazoline Insecticide That Acts via Distinctive Antagonism of Insect Ligand-Gated Chloride Channels. Pestic. Biochem. Physiol. 2018, 151, 67–72. [Google Scholar] [CrossRef]
- Insecticide Resistance Action Committee|IRAC. Available online: https://irac-online.org/ (accessed on 23 September 2023).
- Nakao, T.; Banba, S.; Nomura, M.; Hirase, K. Meta-Diamide Insecticides Acting on Distinct Sites of RDL GABA Receptor from Those for Conventional Noncompetitive Antagonists. Insect Biochem. Mol. Biol. 2013, 43, 366–375. [Google Scholar] [CrossRef]
- Yamato, K.; Nakata, Y.; Takashima, M.; Ozoe, F.; Asahi, M.; Kobayashi, M.; Ozoe, Y. Effects of Intersubunit Amino Acid Substitutions on GABA Receptor Sensitivity to the Ectoparasiticide Fluralaner. Pestic. Biochem. Physiol. 2020, 163, 123–129. [Google Scholar] [CrossRef]
- Blythe, J.; Earley, F.G.P.; Piekarska-Hack, K.; Firth, L.; Bristow, J.; Hirst, E.A.; Goodchild, J.A.; Hillesheim, E.; Crossthwaite, A.J. The Mode of Action of Isocycloseram: A Novel Isoxazoline Insecticide. Pestic. Biochem. Physiol. 2022, 187, 105217. [Google Scholar] [CrossRef]
- Nakao, T.; Banba, S.; Hirase, K. Comparison between the Modes of Action of Novel Meta-Diamide and Macrocyclic Lactone Insecticides on the RDL GABA Receptor. Pestic. Biochem. Physiol. 2015, 120, 101–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Sheng, C.; Liu, G.; Zhang, K.; Jia, Z.; Tang, T.; Mao, X.; Jones, A.K.; Han, Z.; et al. G3′MTMD3 in the Insect GABA Receptor Subunit, RDL, Confers Resistance to Broflanilide and Fluralaner. PLoS Genet. 2023, 19, e1010814. [Google Scholar] [CrossRef]
- Huang, J.; Lee, Y. The Power of Drosophila Genetics in Studying Insect Toxicology and Chemical Ecology. Crop Health 2023, 1, 12. [Google Scholar] [CrossRef]
- Douris, V.; Denecke, S.; Van Leeuwen, T.; Bass, C.; Nauen, R.; Vontas, J. Using CRISPR/Cas9 Genome Modification to Understand the Genetic Basis of Insecticide Resistance: Drosophila and Beyond. Pestic. Biochem. Physiol. 2020, 167, 104595. [Google Scholar] [CrossRef]
- Scott, J.G.; Buchon, N. Drosophila Melanogaster as a Powerful Tool for Studying Insect Toxicology. Pestic. Biochem. Physiol. 2019, 161, 95–103. [Google Scholar] [CrossRef]
- Guo, L.; Qiao, X.; Haji, D.; Zhou, T.; Liu, Z.; Whiteman, N.K.; Huang, J. Convergent Resistance to GABA Receptor Neurotoxins through Plant–Insect Coevolution. Nat. Ecol. Evol. 2023, 7, 1444–1456. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, X.; Zhou, Z.; Guo, L.; Wu, W.; Ma, S.; Zhang, X.; Montell, C.; Huang, J. An Insecticide Target in Mechanoreceptor Neurons. Sci. Adv. 2022, 8, 3132. [Google Scholar] [CrossRef]
- Guo, L.; Fan, X.Y.; Qiao, X.; Montell, C.; Huang, J. An Octopamine Receptor Confers Selective Toxicity of Amitraz on Honeybees and Varroa Mites. eLife 2021, 10, e68268. [Google Scholar] [CrossRef]
- Nakao, T.; Banba, S. Broflanilide: A Meta-Diamide Insecticide with a Novel Mode of Action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Matsui, H.; Nakahira, K. Differential Mechanisms of Action of the Novel γ-Aminobutyric Acid Receptor Antagonist Ectoparasiticides Fluralaner (A1443) and Fipronil. Pest. Manag. Sci. 2015, 71, 91–95. [Google Scholar] [CrossRef]
- Gassel, M.; Wolf, C.; Noack, S.; Williams, H.; Ilg, T. The Novel Isoxazoline Ectoparasiticide Fluralaner: Selective Inhibition of Arthropod γ-Aminobutyric Acid- and l-Glutamate-Gated Chloride Channels and Insecticidal/Acaricidal Activity. Insect Biochem. Mol. Biol. 2014, 45, 111–124. [Google Scholar] [CrossRef]
- Nakao, T.; Banba, S. Mechanisms Underlying the Selectivity of Meta-Diamides between Insect Resistance to Dieldrin (RDL) and Human γ-Aminobutyric Acid (GABA) and Glycine Receptors. Pest. Manag. Sci. 2021, 77, 3744–3752. [Google Scholar] [CrossRef]
- Berticat, C.; Bonnet, J.; Duchon, S.; Agnew, P.; Weill, M.; Corbel, V. Costs and Benefits of Multiple Resistance to Insecticides for Culex Quinquefasciatus Mosquitoes. BMC Evol. Biol. 2008, 8, 104. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Yang, Y.; Li, H.; Wang, X.; Yang, B.; Zhang, J.; Li, C.; Millar, N.S.; Liu, Z. Synergistic and Compensatory Effects of Two Point Mutations Conferring Target-Site Resistance to Fipronil in the Insect GABA Receptor RDL. Sci. Rep. 2016, 6, 32335. [Google Scholar] [CrossRef]
- Foster, S.P.; Harrington, R.; Devonshire, A.L.; Denholm, I.; Devine, G.J.; Kenward, M.G.; Bale, J.S. Comparative Survival of Insecticide-Susceptible and Resistant Peach-Potato Aphids, Myzus Persicae (Sulzer) (Hemiptera: Aphididae), in Low Temperature Field Trials. Bull. Entomol. Res. 1996, 86, 17–27. [Google Scholar] [CrossRef]
- Berticat, C.; Boquien, G.; Raymond, M.; Chevillon, C. Insecticide Resistance Genes Induce a Mating Competition Cost in Culex Pipiens Mosquitoes. Genet Res. 2002, 79, 41–47. [Google Scholar] [CrossRef]
- Edi, C.V.; Djogbénou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.T.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Kétoh, G.K.; Paine, M.J.I.; et al. CYP6 P450 Enzymes and ACE-1 Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito Anopheles Gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef] [PubMed]
| Region | Point-Mutation | Insecticides | Potency Changes | Reference |
|---|---|---|---|---|
| M1 | I276F | Broflanilide | 5.7 | [13] |
| Fluralaner | 0.7 | [14] | ||
| I276C | Fluralaner | 0.06 | [14] | |
| L280C | Broflanilide | 5.7 | [13] | |
| Fluralaner | 23.0 | [14] | ||
| M3 | G335M | Broflanilide | >344.8 | [13] |
| Isocycloseram | 50.3 | [15] | ||
| Fluralaner | >116.8 | [14] | ||
| V339N | Broflanilide | 0.13 | [16] |
| Insecticides | Strain | LC50 (mg/L) | 95% CL | Resistance Ratio |
|---|---|---|---|---|
| Broflanilide | w1118 | 0.14 | 0.10–0.20 | 1 |
| +/+ | 0.14 | 0.13–0.17 | ||
| I276F | 0.16 | 0.14–0.19 | 1.14 | |
| I276C | 0.25 | 0.19–0.31 | 1.79 | |
| G279S | 0.10 | 0.06–0.12 | 0.71 | |
| I276F+G279S | 0.15 | 0.11–0.33 | 1.07 | |
| V339I | 0.13 | 0.12–0.15 | 0.93 | |
| A343T | 0.13 | 0.11–0.14 | 0.93 | |
| Isocycloseram | w1118 | 8.0 | 6.9–9.1 | 1 |
| +/+ | 9.7 | 9.2–10.1 | ||
| I276F | 13.9 | 11.3–17.1 | 1.6 | |
| I276C | 49.3 | 39.0–62.7 | 5.7 | |
| G279S | 22.5 | 18.5–29.7 | 2.6 | |
| I276F+G279S | 15.3 | 11.0–25.1 | 1.8 | |
| V339I | 16.9 | 12.1–26.2 | 1.9 | |
| A343T | 18.6 | 16.2–21.2 | 2.1 | |
| Fluxametamide | w1118 | 2.3 | 1.7–2.9 | 1 |
| +/+ | 5.6 | 4.5–6.9 | ||
| I276F | 4.9 | 3.4–10.0 | 1.5 | |
| I276C | 41.0 | 28.4–55.3 | 12.6 | |
| G279S | 2.5 | 1.7–3.6 | 0.8 | |
| I276F+G279S | 14.7 | 11.7–18.4 | 4.5 | |
| V339I | 5.0 | 4.2–6.1 | 1.6 | |
| A343T | 5.9 | 5.0–7.4 | 1.9 | |
| Fluralaner | w1118 | 0.23 | 0.18–0.28 | 1 |
| +/+ | 0.23 | 0.18–0.28 | ||
| I276F | 0.21 | 0.17–0.25 | 0.91 | |
| I276C | 0.33 | 0.22–0.49 | 1.43 | |
| G279S | 0.10 | 0.09–0.11 | 0.43 | |
| I276F+G279S | 0.49 | 0.37–0.63 | 2.13 | |
| V339I | 0.18 | 0.15–0.21 | 0.78 | |
| A343T | 0.20 | 0.18–0.22 | 0.87 |
| Insecticides | Strain | LC50 (mg/kg) | 95% CL | Resistance Ratio |
|---|---|---|---|---|
| Broflanilide | w1118 | 0.8 | 0.5–1 | 1 |
| +/+ | 0.6 | 0.5–0.9 | ||
| G335M | >1000 | / | >1428 | |
| Isocycloseram | w1118 | 2.5 | 2.0–3.8 | 1 |
| +/+ | 2.8 | 2.1–6.6 | ||
| G335M | >1000 | / | >377 | |
| Fluxametamide | w1118 | 2.5 | 1.9–3.3 | 1 |
| +/+ | 2.7 | 1.8–4.5 | ||
| G335M | >1000 | / | >384 | |
| Fluralaner | w1118 | 2.9 | 2.2–3.6 | 1 |
| +/+ | 2.8 | 2.0–5.8 | ||
| G335M | >1000 | / | >350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Wu, W.; Ma, S.; Chen, J.; Huang, J.; Qiao, X. Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster. Insects 2024, 15, 334. https://doi.org/10.3390/insects15050334
Zhou T, Wu W, Ma S, Chen J, Huang J, Qiao X. Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster. Insects. 2024; 15(5):334. https://doi.org/10.3390/insects15050334
Chicago/Turabian StyleZhou, Tianhao, Weiping Wu, Suhan Ma, Jie Chen, Jia Huang, and Xiaomu Qiao. 2024. "Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster" Insects 15, no. 5: 334. https://doi.org/10.3390/insects15050334
APA StyleZhou, T., Wu, W., Ma, S., Chen, J., Huang, J., & Qiao, X. (2024). Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster. Insects, 15(5), 334. https://doi.org/10.3390/insects15050334







