Investigation of the Tribological Properties of Hybrid Additive-Modified Water-Based Lubricating Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protic Ionic Liquid
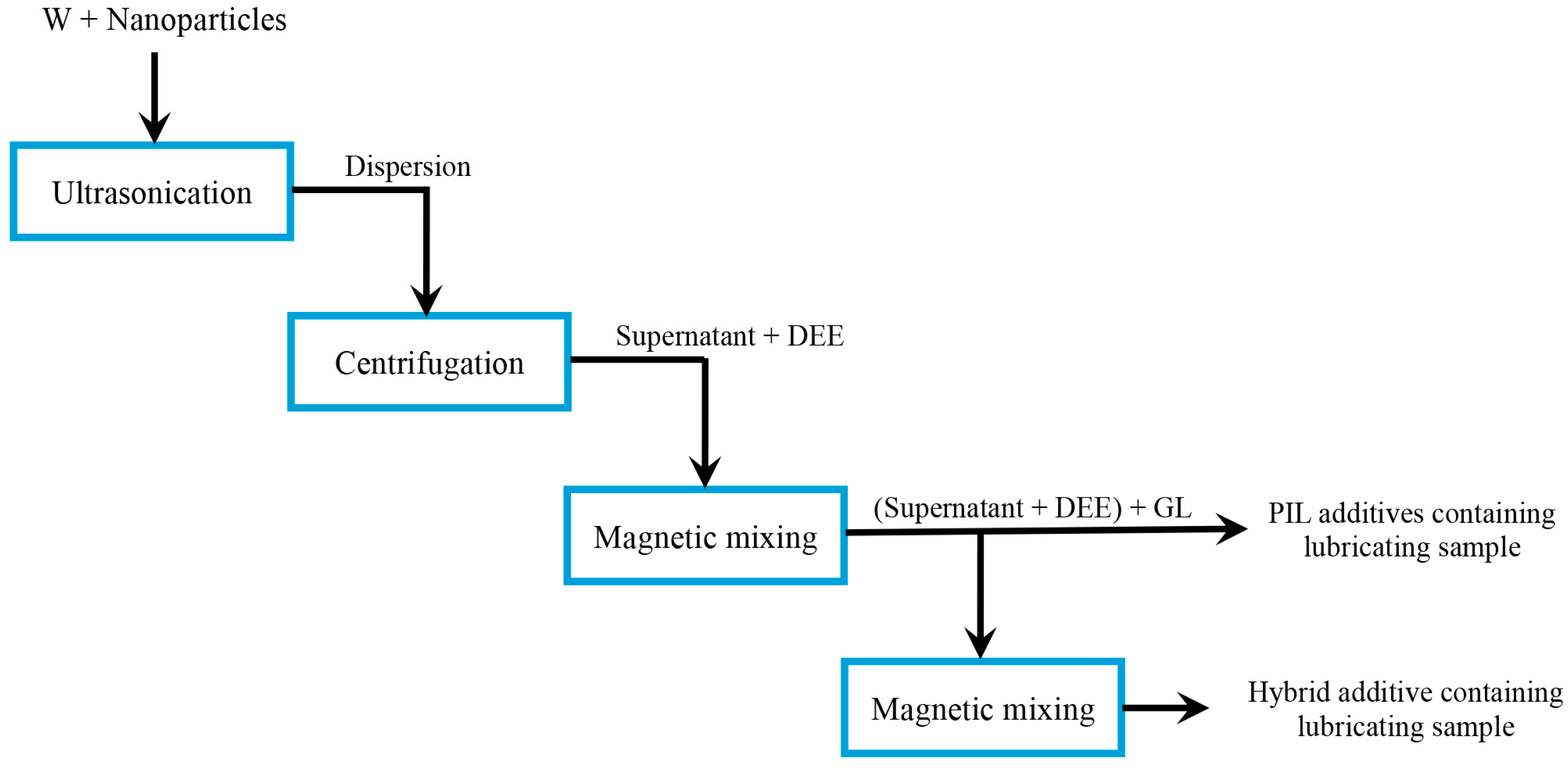
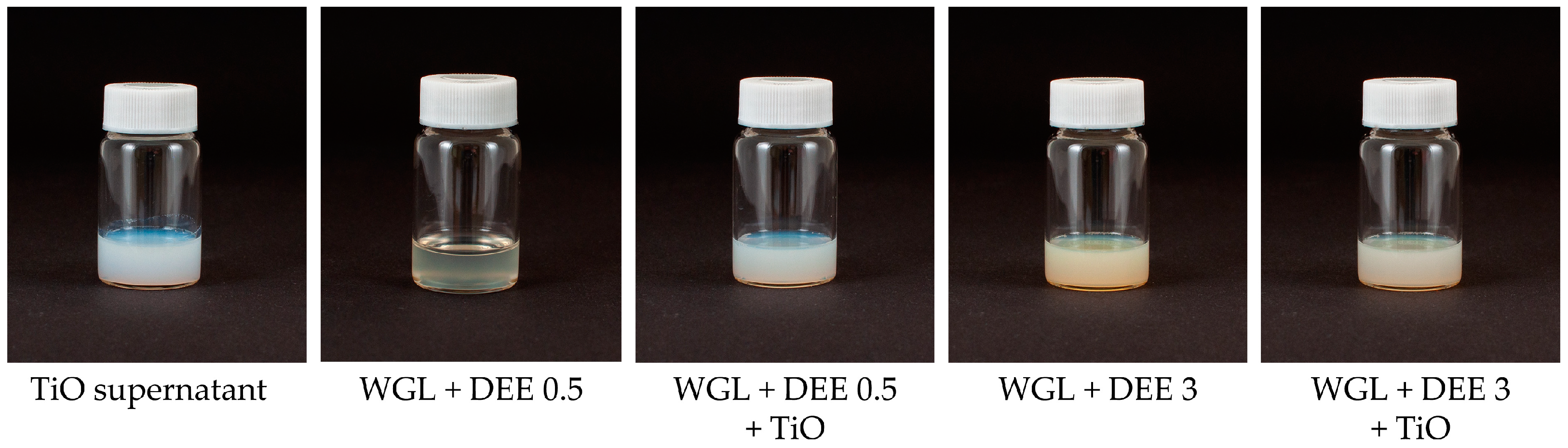
2.2. Preparation of Lubricating Samples
2.3. Physicochemical Properties and Rust Prevention Ability
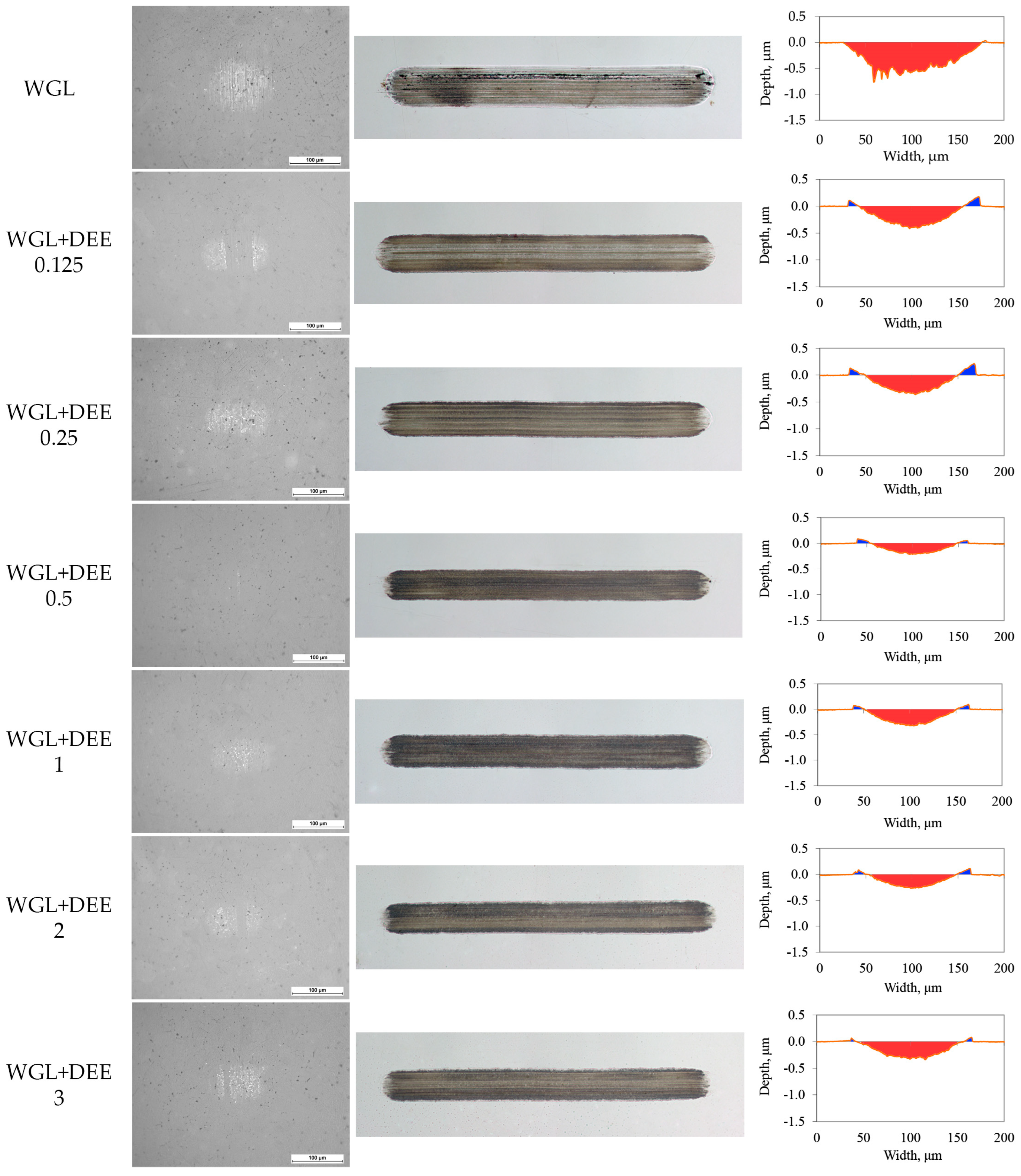
2.4. Tribo-Tests and Worn Surface Analysis
3. Results and Discussion
3.1. Physicochemical Properties and Rust Prevention Ability of Prepared Lubricating Samples
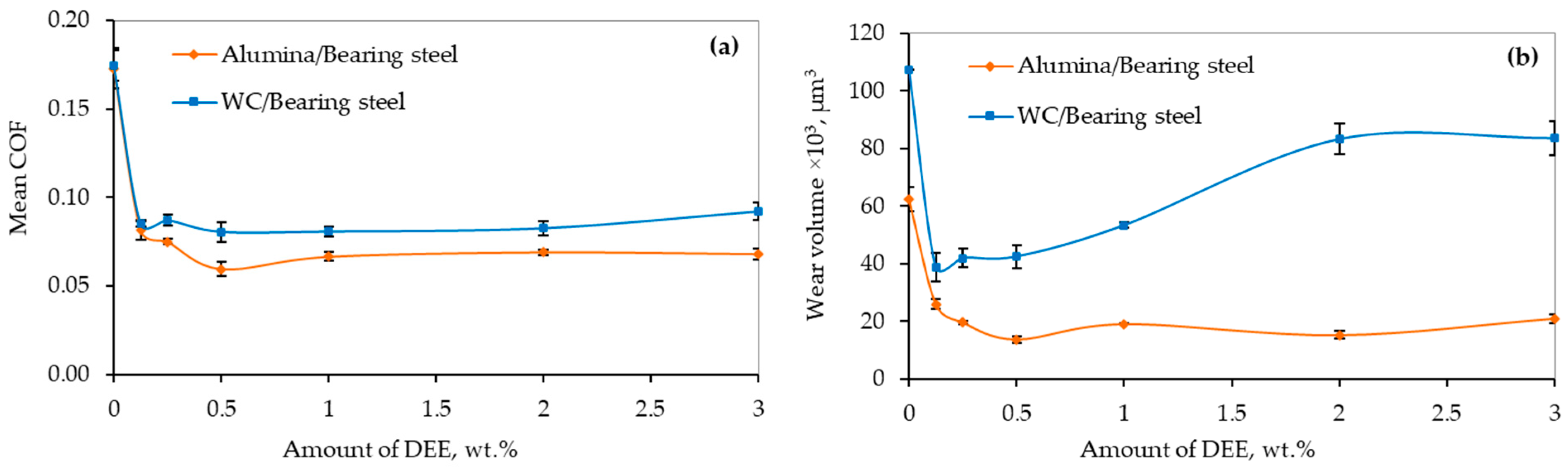
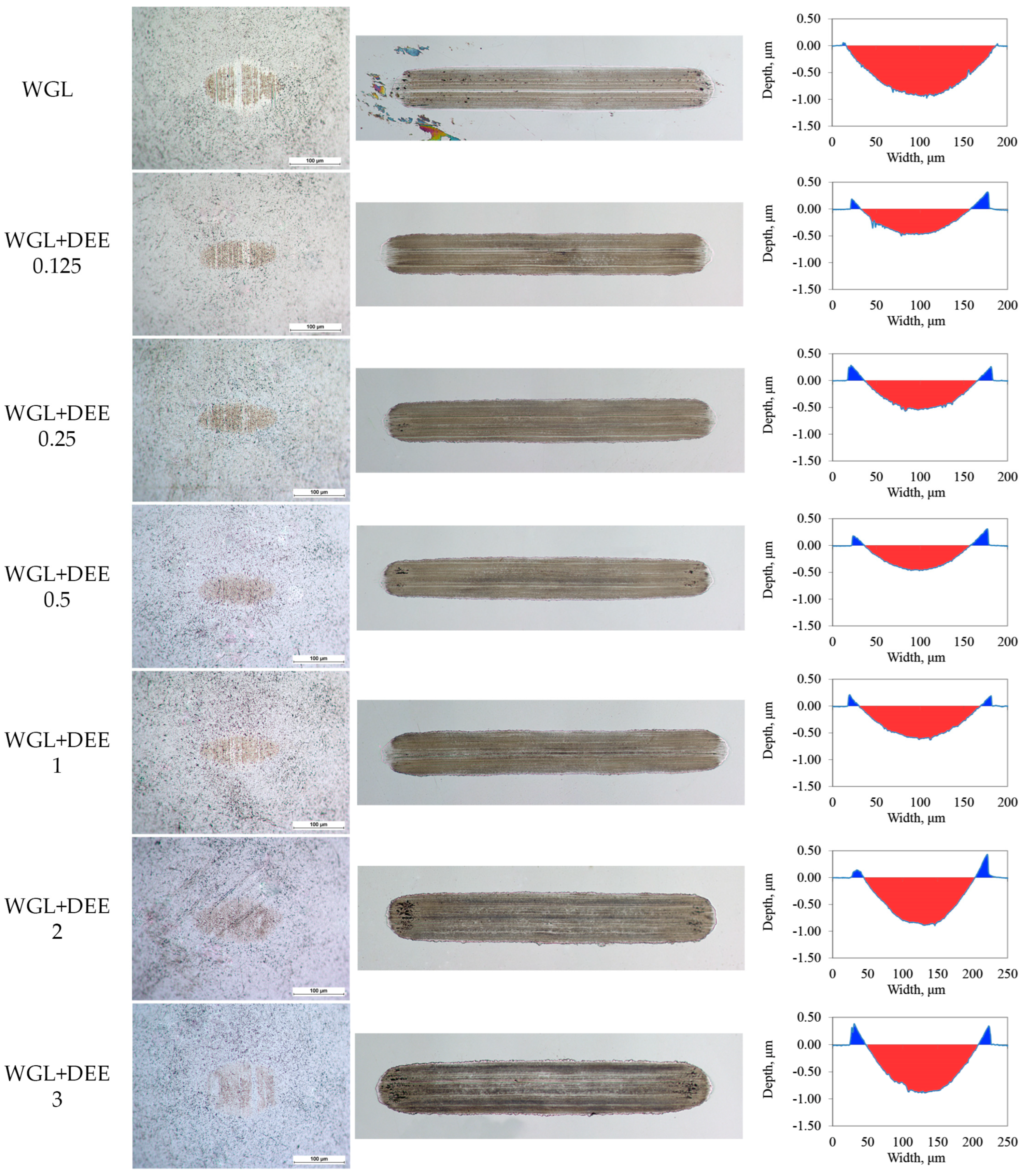
3.2. Lubricity of Protic Ionic Liquid Additive
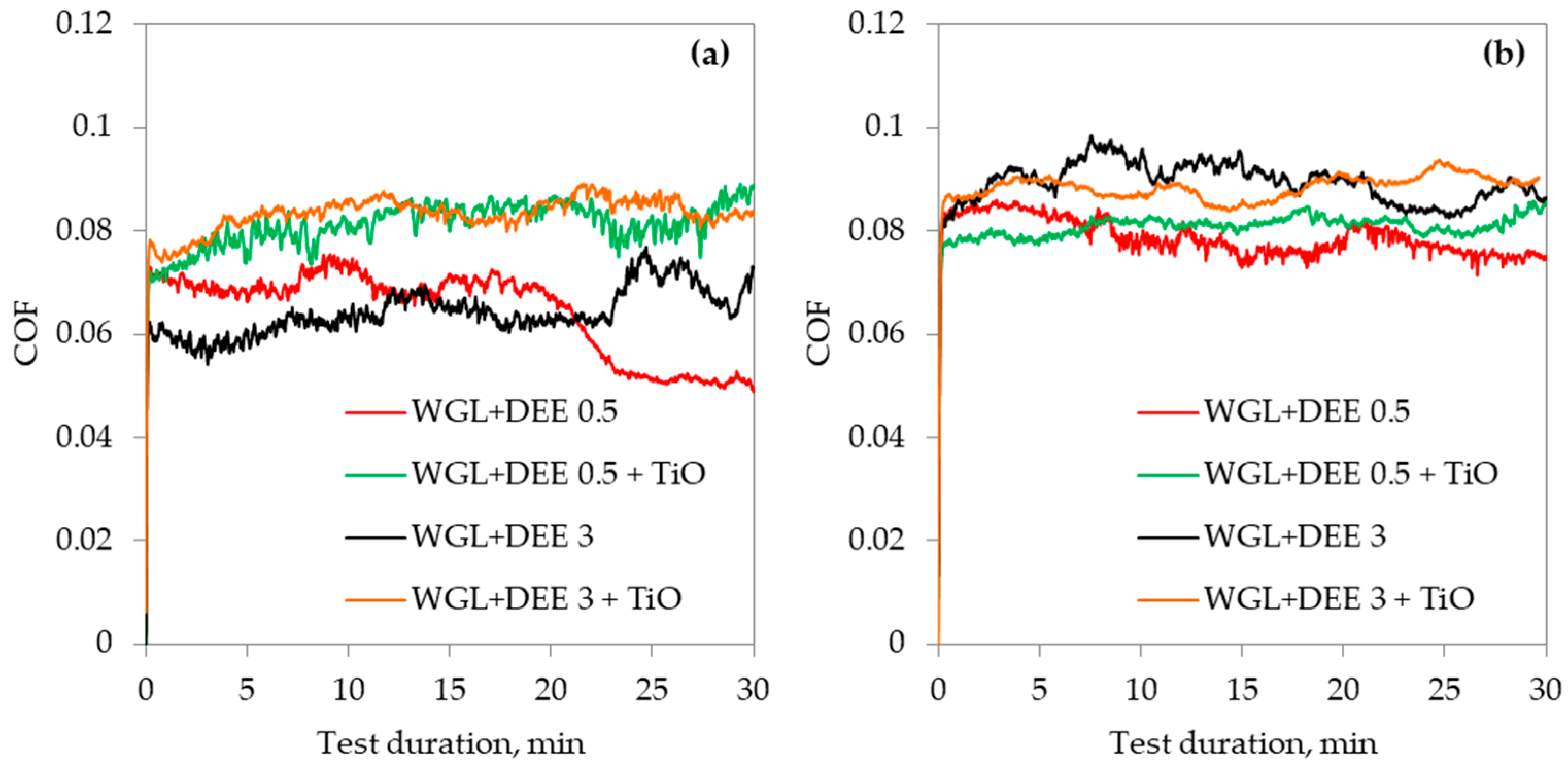
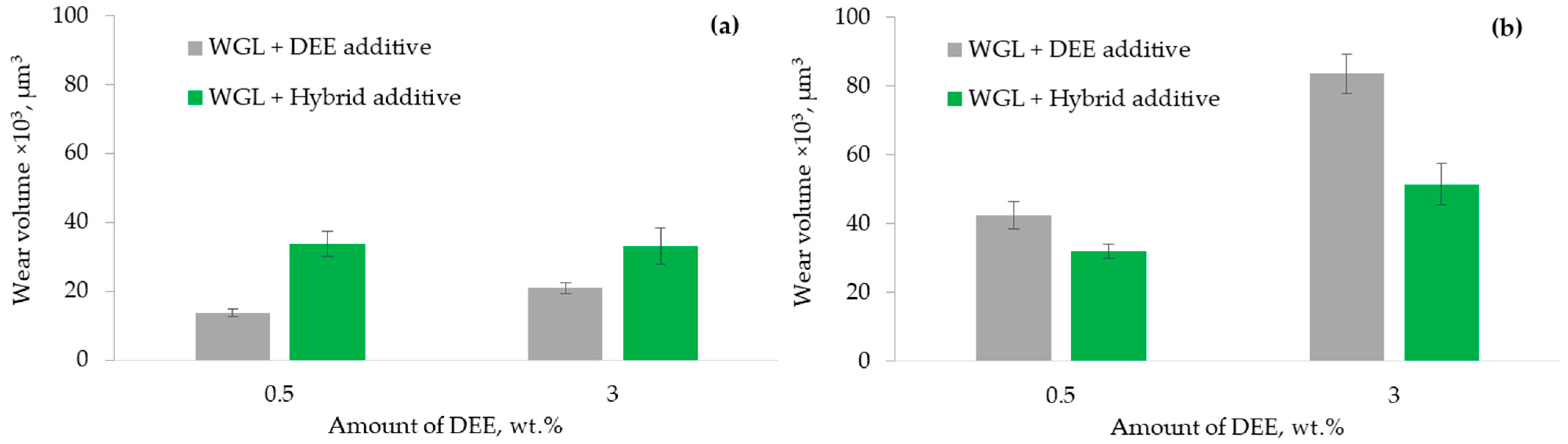
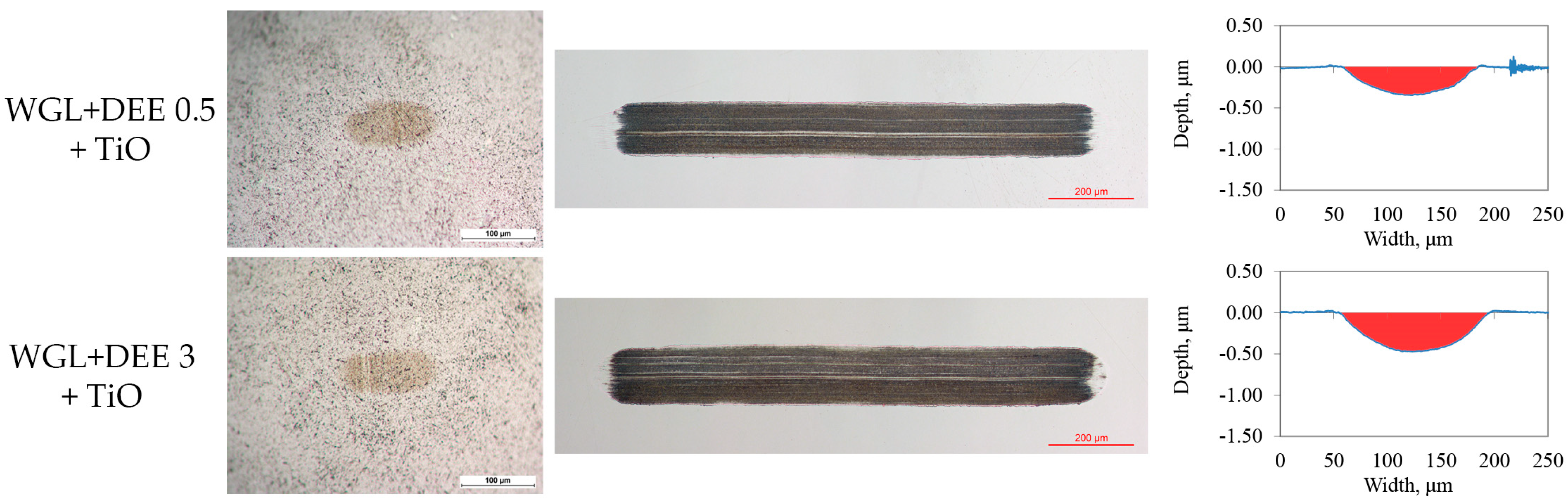
3.3. Lubricity of Hybrid Additives
4. Conclusions
- Bis (2-hydroxyethyl)ammonium erucate protic ionic liquid prevented corrosion when used as an additive in glycerol aqueous solutions. Corrosion was eliminated by loading base fluid with 3 wt. % of protic ionic liquid. The introduction of nanoparticles did not change this behaviour.
- Bis(2-hydroxyethyl)ammonium erucate protic ionic liquid improved the lubricity of water-based lubricating fluid by a few orders of magnitude. A greater improvement was achieved in lubricating the Alumina/Bearing steel friction pair. It was found that 0.5 wt. % concentration of the investigated ionic liquid in the base fluid was the most effective.
- The application of the hybrid additive improved lubricity when it was used to lubricate the WC/Bearing steel friction pair. On the other hand, the lubricity of the Alumina/Bearing steel friction pair diminished.
- It was proposed that the adsorption layer of protic ionic liquid molecules was responsible for preventing corrosion and improving lubricity. During the tribo-interaction, the adsorbed layer could react with metal surfaces, forming either friction polymer or metal soap. With the introduction of titanium oxide, the lubrication mechanism could involve rolling and tribo-sintering of nanoparticles.
- Even though further studies are necessary to understand the lubricity of suggested hybrid additives in various friction pairs, the presented results could be interesting for those who seek environmentally friendly alternatives.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Business Research Company. Metalworking Fluids Global Market Report 2024; The Business Research Company: Hyderabad, India, 2023. [Google Scholar]
- Nune, M.M.R.; Chaganti, P.K. Development, Characterization, and Evaluation of Novel Eco-Friendly Metal Working Fluid. Measurement 2019, 137, 401–416. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Suvin, P.S.; Kailas, S.V.; Mamilla, R.S. Thermal, Rheological, Wettability and Hard Machining Performance of MoS2 and CaF2 Based Minimum Quantity Hybrid Nano-Green Cutting Fluids. J. Mater. Process. Technol. 2019, 266, 125–139. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Suvin, P.S.; Kailas, S.V.; Sankar, M.R. Hard Machining Performance of Indigenously Developed Green Cutting Fluid Using Flood Cooling and Minimum Quantity Cutting Fluid. J Clean Prod. 2019, 206, 108–123. [Google Scholar] [CrossRef]
- Najiha, M.S.; Rahman, M.M.; Yusoff, A.R. Environmental Impacts and Hazards Associated with Metal Working Fluids and Recent Advances in the Sustainable Systems: A Review. Renew. Sustain. Energy Rev. 2016, 60, 1008–1031. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, C.; Zhang, C.; Zhao, S.; Cai, M.; Liu, Z.; Yu, Q. Amino Acid Ionic Liquids as Anticorrosive and Lubricating Additives for Water and Their Environmental Impact. Tribol. Int. 2021, 153, 106663. [Google Scholar] [CrossRef]
- Xie, G.; Liu, S.; Guo, D.; Wang, Q.; Luo, J. Investigation of the Running-in Process and Friction Coefficient under the Lubrication of Ionic Liquid/Water Mixture. Appl. Surf. Sci. 2009, 255, 6408–6414. [Google Scholar] [CrossRef]
- Amiril, S.A.S.; Rahim, E.A.; Syahrullail, S. A Review on Ionic Liquids as Sustainable Lubricants in Manufacturing and Engineering: Recent Research, Performance, and Applications. J. Clean Prod. 2017, 168, 1571–1589. [Google Scholar] [CrossRef]
- Nasser, K.I.; Liñeira del Río, J.M.; Mariño, F.; López, E.R.; Fernández, J. Double Hybrid Lubricant Additives Consisting of a Phosphonium Ionic Liquid and Graphene Nanoplatelets/Hexagonal Boron Nitride Nanoparticles. Tribol. Int. 2021, 163, 107189. [Google Scholar] [CrossRef]
- Nasser, K.I.; Liñeira del Río, J.M.; López, E.R.; Fernández, J. Hybrid Combinations of Graphene Nanoplatelets and Phosphonium Ionic Liquids as Lubricant Additives for a Polyalphaolefin. J. Mol. Liq. 2021, 336, 116266. [Google Scholar] [CrossRef]
- Avilés, M.D.; Pamies, R.; Sanes, J.; Arias-Pardilla, J.; Carrión, F.J.; Bermúdez, M.D. Protic Ammonium Bio-Based Ionic Liquid Crystal Lubricants. Tribol. Int. 2021, 158, 106917. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Treinytė, J. Investigation of Tribological Properties of Environmentally Friendly Ionic Liquids as a Potential Lubricity Improving Additives for Water-Based Lubricants. Ind. Lubr. Tribol. 2022, 74, 294–301. [Google Scholar] [CrossRef]
- Donato, M.T.; Colaço, R.; Branco, L.C.; Saramago, B. A Review on Alternative Lubricants: Ionic Liquids as Additives and Deep Eutectic Solvents. J. Mol. Liq. 2021, 333, 116004. [Google Scholar] [CrossRef]
- Shekhar, H.; Dumpala, R. Overcoming Friction and Steps towards Superlubricity: A Review of Underlying Mechanisms. Appl. Surf. Sci. Adv. 2021, 6, 100175. [Google Scholar] [CrossRef]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the Secrets behind Liquid Superlubricity: A State-of-the-Art Review on Phenomena and Mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Han, T.; Yi, S.; Zhang, C.; Li, J.; Chen, X.; Luo, J.; Banquy, X. Superlubrication Obtained with Mixtures of Hydrated Ions and Polyethylene Glycol Solutions in the Mixed and Hydrodynamic Lubrication Regimes. J. Colloid Interface Sci. 2020, 579, 479–488. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf.-Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Nyholm, N.; Espallargas, N. Functionalized Carbon Nanostructures as Lubricant Additives—A Review. Carbon N. Y. 2023, 201, 1200–1228. [Google Scholar] [CrossRef]
- Lee, G.J.; Rhee, C.K. Enhanced Thermal Conductivity of Nanofluids Containing Graphene Nanoplatelets Prepared by Ultrasound Irradiation. J. Mater. Sci. 2014, 49, 1506–1511. [Google Scholar] [CrossRef]
- Sanukrishna, S.S.; Vishnu, S.; Jose Prakash, M. Experimental Investigation on Thermal and Rheological Behaviour of PAG Lubricant Modified with SiO2 Nanoparticles. J. Mol. Liq. 2018, 261, 411–422. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.; Varman, M.; Kalam, M.; Mufti, R.A.; Zulkifli, N.; Yunus, R.; Zahid, R. Improving the AW/EP Ability of Chemically Modified Palm Oil by Adding CuO and MoS2 Nanoparticles. Tribol. Int. 2015, 88, 271–279. [Google Scholar] [CrossRef]
- Dassenoy, F. Nanoparticles as Additives for the Development of High Performance and Environmentally Friendly Engine Lubricants. Tribol. Online 2019, 14, 237–253. [Google Scholar] [CrossRef]
- Saini, V.; Bijwe, J.; Seth, S.; Ramakumar, S.S.V. Unexplored Solid Lubricity of Titanium Nanoparticles in Oil to Modify the Metallic Interfaces. Appl. Surf. Sci. 2022, 580, 152127. [Google Scholar] [CrossRef]
- Kumar, R.; Gautam, R.K. Tribological Investigation of Sunflower and Soybean Oil with Metal Oxide Nanoadditives. Biomass Convers. Biorefin. 2024, 14, 2389–2401. [Google Scholar] [CrossRef]
- Alves, S.M.; Mello, V.S.; Faria, E.A.; Camargo, A.P.P. Nanolubricants Developed from Tiny CuO Nanoparticles. Tribol. Int. 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological Behavior of Vegetable Oil-Based Lubricants with Nanoparticles of Oxides in Boundary Lubrication Conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Ionic Liquid and Nanoparticle Hybrid Systems: Emerging Applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Liñeira del Río, J.M.; López, E.R.; Fernández, J. Synergy between Boron Nitride or Graphene Nanoplatelets and Tri(Butyl)Ethylphosphonium Diethylphosphate Ionic Liquid as Lubricant Additives of Triisotridecyltrimellitate Oil. J. Mol. Liq. 2020, 301, 112442. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ding, Q.; Li, H.; Qin, B.; Hu, L. Understanding the Synergistic Lubrication Effect of 2-Mercaptobenzothiazolate Based Ionic Liquids and Mo Nanoparticles as Hybrid Additives. Tribol. Int. 2018, 125, 39–45. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Treinytė, J.; Kupčinskas, A.; Gumbytė, M.; Andriušis, A. Improving Tribological Properties of Water/Glycerol Lubricating Fluid by the Synergy of Nanoparticles and Protic Ionic Liquid. Wear 2023, 534, 205133. [Google Scholar] [CrossRef]
- Upendra, M.; Vasu, V. Synergistic Effect Between Phosphonium-Based Ionic Liquid and Three Oxide Nanoparticles as Hybrid Lubricant Additives. J. Tribol. 2020, 142, 052101. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.D.; Saurín, N.; Espinosa, T.; Carrión, F.J.; Bermúdez, M.D. Synergy between Graphene and Ionic Liquid Lubricant Additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Carrión, F.J.; Avilés, M.D.; Nakano, K.; Tadokoro, C.; Nagamine, T.; Bermúdez, M.D. Diprotic Ammonium Palmitate Ionic Liquid Crystal and Nanodiamonds in Aqueous Lubrication. Film Thickness and Influence of Sliding Speed. Wear 2019, 418, 241–252. [Google Scholar] [CrossRef]
- Hao, L.; Hao, W.; Li, P.; Liu, G.; Li, H.; Aljabri, A.; Xie, Z. Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants 2022, 10, 125. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Control of Surface Forces through Hydrated Boundary Layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Kupčinskas, A.; Treinytė, J.; Sendžikienė, E. Synthesis and Tribological Properties of Bis(2-Hydroxyethyl)Ammonium Erucate as a Potential Environmentally Friendly Lubricant and Lubricant Additive. Appl. Sci. 2023, 13, 3401. [Google Scholar] [CrossRef]
- Noor El-Din, M.R.; Mishrif, M.R.; Kailas, S.V.; Ps, S.; Mannekote, J.K. Studying the Lubricity of New Eco-Friendly Cutting Oil Formulation in Metal Working Fluid. Ind. Lubr. Tribol. 2018, 70, 1569–1579. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, H.W.; Chang, H.Y.; Lim, B.T.; Park, H.B.; Kim, Y.S. Corrosion Inhibiting Mechanism of Nitrite Ion on the Passivation of Carbon Steel and Ductile Cast Iron for Nuclear Power Plants. Adv. Mater. Sci. Eng. 2015, 2015, 408138. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, G.; Ding, T.; Xiang, X.; Li, F.; Ren, T.; Liu, S.; Zheng, L. Tribological Properties and Surface Interaction of Novel Water-Soluble Ionic Liquid in Water-Glycol. Tribol. Int. 2017, 116, 440–448. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Li, L.; Bai, P.; Wen, X.; Zhou, X.; Ma, K.; Meng, Y.; Ding, J.; Tian, Y. Wear Mechanism and Evolution of Tribofilm of Ceramic on Steel Pairs under Ester Oil Lubrication in Wide Temperature Range. J. Mater. Res. Technol. 2023, 27, 6984–6996. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Taha-Tijerina, J.; García, A.; Maldonado, D.; González, J.A.; Molina, D.; Palacios, E.; Cantú, P. Antiwear and Extreme Pressure Properties of Nanofluids for Industrial Applications. Tribol. Trans. 2014, 57, 1072–1076. [Google Scholar] [CrossRef]
- Chang, H.; Lan, C.; Chen, C.; Kao, M.; Guo, J. Anti-Wear and Friction Properties of Nanoparticles as Additives in the Lithium Grease. Int. J. Precis. Eng. Manuf. 2014, 15, 2059–2063. [Google Scholar] [CrossRef]
- Song, Z.; Liang, Y.; Fan, M.; Zhou, F.; Liu, W. Ionic Liquids from Amino Acids: Fully Green Fluid Lubricants for Various Surface Contacts. RSC Adv. 2014, 4, 19396–19402. [Google Scholar] [CrossRef]
- Furey, M.J.; Kajdas, C.; Kempinski, R. Applications of the Concept of Tribopolymerisation in Fuels, Lubricants, Metalworking, and “minimalist” Lubrication. Lubr. Sci. 2002, 15, 73–82. [Google Scholar] [CrossRef]
- Wang, Q.J.; Chung, Y.-W. Encyclopedia of Tribology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Xia, W.; Zhao, J.; Wu, H.; Zhao, X.; Zhang, X.; Xu, J.; Jiao, S.; Wang, X.; Zhou, C.; Jiang, Z. Effects of Oil-in-Water Based Nanolubricant Containing TiO2 Nanoparticles in Hot Rolling of 304 Stainless Steel. J. Mater. Process. Technol. 2018, 262, 149–156. [Google Scholar] [CrossRef]




| Investigated Nanoparticles, CAS No., and Abbreviation | Appearance | Particle Size, nm | Molecular Weight, g/mol | Trace Metals, % |
|---|---|---|---|---|
| Titanium (IV) oxide 13463-67-7 [TiO] | White powder | 21 | 79.87 | ≥99.5 |
| Test Temperature, °C | Test Duration, min | Stroke Length, mm | Load, N | Amount of Lubricating Sample, mL | Reciprocation Frequency, Hz |
|---|---|---|---|---|---|
| 30 | 30 | 1 | 4 | 1 | 15 |
| Lubricating Sample | Kinematic Viscosity @ 30 °C, mm2/s | Density @ 30 °C, g/cm3 | pH |
|---|---|---|---|
| WGL | 3.842 ± 0.004 | 1.121 ± 0.0001 | 8.03 ± 0.013 |
| WGL+DEE 0.125 | 4.061 ± 0.003 | 1.123 ± 0.0003 | 8.46 ± 0.007 |
| WGL+DEE 0.25 | 4.336 ± 0.020 | 1.122 ± 0.0002 | 8.52 ± 0.005 |
| WGL+DEE 0.5 | 4.880 ± 0.042 | 1.121 ± 0.0002 | 8.63 ± 0.011 |
| WGL+DEE 1 | 5.330 ± 0.033 | 1.119 ± 0.0003 | 8.88 ± 0.012 |
| WGL+DEE 2 | 6.440 ± 0.049 | 1.118 ± 0.0001 | 9.16 ± 0.007 |
| WGL+DEE 3 | 8.094 ± 0.067 | 1.117 ± 0.0002 | 9.20 ± 0.007 |
| WGL+DEE 0.5 + TiO2 | 4.916 ± 0.015 | 1.122 ± 0.0002 | 8.60 ± 0.013 |
| WGL+DEE 3 + TiO2 | 8.272 ± 0.021 | 1.116 ± 0.0003 | 9.13 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreivaitis, R.; Treinytė, J.; Kupčinskas, A.; Gumbytė, M.; Sendžikienė, E. Investigation of the Tribological Properties of Hybrid Additive-Modified Water-Based Lubricating Fluid. Lubricants 2024, 12, 178. https://doi.org/10.3390/lubricants12050178
Kreivaitis R, Treinytė J, Kupčinskas A, Gumbytė M, Sendžikienė E. Investigation of the Tribological Properties of Hybrid Additive-Modified Water-Based Lubricating Fluid. Lubricants. 2024; 12(5):178. https://doi.org/10.3390/lubricants12050178
Chicago/Turabian StyleKreivaitis, Raimondas, Jolanta Treinytė, Artūras Kupčinskas, Milda Gumbytė, and Eglė Sendžikienė. 2024. "Investigation of the Tribological Properties of Hybrid Additive-Modified Water-Based Lubricating Fluid" Lubricants 12, no. 5: 178. https://doi.org/10.3390/lubricants12050178





