Myositis “Diaphragm Cramp” as a Potential Cause of Respiratory Arrests in Infants. Comment on Salfi, N.C.M. et al. Fatal Deterioration of a Respiratory Syncytial Virus Infection in an Infant with Abnormal Muscularization of Intra-Acinar Pulmonary Arteries: Autopsy and Histological Findings. Diagnostics 2024, 14, 601
Acknowledgments
Conflicts of Interest
References
- Salfi, N.C.M.; Vergine, G.; Poloni, M.; Metalli, S.; Bigucci, B.; Facondini, F.; Pedrazzi, G.; Masciopinto, F.; Bernabè, L.; Sambri, V.; et al. Fatal deterioration of a respiratory syncytial virus infection in an infant with abnormal muscularization of intra-acinar pulmonary arteries: Autopsy and histological findings. Diagnostics 2024, 14, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javouhey, E.; Pouyau, R.; Massenavette, B. Pathophysiology of acute respiratory failure in children with bronchiolitis and effect of CPAP. In Noninvasive Ventilation in High-Risk Infections and Mass Casualty Events; Springer Nature: Berlin/Heidelberg, Germany, 2013; Volume 29, pp. 233–249. [Google Scholar] [CrossRef] [PubMed Central]
- Siren, P.M.; Siren, M.J. Critical diaphragm failure in sudden infant death syndrome. Upsala J. Med. Sci. 2011, 116, 115–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schroeder, A.R.; Mansbach, J.M.; Stevenson, M.; Macias, C.G.; Fisher, E.S.; Barcega, B.; Sullivan, A.F.; Espinola, J.A.; Piedra, P.A.; Camargo, C.A. Apnea in children hospitalized with bronchiolitis. Pediatrics 2013, 132, e1194-201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agyeman, P.; Duppenthaler, A.; Heininger, U.; Aebi, C. Influenza-associated myositis in children. Infection 2004, 32, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kariks, J. Diaphragmatic muscle fibre necrosis in SIDS. Forensic Sci. Int. 1989, 43, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M. Features of diaphragmatic myositis in a case of sudden infant death. Upsala J. Med. Sci. 2011, 116, 220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Rigatto, M.; de Medeiros, N. Diaphragmatic flutter. Report of a case and review of literature. Am. J. Med. 1962, 32, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Zabaleta, I.A.; Sackner, M.A. Diaphragmatic flutter in three babies with bronchopulmonary dysplasia and respiratory syncytial virus bronchiolitis. Pediatr. Pulmonol. 1995, 19, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.S.; Gauda, E.; Crawford, T.; Ogunlesi, F.; Lefton-Greif, M.A.; McGrath-Morrow, S.; Marcus, C.L. Respiratory flutter syndrome: An underrecognized cause of respiratory failure in neonates. Am. J. Respir. Crit. Care Med. 2001, 164, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Kumar, P.P.; Chirla, D.K. Respiratory flutter syndrome in a neonate. Indian Pediatr. 2013, 50, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, V.; Narayanan, K.; Combes, N.; Jost, D.; Jouven, X.; Marijon, E. Electrical cardiac injuries: Current concepts and management. Eur. Heart J. 2018, 39, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Higa de Landoni, J. “Nicotine” Inchem (April 1991), International Programme on Chemical Safety. Available online: http://www.inchem.org (accessed on 1 April 2024).
- Senanayake, N.; Román, G.C. Disorders of neuromuscular transmission due to natural environmental toxins. J. Neurol. Sci. 1992, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- James, T.N.; Riddick, L.; Embry, J.H. Cardiac abnormalities demonstrated postmortem in four cases of accidental electrocution and their potential significance relative to nonfatal electrical injuries of the heart. Am. Heart J. 1990, 120, 143–157. [Google Scholar] [CrossRef] [PubMed]
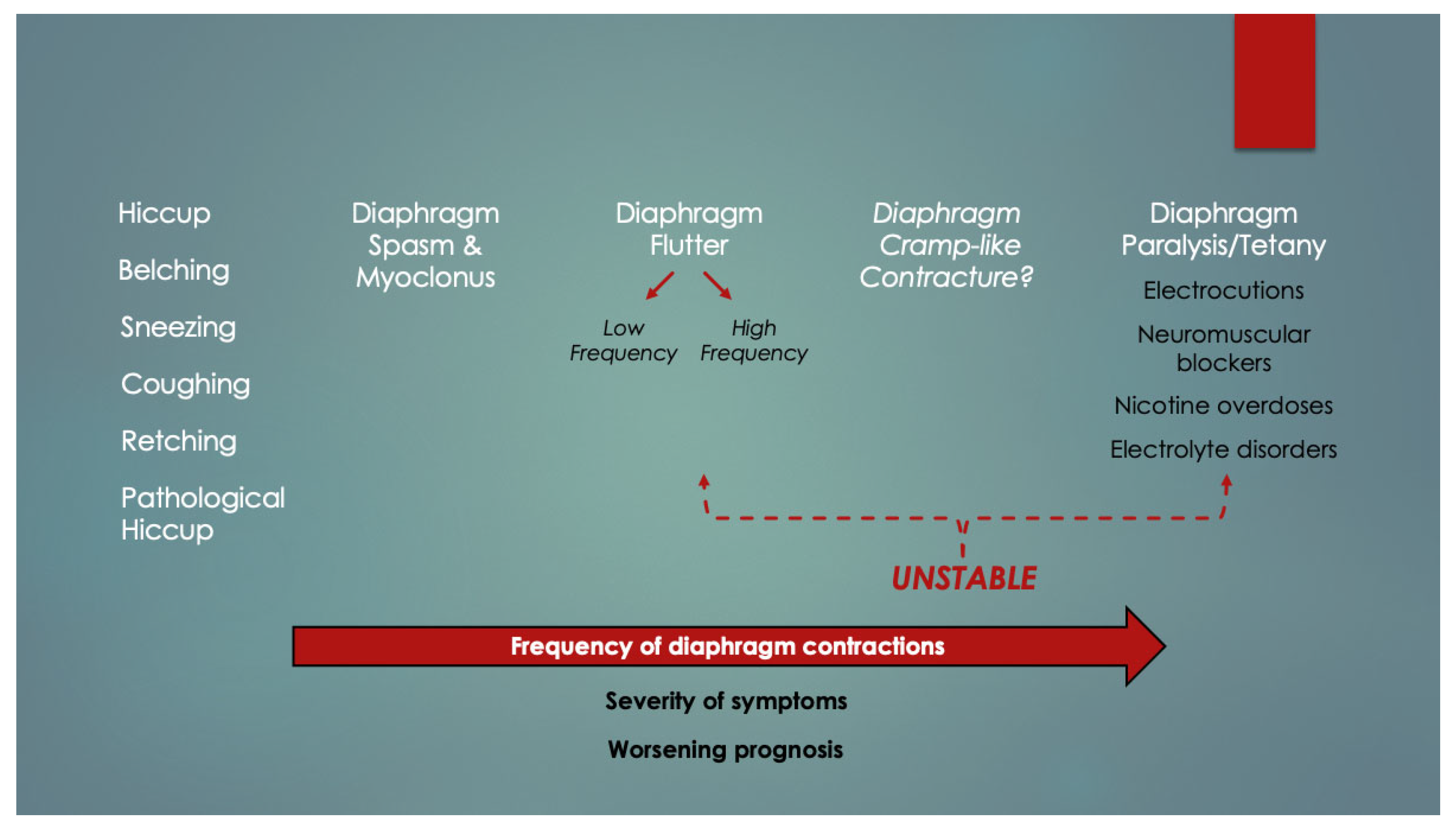
- Gebien, D.J.; Eisenhut, M. Diaphragmatic Cramp-Like Contracture (DCC)—A Novel Terminal Mechanism in Sudden Unexpected Deaths of All Ages. ResearchGate. 2023. Available online: https://tinyurl.com/diaphragm-cramp (accessed on 13 May 2024).
| Characteristic | Apnea Group | No Apnea | p Value | Author |
|---|---|---|---|---|
| Younger gestational age at birth (weeks) | 33 ± 2 | 39 ± 2 | <0.01 | [4] |
| Younger postconceptional age on admission (weeks) | 37 ± 2 | 53 ± 8 | <0.01 | [4] |
| Infiltrates on CXR | 5/5 (100%) | 8/27 (30%) | <0.02 | [4] |
| Increased alveolar-arterial oxygen gradient (mmHg) | 170 ± 96 | 45 ± 20 | <0.01 | [4] |
| Recurrent apneas | 18/38 (47%) | 6/147 (4%) | <0.005 | [5] |
| Low SaO2 (%) | 85 ± 16 | 90 ± 9 | <0.05 | [5] |
| High pCO2 (mmHg) | 55.5 ± 18.0 | 48.8 ± 13.5 | <0.05 | [5] |
| Low pH | 7.31 ± 0.13 | 7.36 ± 0.08 | <0.05 | [5] |
| Atelectasis on CXR | 18/38 (47%) | 37/147 (25%) | NA | [5] |
| Mechanical ventilation | 14/38 (37%) | 14/147 (10%) | NA | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebien, D.J. Myositis “Diaphragm Cramp” as a Potential Cause of Respiratory Arrests in Infants. Comment on Salfi, N.C.M. et al. Fatal Deterioration of a Respiratory Syncytial Virus Infection in an Infant with Abnormal Muscularization of Intra-Acinar Pulmonary Arteries: Autopsy and Histological Findings. Diagnostics 2024, 14, 601. Diagnostics 2024, 14, 1061. https://doi.org/10.3390/diagnostics14101061
Gebien DJ. Myositis “Diaphragm Cramp” as a Potential Cause of Respiratory Arrests in Infants. Comment on Salfi, N.C.M. et al. Fatal Deterioration of a Respiratory Syncytial Virus Infection in an Infant with Abnormal Muscularization of Intra-Acinar Pulmonary Arteries: Autopsy and Histological Findings. Diagnostics 2024, 14, 601. Diagnostics. 2024; 14(10):1061. https://doi.org/10.3390/diagnostics14101061
Chicago/Turabian StyleGebien, Dov Jordan. 2024. "Myositis “Diaphragm Cramp” as a Potential Cause of Respiratory Arrests in Infants. Comment on Salfi, N.C.M. et al. Fatal Deterioration of a Respiratory Syncytial Virus Infection in an Infant with Abnormal Muscularization of Intra-Acinar Pulmonary Arteries: Autopsy and Histological Findings. Diagnostics 2024, 14, 601" Diagnostics 14, no. 10: 1061. https://doi.org/10.3390/diagnostics14101061
APA StyleGebien, D. J. (2024). Myositis “Diaphragm Cramp” as a Potential Cause of Respiratory Arrests in Infants. Comment on Salfi, N.C.M. et al. Fatal Deterioration of a Respiratory Syncytial Virus Infection in an Infant with Abnormal Muscularization of Intra-Acinar Pulmonary Arteries: Autopsy and Histological Findings. Diagnostics 2024, 14, 601. Diagnostics, 14(10), 1061. https://doi.org/10.3390/diagnostics14101061






