Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery?
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Exposure Variable
2.4. Outcome Variable
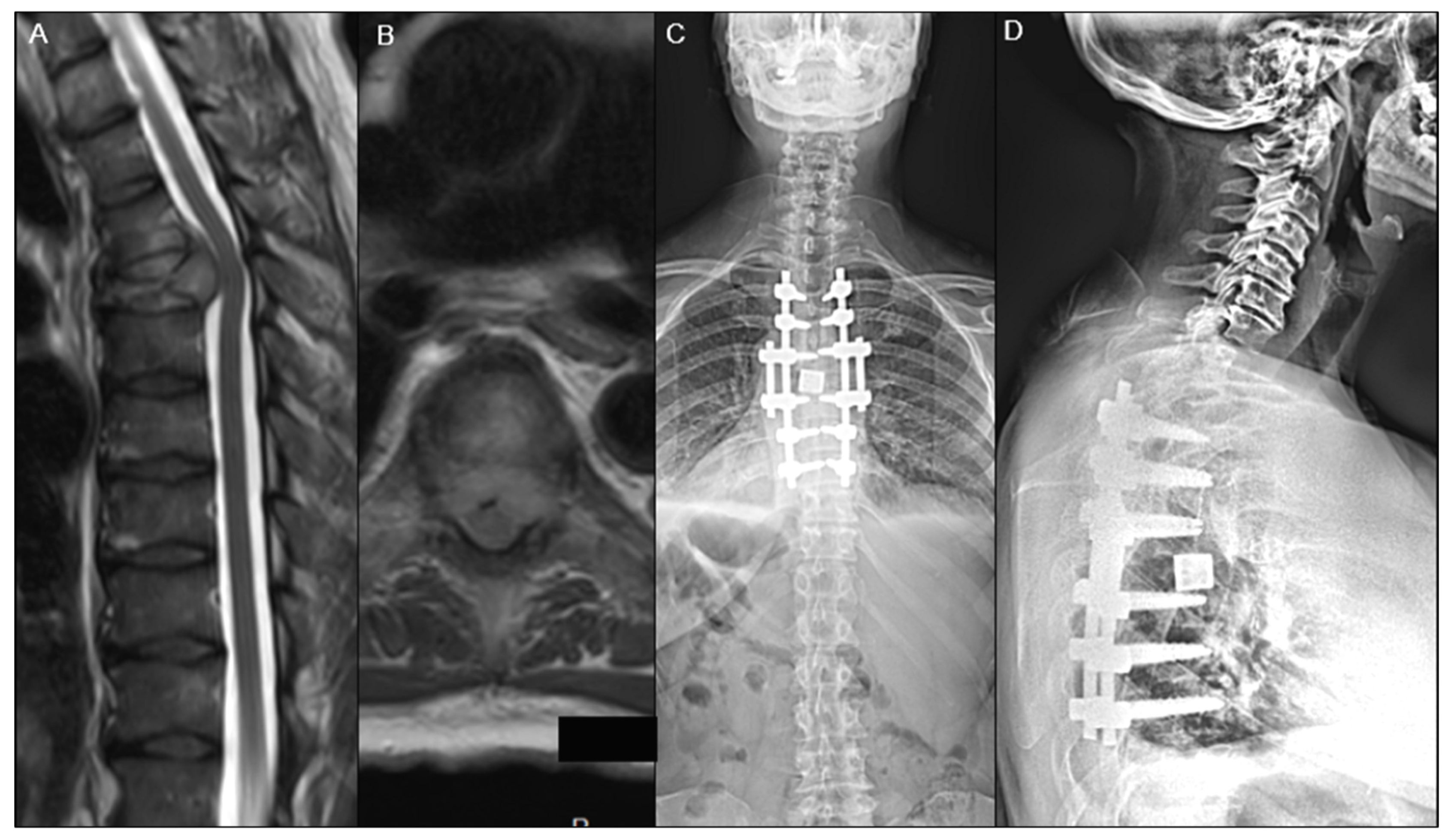
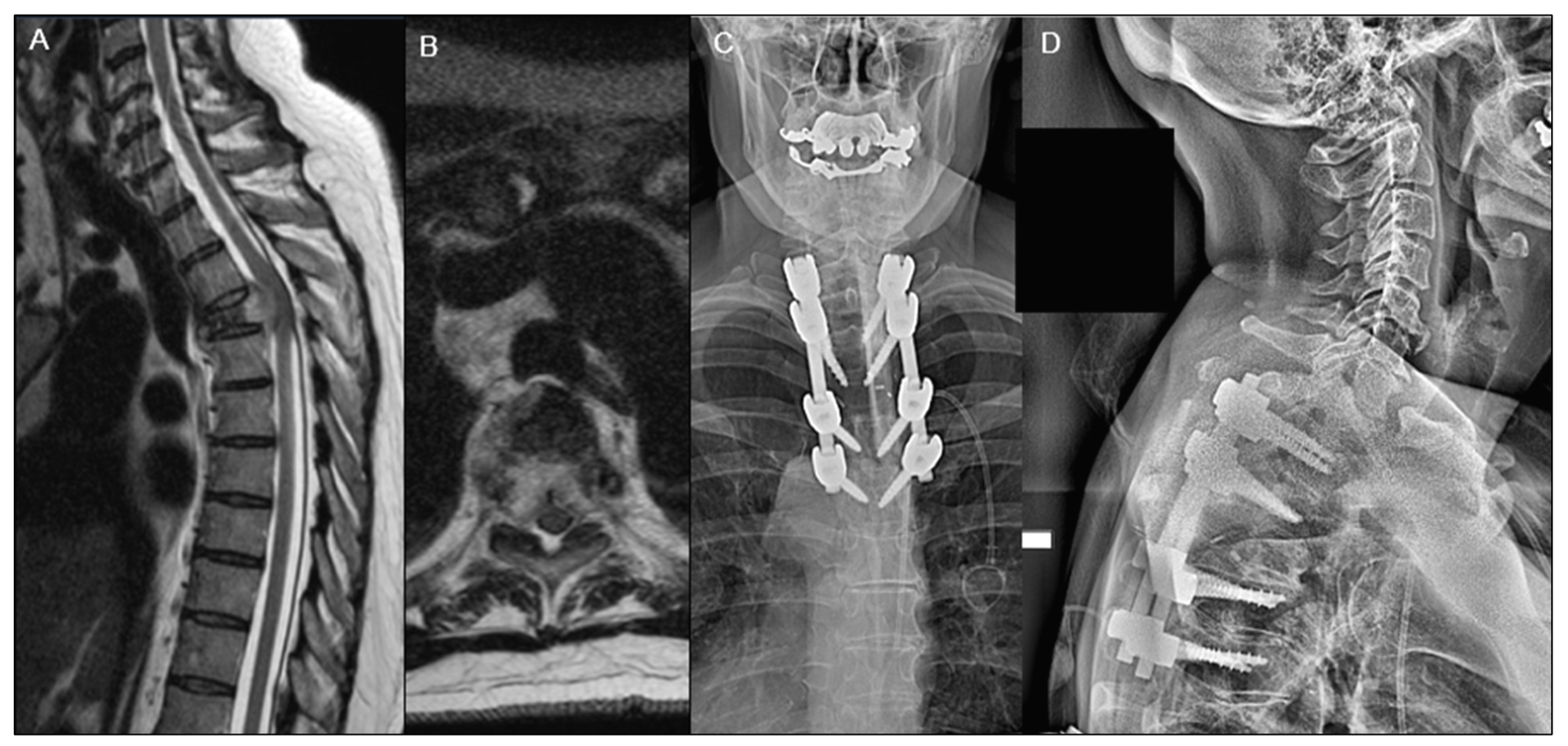
2.5. Surgical Treatment
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Preoperative Factors Associated with ED Presentation
3.3. Perioperative Factors Associated with ED Presentation
3.4. Long-Term Outcomes
3.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobs, W.B.; Perrin, R.G. Evaluation and treatment of spinal metastases: An overview. Neurosurg. Focus 2001, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Kanda, Y.; Sakai, Y.; Yoshikawa, R.; Yurube, T.; Takeoka, Y.; Hara, H.; Akisue, T.; Kuroda, R.; Kakutani, K. Effect of Bone Metastasis Cancer Board on Spinal Surgery Outcomes: A Retrospective Study. Medicina 2023, 59, 2087. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, A.M.; Pasku, D.; Quraishi, N.A. Surgical complications and re-operation rates in spinal metastases surgery: A systematic review. Eur. Spine J. 2021, 30, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Papadopoulos, S.M.; Greenberg, H.S. Metastatic epidural spinal cord compression. Neurol. Clin. 1991, 9, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Bilsky, M.; Fehlings, M.; Fisher, C.; Gokaslan, Z. Spine Oncology—Metastatic Spine Tumors. Neurosurgery 2017, 80, S131–S137. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zanaty, A.; George, K.J. Outcomes and efficiency of managing patients admitted for surgery for spinal metastases. Surg. Neurol. Int. 2022, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Chanbour, H.; Gangavarapu, L.S.; Chen, J.W.; Bendfeldt, G.A.; Younus, I.; Ahmed, M.; Roth, S.G.; Luo, L.Y.; Chotai, S.; Abtahi, A.M.; et al. Unplanned Readmission After Surgery for Cervical Spine Metastases. World Neurosurg. 2023, 171, e768–e776. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Chanbour, H.; Bendfeldt, G.A.; Gangavarapu, L.S.; Karlekar, M.B.; Abtahi, A.M.; Stephens, B.F.; Zuckerman, S.L.; Chotai, S. Palliative Care Consultation Utilization Among Patient Undergoing Surgery for Metastatic Spinal Tumors. World Neurosurg. 2023, 178, e549–e558. [Google Scholar] [CrossRef] [PubMed]
- Chanbour, H.; Chen, J.W.B.; Gangavarapu, L.S.B.; Bendfeldt, G.A.B.; LaBarge, M.E.B.; Ahmed, M.; Roth, S.G.; Chotai, S.; Luo, L.Y.; Abtahi, A.M.; et al. Unplanned Readmission Is Associated With Decreased Overall Survival and Performance After Metastatic Spine Surgery. Spine 2023, 48, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Bendfeldt, G.A.; Chanbour, H.; Chen, J.W.; Gangavarapu, L.S.; LaBarge, M.E.; Ahmed, M.; Jonzzon, S.; Roth, S.G.; Chotai, S.; Luo, L.Y.; et al. Does Low-Grade Versus High-Grade Bilsky Score Influence Local Recurrence and Overall Survival in Metastatic Spine Tumor Surgery? Neurosurgery 2023, 93, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; De la Garza, R.; Dalton, T.; McCray, E.; Pennington, Z.; Erickson, M.; Walsh, K.M.; Yassari, R.; Sciubba, D.M.; Goodwin, A.N.; et al. Insurance status as a mediator of clinical presentation, type of intervention, and short-term outcomes for patients with metastatic spine disease. Cancer Epidemiol. 2022, 76, 102073. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Sleeman, K.E.; Leniz, J.; Wilson, R.; Higginson, I.J.; Verne, J.; Maddocks, M.; Murtagh, F. Socioeconomic position and use of healthcare in the last year of life: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002782. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Torisu, H.; Ono, M.; Kiryu, H.; Furue, M.; Ohmoto, Y.; Nakayama, J.; Nishioka, Y.; Sone, S.; Kuwano, M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: Possible involvement of TNFalpha and IL-1alpha. Int. J. Cancer 2000, 85, 182–188. [Google Scholar] [CrossRef]


| Total N = 311 | ED N = 161 | Clinic N = 150 | p-Value | |
|---|---|---|---|---|
| Age mean ± SD | 60.6 ± 12.0 | 62.1 ± 11.8 | 59.0 ± 12.1 | 0.023 |
| BMI mean ± SD | 27.1 ± 7.0 | 26.7 ± 7.0 | 27.6 ± 6.9 | 0.278 |
| Gender, Male n (%) | 186 (59.8%) | 103 (64.0%) | 83 (44.3%) | 0.133 |
| Race, white n (%) | 271 (87.1%) | 140 (87.0%) | 131 (87.3%) | 0.684 |
| Insurance, n (%) | 0.043 | |||
| Private | 112 (36.0%) | 48 (29.8%) | 64 (42.7%) | |
| Public | 118 (37.9%) | 70 (43.5%) | 48 (32.0%) | |
| Uninsured | 81 (26.0%) | 43 (26.7%) | 38 (25.3%) | |
| Hypertension, n (%) | 173 (55.6%) | 94 (58.4%) | 79 (52.7%) | 0.361 |
| HLD, n (%) | 65 (20.9%) | 37 (23.0%) | 28 (18.7%) | 0.403 |
| Diabetes, n (%) | 72 (23.2%) | 40 (24.8%) | 32 (21.3%) | 0.503 |
| Osteoporosis, n (%) | 9 (2.9%) | 4 (2.5%) | 5 (3.3%) | 0.743 |
| Cardiac, n (%) | 58 (18.6%) | 33 (20.5%) | 25 (16.7%) | 0.467 |
| Psychiatric, n (%) | 56 (18.0%) | 28 (17.4%) | 28 (18.7%) | 0.770 |
| Comorbidities 2+, n (%) | 125 (40.2%) | 76 (47.2%) | 49 (32.7%) | 0.011 |
| Smoking, n (%) | 0.022 | |||
| Never | 172 (55.3%) | 77 (47.8%) | 95 (63.3%) | |
| Current | 59 (19.0%) | 35 (21.7%) | 24 (16.0%) | |
| Prior | 80 (25.7%) | 49 (30.4%) | 31 (20.7%) | |
| Other organ metastasis, n (%) | 170 (54.7%) | 91 (56.5%) | 79 (52.7%) | 0.569 |
| Primary Organ, n (%) | 0.099 | |||
| Breast | 37 (11.9%) | 14 (8.7%) | 23 (15.3%) | |
| Lung | 71 (22.8%) | 44 (27.3%) | 27 (18.0%) | |
| Renal | 40 (12.9%) | 22 (13.7%) | 18 (12.0%) | |
| Others | 163 (52.4%) | 81 (50.3%) | 82 (54.7%) | |
| Time to last follow-up, mean ± SD | 516.6 ± 634.1 | 489.0 ± 579.5 | 542.2 ± 682.9 | 0.568 |
| Total N = 311 | ED N = 161 | Clinic N = 150 | p-Value | |
|---|---|---|---|---|
| Mechanical Pain, n (%) | 168 (54.0%) | 75 (46.6%) | 68 (45.3%) | 0.909 |
| Biologic Pain, n (%) | 154 (49.5%) | 97 (60.2%) | 57 (38.0%) | <0.001 |
| Neurologic Pain, n (%) | 129 (41.5%) | 58 (36.0%) | 71 (47.3%) | 0.050 |
| Sensory Deficit, n (%) | 92 (29.6%) | 54 (33.5%) | 38 (25.3%) | 0.108 |
| Motor Deficit, n (%) | 142 (45.7%) | 80 (49.7%) | 62 (41.3%) | 0.139 |
| Strength, n (%) | 0.533 | |||
| 0 | 27 (8.7%) | 16 (9.9%) | 11 (7.3%) | |
| 1 | 6 (1.9%) | 4 (2.5%) | 2 (1.3%) | |
| 2 | 26 (8.4%) | 17 (10.6%) | 9 (6.0%) | |
| 3 | 24 (7.7%) | 11 (6.8%) | 13 (8.7%) | |
| 4 | 66 (21.2%) | 34 (21.1%) | 32 (21.3%) | |
| 5 | 153 (49.2%) | 74 (46.0%) | 79 (52.7%) | |
| Preop KPS, mean ± SD | 66.2 ± 16.7 | 62.7 ± 17.75 | 70.0 ± 14.6 | <0.001 |
| Tumor Locations, n (%) | 0.351 | |||
| Cervical | 40 (12.9%) | 19 (11.8%) | 21 (14.0%) | |
| Cervico-Thoracic | 11 (3.5%) | 5 (3.1%) | 6 (4.0%) | |
| Thoraco-lumbar | 184 (59.2%) | 103 (64.0%) | 81 (54.0%) | |
| Lumbar | 76 (24.4%) | 34 (21.1%) | 42 (28.0%) | |
| Bilsky score, n (%) | 0.049 | |||
| 0–1 | 80 (25.7%) | 34 (21.1%) | 46 (30.7%) | |
| 2–3 | 218 (70.1%) | 122 (75.8%) | 96 (64.0%) | |
| Tumor Size, mean ± SD | 1.7 ± 1.4 | 1.6 ± 1.3 | 1.8 ± 1.4 | 0.348 |
| Oligometastatic, n (%) | 0.049 | |||
| 1 | 216 (69.5%) | 102 (63.4%) | 114 (76.0%) | |
| <5 | 71 (22.8%) | 45 (28.0%) | 26 (17.3%) | |
| 5+ | 24 (7.7%) | 14 (8.7%) | 10 (6.7%) | |
| Preoperative Embolization, n (%) | 23 (7.4%) | 16 (9.9%) | 7 (4.7%) | 0.088 |
| Preop Chemo, n (%) | 132 (42.4%) | 60 (37.3%) | 72 (48.0%) | 0.066 |
| Postop Chemo, n (%) | 118 (37.9%) | 56 (34.8%) | 62 (41.3%) | 0.244 |
| Preop RT, n (%) | 35 (11.3%) | 13 (8.1%) | 22 (14.7%) | 0.074 |
| Postop RT, n (%) | 127 (40.8%) | 70 (43.5%) | 57 (38.0%) | 0.357 |
| Total N = 311 | ED N = 161 | Clinic N = 150 | p-Value | |
|---|---|---|---|---|
| Instrumented, n (%) | 10 (3.2%) | 155 (96.3%) | 145 (96.7%) | >0.999 |
| Decompressed, n (%) | 292 (93.9%) | 153 (95.0%) | 139 (92.7%) | 0.334 |
| Total Decompressed Levels, mean ± SD | 2.6 ± 1.3 | 2.7 ± 1.3 | 2.4 ± 1.3 | 0.041 |
| Total Instrumented Levels, mean ± SD | 5.3 ± 2.3 | 5.5 ± 2.3 | 5.1 ± 2.4 | 0.227 |
| Transpedicular decompression, n (%) | 164 (52.7%) | 88 (54.7%) | 76 (50.7%) | 0.497 |
| Costotransversectomy, n (%) | 42 (13.5%) | 15 (9.3%) | 27 (18.0%) | 0.031 |
| Corpectomy/vertebrectomy, n (%) | 164 (52.7%) | 88 (54.7%) | 76 (50.7%) | 0.497 |
| Operative Time, mean ± SD | 315.2 ± 125.3 | 308.9 ± 98.9 | 322.0 ± 148.5 | 0.367 |
| EBL (mL), mean ± SD | 830.9 ± 882.2 | 905.9 ± 959.0 | 751.0 ± 787.6 | 0.122 |
| Intraoperative Monitoring, n (%) | 240 (77.2%) | 124 (77.0%) | 116 (77.3%) | >0.999 |
| Intraoperative Monitoring Change, n (%) | 6 (1.9%) | 3 (1.9%) | 3 (2.0%) | >0.999 |
| Total N = 311 | ED N = 161 | Clinic N = 150 | p-Value | |
|---|---|---|---|---|
| Any Complication, n (%) | 91 (29.3%) | 47 (29.2%) | 44 (29.3%) | >0.999 |
| New Neurologic Deficit | 15 (4.8%) | 11 (6.8%) | 4 (2.7%) | 0.113 |
| Cardiac Arrest | 5 (1.6%) | 5 (3.1%) | 0 | 0.061 |
| Hematoma | 2 (0.6%) | 1 (0.6%) | 1 (0.7%) | >0.999 |
| DVT | 12 (3.9%) | 9 (5.6%) | 3 (2.0%) | 0.141 |
| UTI | 14 (4.5%) | 8 (5.0%) | 6 (4.0%) | 0.788 |
| PE | 8 (2.6%) | 4 (2.5%) | 4 (2.7%) | >0.999 |
| Pneumonia | 17 (5.5%) | 7 (4.3%) | 10 (6.7%) | 0.458 |
| Reintubate | 4 (1.3%) | 0 | 4 (2.7%) | 0.053 |
| Sepsis | 14 (4.5%) | 8 (5.0%) | 6 (4.0%) | 0.788 |
| Stroke | 3 (1.0%) | 1 (0.6%) | 2 (1.3%) | 0.611 |
| Mechanical Complication | 9 (2.9%) | 3 (1.9%) | 6 (4.0%) | 0.322 |
| Wound complication | 17 (5.5%) | 7 (4.3%) | 10 (6.7%) | 0.457 |
| Postop Radiculopathy | 10 (3.2%) | 6 (3.7%) | 4 (2.7%) | 0.751 |
| LOS (Days), mean ± SD | 6.9 ± 6.0 | 7.7 ± 6.1 | 6.1 ± 5.8 | 0.020 |
| Postop disposition, n (%) | 0.052 | |||
| Floor | 177 (56.9%) | 83 (51.6%) | 94 (62.7%) | |
| ICU | 134 (43.1%) | 78 (48.4%) | 56 (37.3%) | |
| Discharge Home, n (%) | 188 (60.5%) | 84 (52.2%) | 104 (69.3%) | 0.025 |
| Total N = 311 | ED N = 161 | Clinic N = 150 | p-Value | |
|---|---|---|---|---|
| Reoperation, n (%) | 29 (9.3%) | 13 (8.1%) | 16 (10.7%) | 0.443 |
| Readmission, n (%) | 70 (22.5%) | 34 (21.1%) | 36 (24.0%) | 0.588 |
| Local Recurrence, n (%) | 33 (10.6%) | 16 (9.9%) | 17 (11.3%) | 0.716 |
| Time to local recurrence, mean ± SD | 653.6 ± 839.2 | 870.0 ± 985.3 | 437.2 ± 641.6 | 0.424 |
| KPS postop, mean ± SD | 73.0 ± 16.8 | 72.4 ± 18.1 | 73.6 ± 15.4 | 0.608 |
| KPS last follow-up, mean ± SD | 64.7 ± 20.9 | 62.2 ± 21.5 | 67.1 ± 20.2 | 0.127 |
| MMS postop, mean ± SD | 1.7 ± 1.0 | 1.7 ± 1.0 | 1.6 ± 0.9 | 0.428 |
| MMS last follow-up, mean ± SD | 2.1 ± 1.1 | 2.2 ± 1.2 | 1.9 ± 1.1 | 0.160 |
| Deceased, n (%) | 202 (65.0%) | 106 (65.8%) | 96 (64.0%) | 0.390 |
| Time to death, mean ± SD | 469.4 ± 676.1 | 458.2 ± 604.3 | 481.6 ± 749.9 | 0.005 |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Perioperative Outcome | Independent Variable | β/OR/HR (95%CI) | p-Value | β/OR/HR (95%CI) | p-Value |
| LR | ED | 0.86 (0.41–1.77) | 0.690 | 1.18 (0.55–2.54) | 0.665 |
| Time to LR | 0.68 (0.26–1.75) | 0.426 | 0.98 (0.37–2.58) | 0.980 | |
| Death | 1.27 (0.78–2.06) | 0.328 | 1.35 (0.81–2.25) | 0.247 | |
| Time to Death | 1.52 (1.13–2.06) | 0.005 | 1.40 (1.01–1.93) | 0.041 | |
| KPS Last | −4.90 (−11.23, 1.41) | 0.127 | −4.41 (−10.75, 1.91) | 0.170 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeoli, T.; Chanbour, H.; Ahluwalia, R.; Abtahi, A.M.; Stephens, B.F.; Zuckerman, S.L. Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery? Diagnostics 2024, 14, 1058. https://doi.org/10.3390/diagnostics14101058
Zeoli T, Chanbour H, Ahluwalia R, Abtahi AM, Stephens BF, Zuckerman SL. Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery? Diagnostics. 2024; 14(10):1058. https://doi.org/10.3390/diagnostics14101058
Chicago/Turabian StyleZeoli, Tyler, Hani Chanbour, Ranbir Ahluwalia, Amir M. Abtahi, Byron F. Stephens, and Scott L. Zuckerman. 2024. "Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery?" Diagnostics 14, no. 10: 1058. https://doi.org/10.3390/diagnostics14101058
APA StyleZeoli, T., Chanbour, H., Ahluwalia, R., Abtahi, A. M., Stephens, B. F., & Zuckerman, S. L. (2024). Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery? Diagnostics, 14(10), 1058. https://doi.org/10.3390/diagnostics14101058






