Novel Insights into Postoperative Surveillance in Resected Pancreatic Cystic Neoplasms—A Review
Abstract
1. Introduction
2. Search Strategy
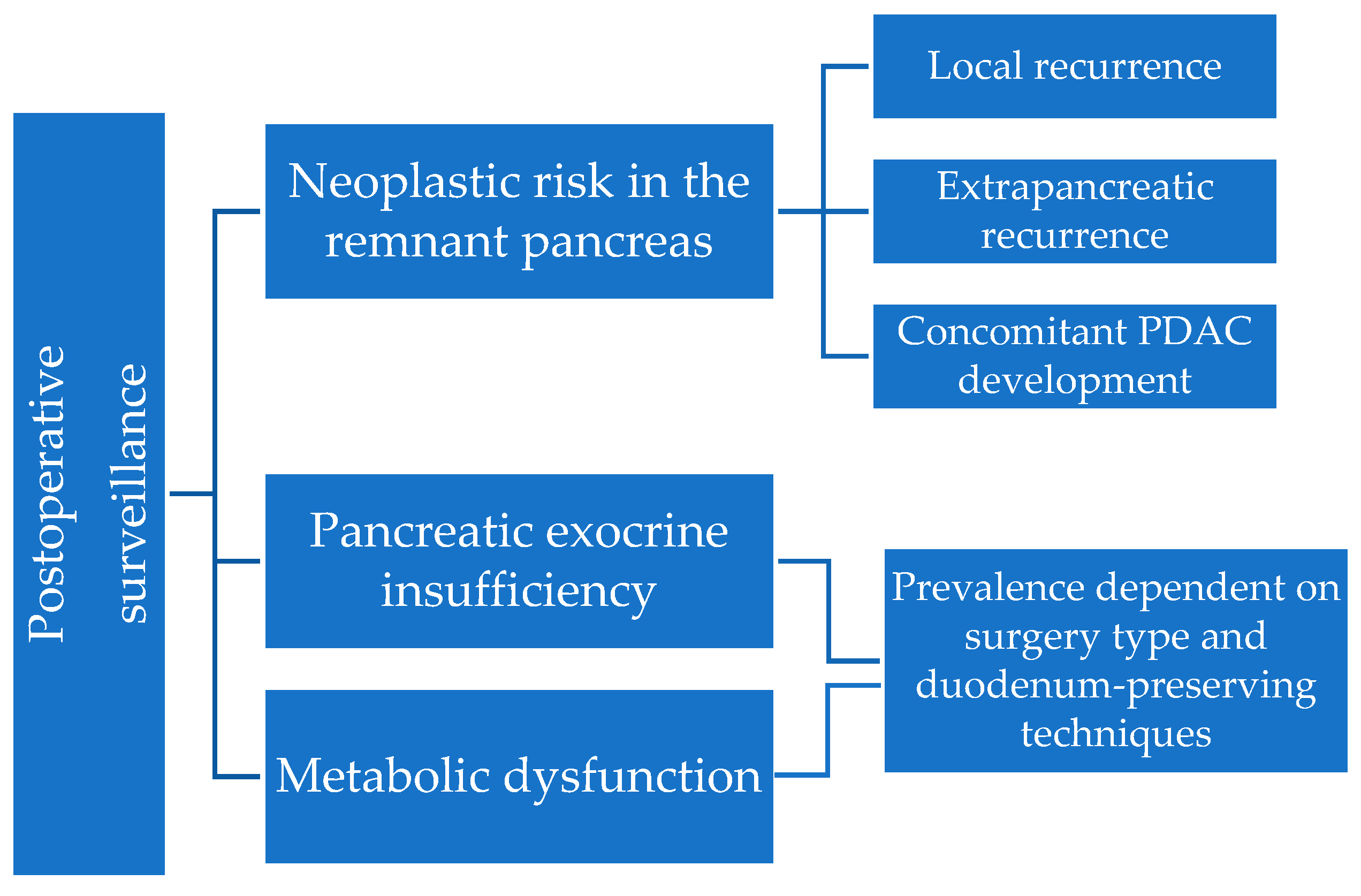
3. Surveillance of the Remnant Pancreas
3.1. Mechanisms and Risk Factors for Recurrence
- Recurrence of the initial lesion—either through positive margins after surgery, with residual microscopic disease which progresses over time, or through the intraductal spread of neoplastic cells, which leads to a new lesion in the remaining parenchyma, distant from the resection site, but with a similar genetic background.
- Progression of multifocal disease—either through the progression of residual lesions, which were accurately detected preoperatively but did not show indication for resection, or through the occurrence of a genetically non-related neoplastic lesion, independently of the index cyst.
3.2. Initial Indication for Surgery and the Results of the Resected Specimen
3.3. Surveillance Intervals and Methods
4. Pancreatic Exocrine Insufficiency after Pancreatic Surgery for PCL
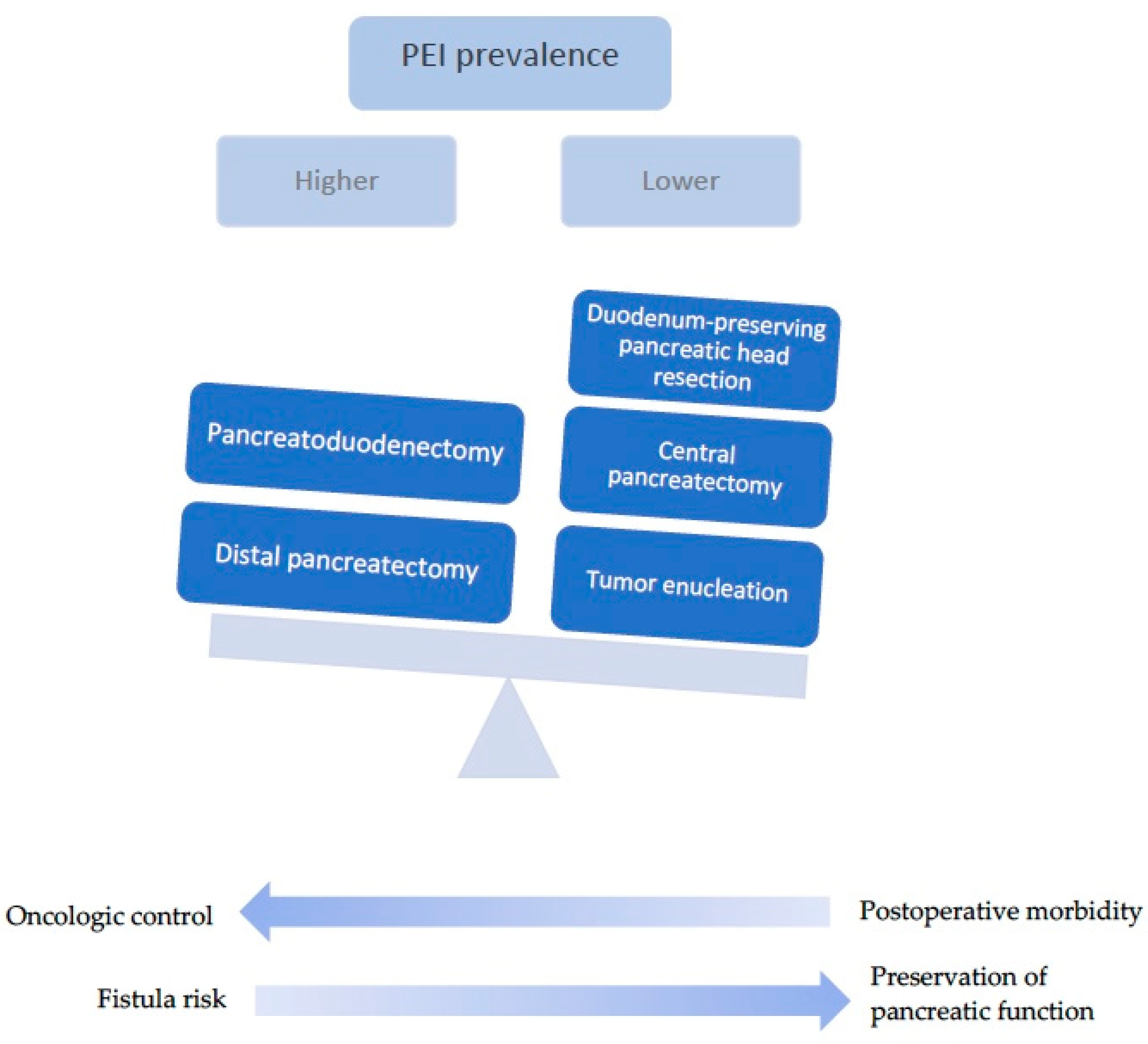
4.1. Type of Surgery
| Resection Type | Details |
|---|---|
| Pancreaticoduodenectomy (Whipple’s procedure) | Resection of the pancreatic head along with the duodenum, gallbladder and distal bile duct Radical surgery with lymphadenopathy, indicated for invasive carcinoma Technically superior due to the ease of additional resection in case of positive margins in intraoperative frozen section Associated with potentially significant morbidity and high metabolic risk |
| Distal pancreatectomy/Left pancreatic resection | Resection of the distal portion of the pancreas Commonly associated with splenectomy Less invasive and harbors lower risk of metabolic dysfunction Carries a risk of pancreatic fistula Can limit the acquisition of further margins if the transection was done at the pancreatic neck |
| Duodenum-preserving pancreatic head resection | Resection of the pancreatic head with the preservation of duodenum and bile duct Indicated for benign, premalignant or low-malignant lesions of the pancreatic head Lower risk of postoperative morbidity and metabolic dysfunction Potential for pancreatic fistula |
| Central pancreatectomy | Segmental resection at the level of the pancreatic body Suitable for benign or low-grade malignant neoplasms High risk for pancreatic fistula |
| Tumor enucleation | Removal of tumor from adjacent parenchyma, with maximum preservation of pancreatic tissue Best suited for small, well delineated tumors with preoperative benign features |
| Total pancreatectomy | Removal of the entire pancreas Considered in diffuse disease that affects the entire parenchyma; however, even in multifocal IPMNs, only the high-risk lesion might be surgically targeted to avoid prophylactic total pancreatectomy due to profound postoperative metabolic dysfunction. Indication should also be determined based on economic and social factors (limited access to follow-up; limited insulin availability) that could additionally negatively impact metabolic outcomes. |
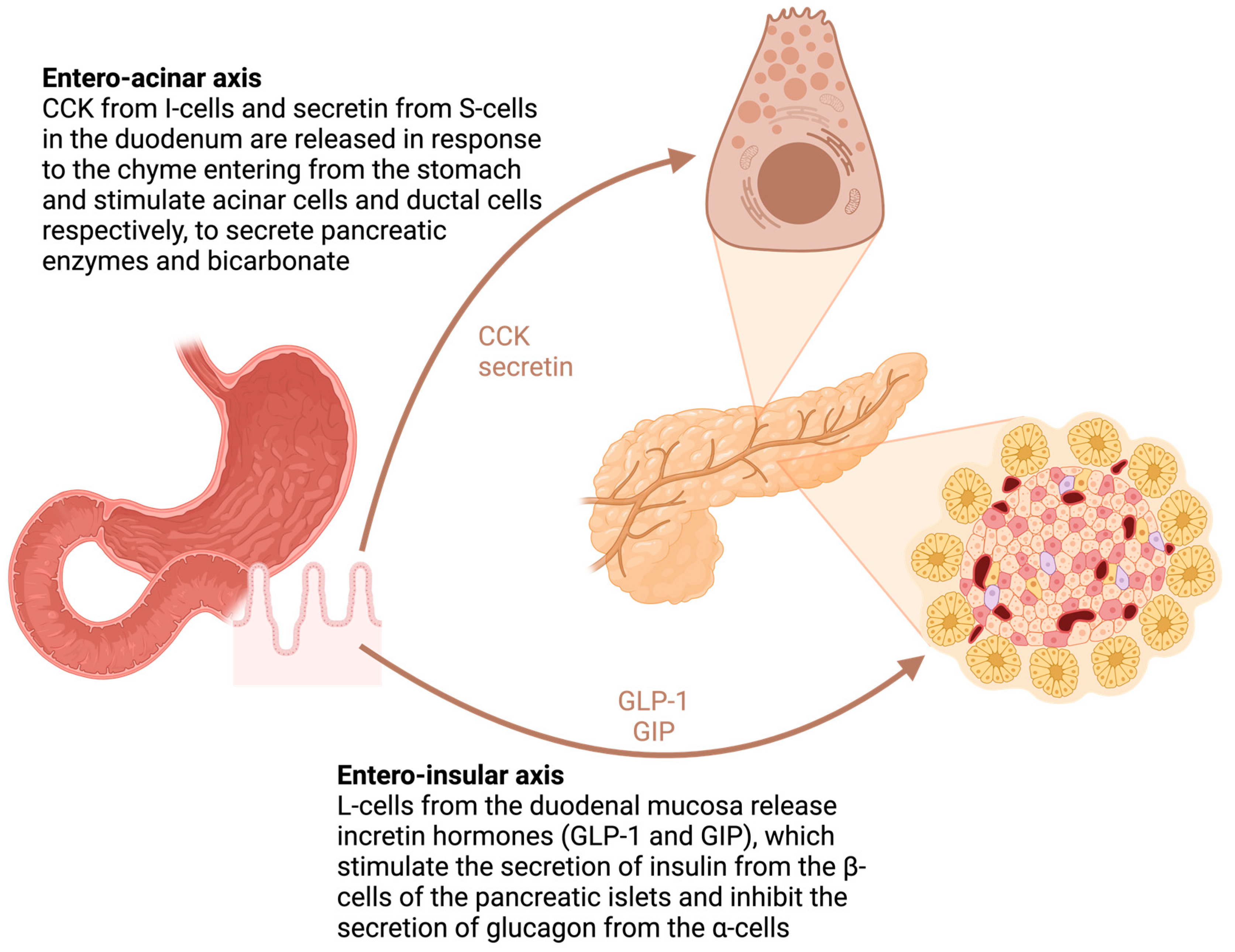
4.2. The Role of the Duodenum
4.3. PEI Evolution in Time
4.4. The Impact of PEI after Surgery
5. Metabolic Dysfunction—Diabetes and Hepatosteatosis
5.1. Diabetes after Pancreatic Resection
5.2. Steatotic Liver Disease
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S.; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.-Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2023, 24, 255–270. [Google Scholar] [CrossRef]
- Salvia, R.; Burelli, A.; Nepi, A.; Caravati, A.; Tomelleri, C.; Dall’Olio, T.; Casciani, F.; Crinò, S.F.; Perri, G.; Marchegiani, G. Pancreatic cystic neoplasms: Still high rates of preoperative misdiagnosis in the guidelines and endoscopic ultrasound era. Surgery 2023, 174, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Vlăduţ, C.; Bilous, D.; Ciocîrlan, M. Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines. J. Clin. Med. 2023, 12, 4020. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Visrodia, K.; Farrell, J.J.; Gonda, T.A. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J. Gastroenterol. 2019, 25, 4405–4413. [Google Scholar] [CrossRef]
- Lee, L.S. Updates in diagnosis and management of pancreatic cysts. World J. Gastroenterol. 2021, 27, 5700–5714. [Google Scholar] [CrossRef]
- Koehler, B.; Ryoo, D.Y.; Krishna, S.G. A Review of Endoscopic Ultrasound-Guided Chemoablative Techniques for Pancreatic Cystic Lesions. Diagnostics 2023, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Papaefthymiou, A.; Johnson, G.J.; Maida, M.; Gkolfakis, P.; Ramai, D.; Facciorusso, A.; Arvanitakis, M.; Ney, A.; Fusai, G.K.; Saftoiu, A.; et al. Performance and Safety of EUS Ablation Techniques for Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2627. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.P.; Baugh, K.A.; Brubaker, L.S.; Van Buren, G.; Villafane-Ferriol, N.; McElhany, A.L.; Mohamed, S.; Silberfein, E.J.; Hsu, C.; Massarweh, N.N.; et al. Long-Term Assessment of Pancreatic Function After Pancreatectomy for Cystic Neoplasms. J. Surg. Res. 2020, 247, 547–555. [Google Scholar] [CrossRef]
- Marchegiani, G.; Crippa, S.; Perri, G.; Rancoita, P.M.V.; Caravati, A.; Belfiori, G.; Dall’Olio, T.; Aleotti, F.; Partelli, S.; Bassi, C.; et al. Surgery for Intraductal Papillary Mucinous Neoplasms of the Pancreas: Preoperative Factors Tipping the Scale of Decision-Making. Ann. Surg. Oncol. 2022, 29, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Shahrokni, A.; Brennan, M.F. Surgical resection for intraductal papillary mucinous neoplasm in the older population. Eur. J. Surg. Oncol. 2022, 48, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Sahora, K.; Mino-Kenudson, M.; Brugge, W.; Thayer, S.P.; Ferrone, C.R.; Sahani, D.; Pitman, M.B.; Warshaw, A.L.; Lillemoe, K.D.; Fernandez-del Castillo, C.F. Branch duct intraductal papillary mucinous neoplasms: Does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann. Surg. 2013, 258, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Fogliati, A.; Valente, R.; Sadr-Azodi, O.; Arnelo, U.; Capurso, G.; Halimi, A.; Partelli, S.; Ateeb, Z.; Arcidiacono, P.G.; et al. A tug-of-war in intraductal papillary mucinous neoplasms management: Comparison between 2017 International and 2018 European guidelines. Dig. Liver Dis. 2021, 53, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Sighinolfi, M.; Quan, S.Y.; Lee, Y.; Ibaseta, A.; Pham, K.; Dua, M.M.; Poultsides, G.A.; Visser, B.C.; Norton, J.A.; Park, W.G. Fukuoka and AGA Criteria Have Superior Diagnostic Accuracy for Advanced Cystic Neoplasms than Sendai Criteria. Dig. Dis. Sci. 2017, 62, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-M.; Yin, S.; Siddiqui, A.A.; Salem, R.R.; Schrope, B.; Sethi, A.; Poneros, J.M.; Gress, F.G.; Genkinger, J.M.; Do, C.; et al. Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine 2017, 96, e7900. [Google Scholar] [CrossRef]
- Ma, G.K.; Goldberg, D.S.; Thiruvengadam, N.; Chandrasekhara, V.; Kochman, M.L.; Ginsberg, G.G.; Vollmer, C.M.; Ahmad, N.A. Comparing American Gastroenterological Association Pancreatic Cyst Management Guidelines with Fukuoka Consensus Guidelines as Predictors of Advanced Neoplasia in Patients with Suspected Pancreatic Cystic Neoplasms. J. Am. Coll. Surg. 2016, 223, 729–737.e1. [Google Scholar] [CrossRef]
- Wong, J.; Weber, J.; Centeno, B.A.; Vignesh, S.; Harris, C.L.; Klapman, J.B.; Hodul, P. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J. Gastrointest. Surg. 2013, 17, 78–84; discussion 84–85. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Klauss, M.; Bergmann, F.; Hackert, T.; Hartwig, W.; Strobel, O.; Bundy, B.D.; Büchler, M.W.; Werner, J. Small (Sendai negative) branch-duct IPMNs: Not harmless. Ann. Surg. 2012, 256, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, D.; Cortesi, P.; Facchetti, R.; de Pretis, N.; Pérez-Cuadrado-Robles, E.; Uribarri-Gonzalez, L.; Ateeb, Z.; Belfiori, G.; Arcidiacono, P.G.; Mantovani, L.G.; et al. Real-world costs and dynamics of surveillance in patients who underwent surgery for low-risk branch duct intraductal papillary mucinous neoplasms. Eur. J. Surg. Oncol. 2023, 49, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Pollini, T.; Burelli, A.; Han, Y.; Jung, H.-S.; Kwon, W.; Rocha Castellanos, D.M.; Crippa, S.; Belfiori, G.; Arcidiacono, P.G.; et al. Surveillance for Presumed BD-IPMN of the Pancreas: Stability, Size, and Age Identify Targets for Discontinuation. Gastroenterology 2023, 165, 1016–1024.e5. [Google Scholar] [CrossRef] [PubMed]
- Chhoda, A.; Singh, S.; Sheth, A.H.; Grimshaw, A.A.; Gunderson, C.G.; Sharma, P.; Kunstman, J.W.; Sharma, A.; Ahuja, N.; Gonda, T.A.; et al. Benefit of Extended Surveillance of Low-Risk Pancreatic Cysts After 5-Year Stability: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 1430–1446. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of high-risk stigmata and worrisome features with advanced neoplasia in intraductal papillary mucinous neoplasms (IPMN): A systematic review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Levink, I.; Bruno, M.J.; Cahen, D.L. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr. Treat. Options Gastroenterol. 2018, 16, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, A.; Thayaparan, V.; Hamad, A.; Tarver, W.; Cloyd, J.M.; Kim, A.C.; Gebhard, R.; Pawlik, T.M.; Reames, B.N.; Ejaz, A. Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance. J. Clin. Med. 2024, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Fuji, T.; Umeda, Y.; Takagi, K.; Yoshida, R.; Yoshida, K.; Yasui, K.; Matsumoto, K.; Kato, H.; Yagi, T.; Fujiwara, T. Optimal surveillance of intraductal papillary mucinous neoplasms of the pancreas focusing on remnant pancreas recurrence after surgical resection. BMC Cancer 2022, 22, 588. [Google Scholar] [CrossRef]
- Sereni, E.; Luchini, C.; Salvia, R.; Pea, A. Molecular and clinical patterns of local progression in the pancreatic remnant following resection of pancreatic intraductal papillary mucinous neoplasm (IPMN). Chin. Clin. Oncol. 2019, 8, 21. [Google Scholar] [CrossRef]
- Pea, A.; Yu, J.; Rezaee, N.; Luchini, C.; He, J.; Dal Molin, M.; Griffin, J.F.; Fedor, H.; Fesharakizadeh, S.; Salvia, R.; et al. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann. Surg. 2017, 266, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallego, C.; Miyasaka, Y.; Hozaka, Y.; Nishino, H.; Kawamoto, M.; Vieira, D.L.; Ohtsuka, T.; Wolfgang, C. Surveillance after resection of non-invasive intraductal papillary mucinous neoplasms (IPMN). A systematic review. Pancreatology 2023, 23, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Al Efishat, M.; Attiyeh, M.A.; Eaton, A.A.; Gönen, M.; Basturk, O.; Klimstra, D.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Balachandran, V.; et al. Progression Patterns in the Remnant Pancreas after Resection of Non-Invasive or Micro-Invasive Intraductal Papillary Mucinous Neoplasms (IPMN). Ann. Surg. Oncol. 2018, 25, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Hirono, S.; Shimizu, Y.; Ohtsuka, T.; Kin, T.; Hara, K.; Kanno, A.; Koshita, S.; Hanada, K.; Kitano, M.; Inoue, H.; et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J. Gastroenterol. 2020, 55, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, Y.; Kitago, M.; Matsui, J. Independent predictors of secondary invasive pancreatic remnant tumors after initial resection of an intraductal papillary mucinous neoplasm: A nationwide large-scale survey in Japan. Surg. Today 2020, 50, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Pflüger, M.J.; Griffin, J.F.; Hackeng, W.M.; Kawamoto, S.; Yu, J.; Chianchiano, P.; Shin, E.; Lionheart, G.; Tsai, H.-L.; Wang, H.; et al. The Impact of Clinical and Pathological Features on Intraductal Papillary Mucinous Neoplasm Recurrence After Surgical Resection: Long-Term Follow-Up Analysis. Ann. Surg. 2022, 275, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Habib, J.R.; Blair, A.; Rezaee, N.; Kinny-Köster, B.; Cameron, J.L.; Hruban, R.H.; Weiss, M.J.; Fishman, E.K.; Lafaro, K.J.; et al. Invasive and Noninvasive Progression After Resection of Noninvasive Intraductal Papillary Mucinous Neoplasms. Ann. Surg. 2022, 276, 370–377. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cameron, J.L.; Ahuja, N.; Makary, M.A.; Hirose, K.; Choti, M.A.; Schulick, R.D.; Hruban, R.H.; Pawlik, T.M.; Wolfgang, C.L. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J. Am. Coll. Surg. 2013, 216, 657–665; discussion 665–667. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Habeeb, T.A.A.M.; Kechagias, A.; Isik, A.; Aiolfi, A.; Shaker, S.E.; Samir, A.; Sheded, M.M.; Khalifa, M.R.; Haggag, R.; et al. Outcomes of Surgical Resection of Pancreatic Cystic Neoplasms Based on the European Expert Consensus Statement: A Prospective Observational Study. Surg. Gastroenterol. Oncol. 2022, 27, 264. [Google Scholar] [CrossRef]
- Hirono, S.; Kawai, M.; Okada, K.-I.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Ueno, M.; Yanagisawa, A.; Yamaue, H. Long-term surveillance is necessary after operative resection for intraductal papillary mucinous neoplasm of the pancreas. Surgery 2016, 160, 306–317. [Google Scholar] [CrossRef]
- Habib, J.R.; Kinny-Köster, B.; Amini, N.; Shoucair, S.; Cameron, J.L.; Thompson, E.D.; Fishman, E.K.; Hruban, R.H.; Javed, A.A.; He, J.; et al. Predictors, Patterns, and Timing of Recurrence Provide Insight into the Disease Biology of Invasive Carcinomas Arising in Association with Intraductal Papillary Mucinous Neoplasms. J. Gastrointest. Surg. 2022, 26, 2311–2320. [Google Scholar] [CrossRef]
- Haeberle, L.; Busch, M.; Kirchner, J.; Fluegen, G.; Antoch, G.; Knoefel, W.T.; Esposito, I. Pancreatic ductal adenocarcinoma concomitant with pancreatic metastases of clear-cell renal cell carcinoma: A case report. J. Med. Case Rep. 2021, 15, 314. [Google Scholar] [CrossRef]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2023, 164, 117–133.e7. [Google Scholar] [CrossRef] [PubMed]
- Zelga, P.; Hernandez-Barco, Y.G.; Qadan, M.; Ferrone, C.R.; Kambadakone, A.; Horick, N.; Jah, A.; Warshaw, A.L.; Lillemoe, K.D.; Balakrishnan, A.; et al. Number of Worrisome Features and Risk of Malignancy in Intraductal Papillary Mucinous Neoplasm. J. Am. Coll. Surg. 2022, 234, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Mino-Kenudson, M.; Ferrone, C.R.; Morales-Oyarvide, V.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F.-D. Patterns of Recurrence After Resection of IPMN: Who, When, and How? Ann. Surg. 2015, 262, 1108–1114. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, Y.; Kang, J.S.; Choi, Y.J.; Byun, Y.; Kim, H.; Lee, K.B.; Kim, H.; Kwon, W.; Jang, J.-Y. Fate of Patients With Intraductal Papillary Mucinous Neoplasms of Pancreas After Resection According to the Pathology and Margin Status: Continuously Increasing Risk of Recurrence Even After Curative Resection Suggesting Necessity of Lifetime Surveillance. Ann. Surg. 2022, 276, e231–e238. [Google Scholar] [CrossRef]
- Tamura, K.; Ohtsuka, T.; Ideno, N.; Aso, T.; Shindo, K.; Aishima, S.; Ohuchida, K.; Takahata, S.; Ushijima, Y.; Ito, T.; et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: A retrospective review. Ann. Surg. 2014, 259, 360–368. [Google Scholar] [CrossRef]
- Koh, Y.-X.; Chok, A.-Y.; Zheng, H.-L.; Tan, C.-S.; Goh, B.K.P. Systematic review and meta-analysis comparing the surgical outcomes of invasive intraductal papillary mucinous neoplasms and conventional pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2782–2800. [Google Scholar] [CrossRef] [PubMed]
- Sauvanet, A.; Couvelard, A.; Belghiti, J. Role of frozen section assessment for intraductal papillary and mucinous tumor of the pancreas. World J. Gastrointest. Surg. 2010, 2, 352–358. [Google Scholar] [CrossRef]
- Arnelo, U.; Valente, R.; Scandavini, C.M.; Halimi, A.; Mucelli, R.M.P.; Rangelova, E.; Svensson, J.; Schulick, R.D.; Torphy, R.J.; Fagerström, N.; et al. Intraoperative pancreatoscopy can improve the detection of skip lesions during surgery for intraductal papillary mucinous neoplasia: A pilot study. Pancreatology 2023, 23, 704–711. [Google Scholar] [CrossRef]
- de Jong, D.M.; Stassen, P.M.C.; Groot Koerkamp, B.; Ellrichmann, M.; Karagyozov, P.I.; Anderloni, A.; Kylänpää, L.; Webster, G.J.M.; van Driel, L.M.J.W.; Bruno, M.J.; et al. The role of pancreatoscopy in the diagnostic work-up of intraductal papillary mucinous neoplasms: A systematic review and meta-analysis. Endoscopy 2023, 55, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Krishna, S.G.; Pannala, R. Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics 2023, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chennatt, J.J.; Mandal, C.; Gupta, J.; Krishnasamy, S.; Bose, B.; Solanki, P.; Sunil, H.; Singh, S.K.; Gupta, S. Approach to Cystic Lesions of the Pancreas: Review of Literature. Cureus 2023, 15, e36827. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Mino-Kenudson, M.; Sahora, K.; Morales-Oyarvide, V.; Thayer, S.; Ferrone, C.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-Del Castillo, C. IPMN involving the main pancreatic duct: Biology, epidemiology, and long-term outcomes following resection. Ann. Surg. 2015, 261, 976–983. [Google Scholar] [CrossRef] [PubMed]
- van Oosten, A.F.; Groot, V.P.; Dorland, G.; Burkhart, R.A.; Wolfgang, C.L.; van Santvoort, H.C.; He, J.; Molenaar, I.Q.; Daamen, L.A. Dynamics of Serum CA19-9 in Patients Undergoing Pancreatic Cancer Resection. Ann. Surg. 2024, 279, 493–500. [Google Scholar] [CrossRef]
- Levink, I.J.M.; Jaarsma, S.C.; Koopmann, B.D.M.; van Riet, P.A.; Overbeek, K.A.; Meziani, J.; Sprij, M.L.J.A.; Casadei, R.; Ingaldi, C.; Polkowski, M.; et al. The additive value of CA19.9 monitoring in a pancreatic cyst surveillance program. United Eur. Gastroenterol. J. 2023, 11, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Peng, L.; Jin, Z.; Sun, L.; Song, B. The pathological features and prognoses of intraductal papillary mucinous neoplasm and mucinous cystic neoplasm after surgical resection: A single institution series. World J. Surg. Oncol. 2020, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Ohtsuka, T.; Tamura, K.; Mori, Y.; Shindo, K.; Yamada, D.; Takahata, S.; Ishigami, K.; Ito, T.; Tokunaga, S.; et al. Predictive Factors for the Metachronous Development of High-risk Lesions in the Remnant Pancreas After Partial Pancreatectomy for Intraductal Papillary Mucinous Neoplasm. Ann. Surg. 2016, 263, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Nista, E.C.; Schepis, T.; Candelli, M.; Giuli, L.; Pignataro, G.; Franceschi, F.; Gasbarrini, A.; Ojetti, V. Humoral Predictors of Malignancy in IPMN: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 12839. [Google Scholar] [CrossRef]
- Kane, L.E.; Mellotte, G.S.; Conlon, K.C.; Ryan, B.M.; Maher, S.G. Multi-Omic Biomarkers as Potential Tools for the Characterisation of Pancreatic Cystic Lesions and Cancer: Innovative Patient Data Integration. Cancers 2021, 13, 769. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Wu, H.; Allison, H.R.; Easler, J.J.; Sherman, S.; Al-Haddad, M.A.; Dewitt, J.M.; Schmidt, C.M. Biomarker Risk Score Algorithm and Preoperative Stratification of Patients with Pancreatic Cystic Lesions. J. Am. Coll. Surg. 2021, 233, 426–434.e4. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Mayer, B.; Vasilescu, C.; Poch, B. Long-term Metabolic Morbidity and Steatohepatosis Following Standard Pancreatic Resections and Parenchyma-sparing, Local Extirpations for Benign Tumor: A Systematic Review and Meta-analysis. Ann. Surg. 2022, 275, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Maignan, A.; Ouaïssi, M.; Turrini, O.; Regenet, N.; Loundou, A.; Louis, G.; Moutardier, V.; Dahan, L.; Pirrò, N.; Sastre, B.; et al. Risk factors of exocrine and endocrine pancreatic insufficiency after pancreatic resection: A multi-center prospective study. J. Visc. Surg. 2018, 155, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Mantovani, W.; Crippa, S.; Mascetta, G.; Salvia, R.; Pederzoli, P. Pancreatic insufficiency after different resections for benign tumours. Br. J. Surg. 2008, 95, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Mayer, B.; Poch, B. Duodenum-Preserving Pancreatic Head Resection for Benign and Premalignant Tumors-a Systematic Review and Meta-analysis of Surgery-Associated Morbidity. J. Gastrointest. Surg. 2023, 27, 2611–2627. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, J.; Anderson, B.; Liu, J.; Williams, G.A.; Chapman, W.C.; Doyle, M.M.; Khan, A.S.; Sanford, D.E.; Hammill, C.W.; Strasberg, S.M.; et al. Long Term Endocrine and Exocrine Insufficiency after Pancreatectomy. J. Gastrointest. Surg. 2019, 23, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Iacono, C.; Verlato, G.; Ruzzenente, A.; Campagnaro, T.; Bacchelli, C.; Valdegamberi, A.; Bortolasi, L.; Guglielmi, A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br. J. Surg. 2013, 100, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Xu, J.; Li, X.; Chen, W.; Gao, S.-L.; Shen, Y.; Zhang, M.; Wu, J.; Que, R.-S.; Yu, J.; et al. Central pancreatectomy for benign or low-grade malignant pancreatic tumors in the neck and body of the pancreas. World J. Gastrointest. Surg. 2022, 14, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Yang, Q.; Hu, H.-J.; Liu, F.; Karn, H.R.; Ma, W.-J.; Ran, C.-D.; Li, F.-Y. Overall Postoperative Morbidity and Pancreatic Fistula Are Relatively Higher after Central Pancreatectomy than Distal Pancreatic Resection: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2020, 2020, 7038907. [Google Scholar] [CrossRef]
- Bi, S.; Liu, Y.; Dai, W.; Pang, L.; Yang, S.; Zheng, Y.; Zhang, X.; Wu, S.; Kong, J. Effectiveness and safety of central pancreatectomy in benign or low-grade malignant pancreatic body lesions: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 2025–2036. [Google Scholar] [CrossRef]
- Crippa, S.; Salvia, R.; Warshaw, A.L.; Domínguez, I.; Bassi, C.; Falconi, M.; Thayer, S.P.; Zamboni, G.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: Lessons from 163 resected patients. Ann. Surg. 2008, 247, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Han, Y.; Byun, Y.; Kim, H.; Kwon, W.; Jang, J.-Y. Central Pancreatectomy Versus Distal Pancreatectomy and Pancreaticoduodenectomy for Benign and Low-Grade Malignant Neoplasms: A Retrospective and Propensity Score-Matched Study with Long-Term Functional Outcomes and Pancreas Volumetry. Ann. Surg. Oncol. 2020, 27, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Asano, Y.; Ito, M.; Arakawa, S.; Horiguchi, A. Recent trends in organ-preserving pancreatectomy: Its problems and clinical advantages compared with other standard pancreatectomies. Ann. Gastroenterol. Surg. 2024, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, J.G.; Werner, J. Surgery of Cystic Tumors of the Pancreas—Why, When, and How? Visc. Med. 2018, 34, 206–210. [Google Scholar] [CrossRef]
- Chincarini, M.; Zamboni, G.A.; Pozzi Mucelli, R. Major pancreatic resections: Normal postoperative findings and complications. Insights Imaging 2018, 9, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Mayer, B.; Poch, B. Resection of the duodenum causes long-term endocrine and exocrine dysfunction after Whipple procedure for benign tumors—Results of a systematic review and meta-analysis. HPB 2020, 22, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kanda, M.; Kodera, Y.; Nagai, S.; Sahin, T.T.; Kanzaki, A.; Yamada, S.; Sugimoto, H.; Nomoto, S.; Morita, S.; et al. Comparison of Pancreatic Head Resection With Segmental Duodenectomy and Pylorus-Preserving Pancreatoduodenectomy for Benign and Low-Grade Malignant Neoplasms of the Pancreatic Head. Pancreas 2011, 40, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.-W.; Dinh, K.H.; Sullivan, M.; Wassef, W.Y.; Zivny, J.; Whalen, G.F.; LaFemina, J. Thirty-day outcomes underestimate endocrine and exocrine insufficiency after pancreatic resection. HPB 2016, 18, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Alvino, D.M.; Castillo, C.F.-D.; Nipp, R.D.; Traeger, L.N.; Ruddy, M.; Lubitz, C.C.; Johnson, C.D.; Chang, D.C.; Warshaw, A.L.; et al. Health-related Quality of Life and Functional Outcomes in 5-year Survivors After Pancreaticoduodenectomy. Ann. Surg. 2017, 266, 685–692. [Google Scholar] [CrossRef]
- Pezzilli, R.; Caccialanza, R.; Capurso, G.; Brunetti, O.; Milella, M.; Falconi, M. Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer. Cancers 2020, 12, 275. [Google Scholar] [CrossRef]
- Sabater, L.; Ausania, F.; Bakker, O.J.; Boadas, J.; Domínguez-Muñoz, J.E.; Falconi, M.; Fernández-Cruz, L.; Frulloni, L.; González-Sánchez, V.; Lariño-Noia, J.; et al. Evidence-based Guidelines for the Management of Exocrine Pancreatic Insufficiency After Pancreatic Surgery. Ann. Surg. 2016, 264, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Layer, P.; Kashirskaya, N.; Gubergrits, N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J. Gastroenterol. 2019, 25, 2430–2441. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tirgoviste, C.; Gagniuc, P.A.; Gubceac, E.; Mardare, L.; Popescu, I.; Dima, S.; Militaru, M. A 3D map of the islet routes throughout the healthy human pancreas. Sci. Rep. 2015, 5, 14634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Misawa, R.; Zielinski, M.C.; Cowen, P.; Jo, J.; Periwal, V.; Ricordi, C.; Khan, A.; Szust, J.; Shen, J.; et al. Regional Differences in Islet Distribution in the Human Pancreas—Preferential Beta-Cell Loss in the Head Region in Patients with Type 2 Diabetes. PLoS ONE 2013, 8, e67454. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.K.; Singh, S.R.; Mishra, P.R. Redefining the tail of pancreas based on the islets microarchitecture and inter-islet distance. Medicine 2021, 100, e25642. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Xavier, G. The Cells of the Islets of Langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Kohoutova, D.; Skrha, J.; Bunganic, B.; Ngo, O.; Suchanek, S.; Skrha, P.; Zavoral, M. Diabetes Mellitus in Pancreatic Cancer: A Distinct Approach to Older Subjects with New-Onset Diabetes Mellitus. Cancers 2023, 15, 3669. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bian, X.; Wei, S.; He, M.; Yang, Y. The relationship between pancreatic cancer and type 2 diabetes: Cause and consequence. Cancer Manag. Res. 2019, 11, 8257–8268. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Nahm, C.B.; Jamieson, N.B.; Samra, J.; Clifton-Bligh, R.; Mittal, A.; Tsang, V. Risk factors for development of diabetes mellitus (Type 3c) after partial pancreatectomy: A systematic review. Clin. Endocrinol. 2020, 92, 396–406. [Google Scholar] [CrossRef]
- Scholten, L.; Mungroop, T.H.; Haijtink, S.A.L.; Issa, Y.; van Rijssen, L.B.; Koerkamp, B.G.; van Eijck, C.H.; Busch, O.R.; DeVries, J.H.; Besselink, M.G. New-onset diabetes after pancreatoduodenectomy: A systematic review and meta-analysis. Surgery 2018, 164, 6–16. [Google Scholar] [CrossRef]
- Lund, A.; Bagger, J.I.; Wewer Albrechtsen, N.J.; Christensen, M.; Grøndahl, M.; Hartmann, B.; Mathiesen, E.R.; Hansen, C.P.; Storkholm, J.H.; van Hall, G.; et al. Evidence of Extrapancreatic Glucagon Secretion in Man. Diabetes 2016, 65, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Isaza, J.; Curt, J.R.; Woodward, E.R. Distension of the pyloric antrum as a stimulus for gastrin release. Arch. Surg. 1970, 100, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Powley, T.L.; Hudson, C.N.; McAdams, J.L.; Baronowsky, E.A.; Martin, F.N.; Mason, J.K.; Phillips, R.J. Organization of vagal afferents in pylorus: Mechanoreceptors arrayed for high sensitivity and fine spatial resolution? Auton. Neurosci. 2014, 183, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Sugimoto, H.; Yamada, S.; Fujii, T.; Nomoto, S.; Takeda, S.; Kodera, Y.; Nakao, A. Risk factors for hepatic steatosis after pancreatectomy: A retrospective observational cohort study of the importance of nutritional management. Pancreas 2012, 41, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Horiuchi, A.; Yokoyama, T.; Kaneko, G.; Horigome, N.; Yamaura, T.; Nagaya, T.; Komatsu, M.; Sano, K.; Miyagawa, S.-I.; et al. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J. Gastroenterol. 2011, 46, 758–768. [Google Scholar] [CrossRef]
- Nagaya, T.; Tanaka, N.; Kimura, T.; Kitabatake, H.; Fujimori, N.; Komatsu, M.; Horiuchi, A.; Yamaura, T.; Umemura, T.; Sano, K.; et al. Mechanism of the development of nonalcoholic steatohepatitis after pancreaticoduodenectomy. BBA Clin. 2015, 3, 168–174. [Google Scholar] [CrossRef][Green Version]
- Nakamura, M.; Nakata, K.; Matsumoto, H.; Ohtsuka, T.; Yoshida, K.; Tokunaga, S.; Hino, K. Acyl/free carnitine ratio is a risk factor for hepatic steatosis after pancreatoduodenectomy and total pancreatectomy. Pancreatology 2017, 17, 135–138. [Google Scholar] [CrossRef]
- Yasukawa, K.; Shimizu, A.; Yokoyama, T.; Kubota, K.; Notake, T.; Seki, H.; Kobayashi, A.; Soejima, Y. Preventive Effect of High-Dose Digestive Enzyme Management on Development of Nonalcoholic Fatty Liver Disease after Pancreaticoduodenectomy: A Randomized Controlled Clinical Trial. J. Am. Coll. Surg. 2020, 231, 658–669. [Google Scholar] [CrossRef]
- Shah, P.; Patel, V.; Ashkar, M. De novo non-alcoholic fatty liver disease after pancreatectomy: A systematic review. World J. Clin. Cases 2022, 10, 12946–12958. [Google Scholar] [CrossRef]
- Patel, V.; Shah, P.; Ludwig, D.R.; Hammill, C.W.; Ashkar, M. Development of de novo nonalcoholic fatty liver disease following pancreatectomy. Medicine 2023, 102, e32782. [Google Scholar] [CrossRef]
- Li, Z.; Weinstein, J.; Redstone, E.; Mitchell, D.G. Hepatic Steatosis After Partial Pancreatectomy in a Cohort of Patients with Intraductal Papillary Mucinous Neoplasm. J. Clin. Exp. Hepatol. 2023, 13, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Firkins, S.A.; Hart, P.A.; Porter, K.; Chiang, C.; Cloyd, J.M.; Dillhoff, M.; Lara, L.F.; Manilchuk, A.; Papachristou, G.I.; Pawlik, T.M.; et al. Incidence and Risk Factors for New-Onset Diabetes Mellitus After Surgical Resection of Pancreatic Cystic Lesions: A MarketScan Study. Pancreas 2022, 51, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; Zhen, T.-T.; Wu, Y.; Wang, M.; Qin, T.-T.; Zhang, H.; Qin, R.-Y. Quality of life after pancreatic surgery. World J. Gastroenterol. 2024, 30, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Ferrone, C.R. ASO Author Reflections: Long-Term Impact of Pancreatoduodenectomy on Pancreas-Specific Quality of Life. Ann. Surg. Oncol. 2021, 28, 4225–4226. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Yamaguchi, K.; Chijiiwa, K.; Kinukawa, N.; Tanaka, M. Quality of life after pylorus-preserving pancreatoduodenectomy. Am. J. Surg. 2001, 182, 230–236. [Google Scholar] [CrossRef]

| Type of Surgery | Partial Pancreatectomy | Radical Pancreatectomy with Lymph Node Dissection | Organ-Preserving Pancreatectomy without Lymphadenectomy * | Comments | |
|---|---|---|---|---|---|
| IPMN Subtype | |||||
| BD-IPMN | Usually preferred | Only when IC is suspected or confirmed | Only if the suspicion for IC is low based on preoperative features and/or intraoperative findings | Minimally invasive approaches (laparoscopic or robotic pancreatectomy) can be utilized. Goal: negative surgical margins ** Pancreatoscopy should be performed preoperatively, but is not recommended as a routine examination. | |
| Mixed IPMN | Same as for BD-IPMN | Same as for BD-IPMN | |||
| MD-IPMN | Same as for BD-IPMN | Same as for BD-IPMN | |||
| Surgery Type | NOD (%) | Steatosis/NAFLD (%) | PEI (%) | Other Postop. Findings |
|---|---|---|---|---|
| PD | 15 (Beger, 2020 [76]) 15.7 (Beger, 2022 [62]) 27.8 (Patel, 2023 [100]) 9–24 (Wu, 2020 [89]) | 23.8 (Beger, 2022) 16–26 (Shah P, 2022) 11.1 (Patel, 2023) | 44.9 (Beger, 2020) 44.3 (Beger, 2022) 44.4 (Patel, 2023) | Significant decrease in fasting basal and stimulated levels of gastrin, motilin, insulin, C-peptide, secretin, PP and GIP after mean 7.8 mo. Significantly lower levels of gastrin, secretin and CCK compared to PPPD (p < 0.05) Stimulated CCK secretion is significantly reduced compared to PPPD (p < 0.0001) and DPPHR (p = 0.011) (Beger, 2020) |
| DPPHR | 6 (Beger, 2020) 5 (Beger, 2022) | 3 (Beger, 2022) | 6.8 (Beger, 2020) 6.7 (Beger, 2022) | Normal levels of fasting motilin and secretin; stimulated response of insulin, gastrin, motilin, CCK and secretin comparable to preop. (Beger, 2020) |
| LP/DP | 23.3 (Beger, 2022) 54.1 (Patel, 2023) 3–40 (Wu, 2020) | 8.3 (Patel, 2023) | 17 (Beger, 2022) 25 (Patel, 2023) | |
| PPPD | 19.7 (Beger, 2022) | Significantly increased fasting basal and stimulated secretion of GLP-1 and glucagon (p < 0.05) (Beger, 2020) | ||
| PMSR/CP | 5.6 (Beger, 2022) 0–14 (Wu, 2020) | 8 (Beger, 2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaban, D.V.; Coman, L.-I.; Balaban, M.; Costache, R.S.; Jinga, M. Novel Insights into Postoperative Surveillance in Resected Pancreatic Cystic Neoplasms—A Review. Diagnostics 2024, 14, 1056. https://doi.org/10.3390/diagnostics14101056
Balaban DV, Coman L-I, Balaban M, Costache RS, Jinga M. Novel Insights into Postoperative Surveillance in Resected Pancreatic Cystic Neoplasms—A Review. Diagnostics. 2024; 14(10):1056. https://doi.org/10.3390/diagnostics14101056
Chicago/Turabian StyleBalaban, Daniel Vasile, Laura-Ioana Coman, Marina Balaban, Raluca Simona Costache, and Mariana Jinga. 2024. "Novel Insights into Postoperative Surveillance in Resected Pancreatic Cystic Neoplasms—A Review" Diagnostics 14, no. 10: 1056. https://doi.org/10.3390/diagnostics14101056
APA StyleBalaban, D. V., Coman, L.-I., Balaban, M., Costache, R. S., & Jinga, M. (2024). Novel Insights into Postoperative Surveillance in Resected Pancreatic Cystic Neoplasms—A Review. Diagnostics, 14(10), 1056. https://doi.org/10.3390/diagnostics14101056








