Simplified Assessment of Radioiodine Biokinetics for Thyroid Cancer Patients: A Practical Approach Using Continuous External Radiation Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. External Radiation Dose Rate Measurements and Whole-Body Radiation Retention Ratio
2.3. Biokinetic Modelling of RAI
2.4. Radiation Exposure Estimation
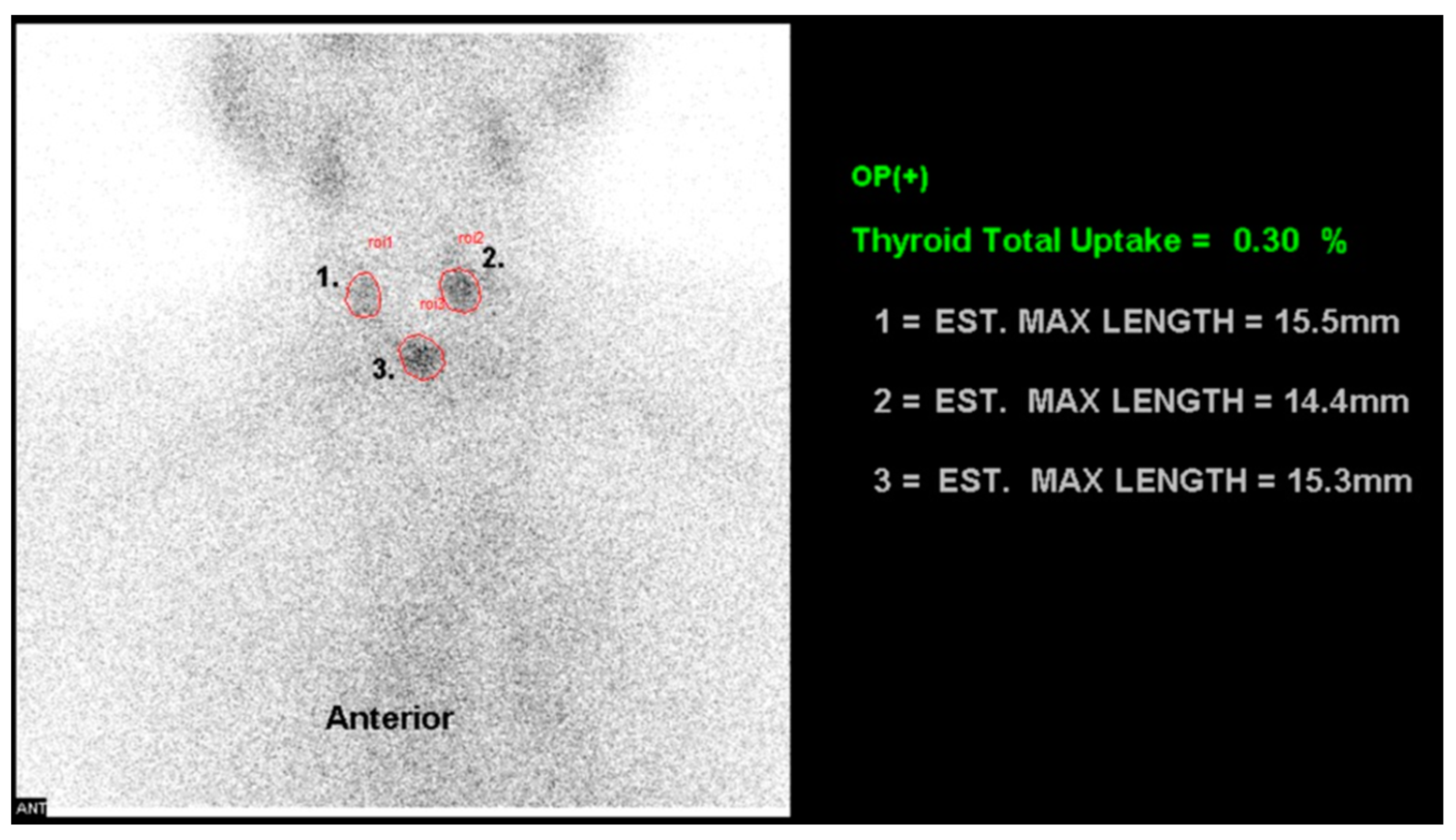
2.5. 99mTc-pertechnetate Uptake Ratio
2.6. Estimated Glomerular Filtration Rate
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.M.; Alexander, E.K.; Bible, K.C.; Doherty, G.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.; Sawka, A.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Ilisea, R.; Smith, Y.; Viersinga, V. European consensus on the management of patients with differentiated carcinoma of the thyroid from follicular epithelium. Vestn. Khirurgii Im. II Grek. 2008, 167, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006; p. 422. [Google Scholar]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef] [PubMed]
- NCRP. Implications of Recent Epidemiologic Studies for the Linear Nonthreshold Model and Radiation Protection; NCRP: Bethesda, MD, USA, 2018. [Google Scholar]
- Shore, R.E.; Beck, H.L.; Boice, J.D.; Caffrey, E.A.; Davis, S.; Grogan, H.A.; Mettler, F.A.; Preston, R.J.; Till, J.E.; Wakeford, R.; et al. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J. Radiol. Prot. 2018, 38, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. EPA Radiogenic Cancer Risk Models and Projections for the US Population; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Advisory Committee on the Medical Use of Isotopes (ACMUI) to the NRC. Patient Release Report; Available on the ACMUI’s Public Website under Related Information. Available online: http://www.nrc.gov/about-nrc/regulatory/advisory/acmui.html (accessed on 13 December 2010).
- International Atomic Energy Agency. Release of Patients After Radionuclide Therapy; International Atomic Energy Agency: Vienna, 2009. [Google Scholar]
- The 2007 Recommendations of the International Commission on Radiological Protection. Preface, Executive Summary and Glossary. Ann. ICRP 2007, 37, 9–34. [Google Scholar] [CrossRef] [PubMed]
- U.S. Nuclear Regulatory Commission. Release of Patients Administered Radioactive Material Publication Regulatory Guide 8.39; US Government Printing Office: Washington, DC, USA, 1997.
- Van Nostrand, D.; Atkins, F.; Yeganeh, F.; Acio, E.; Bursaw, R.; Wartofsky, L. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid 2002, 12, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Hänscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J. Nucl. Med. 2006, 47, 648–654. [Google Scholar] [PubMed]
- Benua, R.S.; Cicale, N.R.; Sonenberg, M.; Rawson, R.W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1962, 87, 171–182. [Google Scholar] [PubMed]
- Maxon, H.R.; Thomas, S.R.; Hertzberg, V.S.; Kereiakes, J.G.; Chen, I.-W.; Sperling, M.I.; Saenger, E.L. Relation between Effective Radiation Dose and Outcome of Radioiodine Therapy for Thyroid Cancer. N. Engl. J. Med. 1983, 309, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Zanzonico, P.B.; Siegel, J.A.; St Germain, J. A generalized algorithm for determining the time of release and the duration of post-release radiation precautions following radionuclide therapy. Health Phys. 2000, 78, 648–659. [Google Scholar] [CrossRef] [PubMed]
- NCRP. Scientific Committee 91-1 on Precautions in the Management of Patients Who Have Received Therapeutic Amounts of Radioactivity. Management of Radionuclide Therapy Patients; NCRP: Bethesda, MD, USA, 2007. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Pearson: Reading, MA, USA, 1977; Volume 2. [Google Scholar]
- Benua, R.S. A method and rationale for treating metastatic thyroid carcinoma with the largest safe dose of I-131. Front. Thyroidol. 1986, 2, 1317–1321. [Google Scholar]
- Tenhunen, M.; Lehtonen, S.; Heikkonen, J.; Halonen, P.; Maenpaa, H. First-day iodine kinetics is useful for individualizing radiation safety precautions for thyroid carcinoma patients. Nucl. Med. Commun. 2013, 34, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, W.; Huang, R.; Tian, R.; Zeng, Y.; Kuang, A. Thyroid cancer: Radiation safety precautions in 131I therapy based on actual biokinetic measurements. Radiology 2014, 273, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; Wexler, J.A.; et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: Practice recommendations of the American Thyroid Association. Thyroid 2011, 21, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Suriano, S.; Ricci, R.; Ceriani, L.; Anton Verburg, F. Postsurgical thyroid remnant estimation by (99m) Tc-pertechnetate scintigraphy predicts radioiodine ablation effectiveness in patients with differentiated thyroid carcinoma. Head Neck 2011, 33, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, D.; Cuhaci, F.N.; Ozdemir, E.; Aydin, C.; Ersoy, R.; Turkolmez, S.; Cakir, B. The role of postoperative Tc-99m pertechnetate scintigraphy in estimation of remnant mass and prediction of successful ablation in patients with differentiated thyroid cancer. Nucl. Med. Commun. 2016, 37, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kueh, S.S.; Roach, P.J.; Schembri, G.P. Role of Tc-99m pertechnetate for remnant scintigraphy post-thyroidectomy. Clin. Nucl. Med. 2010, 35, 671–674. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; p. 360. [Google Scholar]
- Myssayev, A.; Myssayev, A.; Ideguchi, R.; Nishi, K.; Fukuda, N.; Ito, A.; Haraguchi, A.; Horie, I.; Kawakami, A.; Kudo, T. Estimation of Clearance Rate of Iodine-131 from Thyroid Cancer Patients Based on Individual Patient’s Characteristic. Res. Sq. 2020. [Google Scholar] [CrossRef]

| Characteristic | N (%) | Mean ± SD | Range |
|---|---|---|---|
| Age | 46.91 ± 14.85 | 26–76 | |
| Sex | |||
| Women | 23 (65.7%) | ||
| Men | 12 (34.3%) | ||
| Pathology | |||
| Papillary carcinoma | 33 (94.3%) | ||
| Follicular carcinoma | 1 (2.9%) | ||
| Hurthle cell carcinoma | 1 (2.9%) | ||
| T stage | |||
| 1 | 11 (31.4%) | ||
| 2 | 12 (34.3%) | ||
| 3 | 10 (28.6%) | ||
| 4 | 2 (5.7%) | ||
| N stage | |||
| No lymph node metastasis (N0) | 19 (54.3%) | ||
| Lymph node metastases (N1) | 16 (45.7%) | ||
| Preparation of TSH stimulation | |||
| rh-TSH a administration | 30 (85.7%) | ||
| T4 withdrawal | 5 (14.3%) | ||
| Administered dose of RAI b (GBq) | 35 | 3.74 ± 1.17 | 1.11–5.55 |
| 1.11 GBq | 5 (14.3%) | ||
| 3.7 GBq | 12 (34.3%) | ||
| 4.44 GBq | 17 (48.6%) | ||
| 5.55 GBq | 1 (2.9%) | ||
| RRrelease c (%) | 9.61 ± 5.57 | 2.50–29.12 | |
| TcUR d (%) | 0.36 ± 0.50 | 0.04–2.49 | |
| BMI | 23.23 ± 3.39 | 17.69–33.41 | |
| TSH (mIU/mL) | 164.01 ± 80.80 | 38.61–375.00 | |
| Creatinine (mg/dL) | 0.82 ± 0.17 | 0.5–1.2 | |
| BUN e (mg/dL) | 10.37 ± 2.28 | 7–15 | |
| eGFR f (mL/min per 1.73 m2) | 87.62 ± 17.51 | 61.3–129.1 | |
| Derived biokinetic parameters of RAI | |||
| Ft (RAI uptake fraction of the thyroid tissue) | 0.08 ± 0.06 | 0.006–0.288 | |
| Tet (effective half-life of RAI in extra-thyroid tissue) | 7.57 ± 1.45 | 4.180–10.228 |
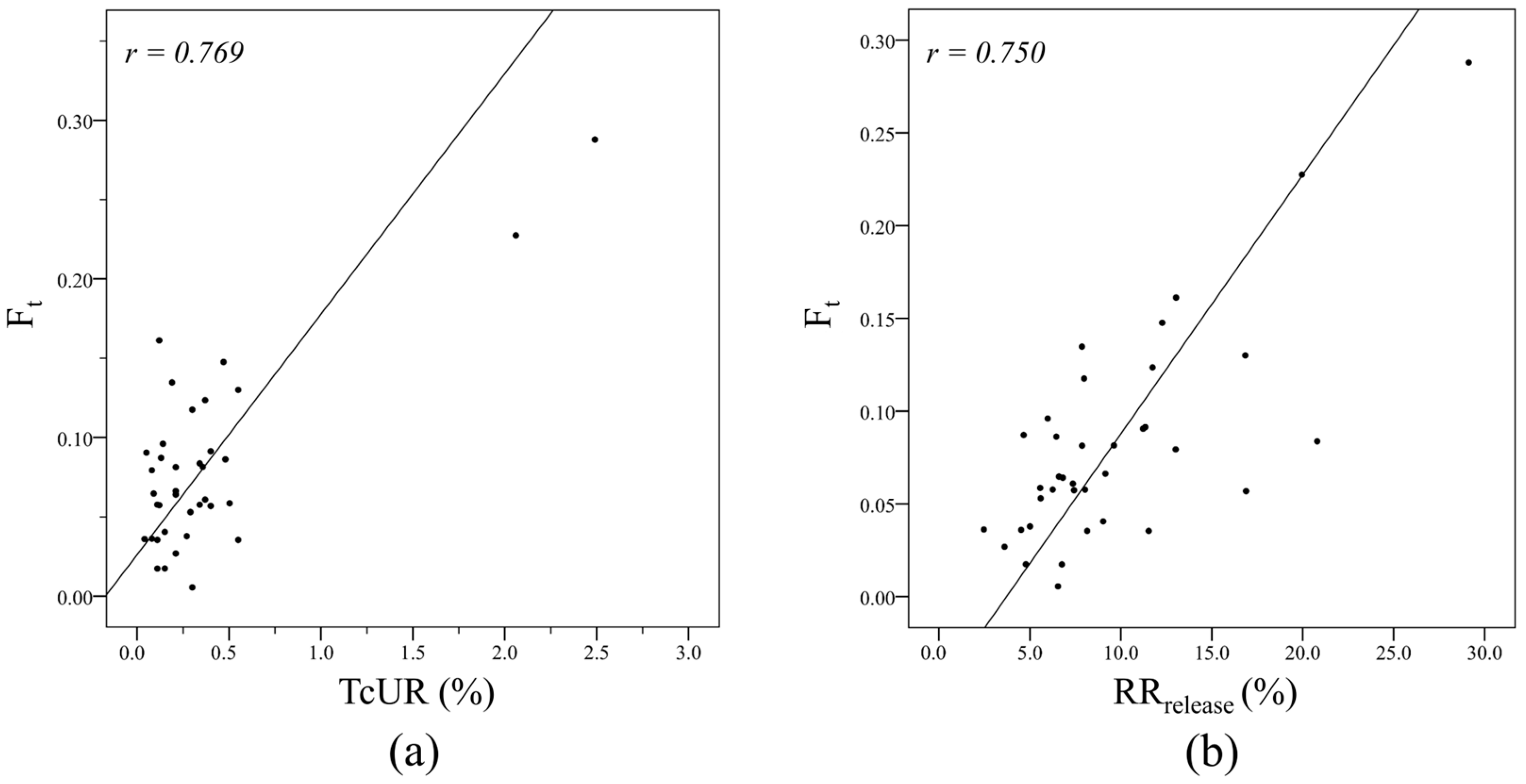
| Variable | Correlation Coefficient, r | p Value |
|---|---|---|
| TcUR b | 0.769 | <0.001 |
| RRrelease c | 0.750 | <0.001 |
| Variable | Correlation Coefficient, r | p Value |
|---|---|---|
| Age | 0.160 | 0.345 |
| BMI b | 0.374 | 0.022 * |
| BUN c level | 0.371 | 0.024 * |
| Creatinine level | 0.629 | <0.001 *** |
| eGFR d | −0.400 | 0.017 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-K.; Lin, L.-F.; Cheng, C.-Y.; Wong, C.-Y.O.; Wang, W.-H.; Shen, D.H.-Y.; Su, S.-L.; Chen, E.-S.; Chen, T.-Y.; Chen, I.-F. Simplified Assessment of Radioiodine Biokinetics for Thyroid Cancer Patients: A Practical Approach Using Continuous External Radiation Monitoring. Diagnostics 2024, 14, 1010. https://doi.org/10.3390/diagnostics14101010
Tsai Y-K, Lin L-F, Cheng C-Y, Wong C-YO, Wang W-H, Shen DH-Y, Su S-L, Chen E-S, Chen T-Y, Chen I-F. Simplified Assessment of Radioiodine Biokinetics for Thyroid Cancer Patients: A Practical Approach Using Continuous External Radiation Monitoring. Diagnostics. 2024; 14(10):1010. https://doi.org/10.3390/diagnostics14101010
Chicago/Turabian StyleTsai, Yao-Kuang, Li-Fan Lin, Cheng-Yi Cheng, Ching-Yee Oliver Wong, Wei-Hsung Wang, Daniel Hueng-Yuan Shen, Sui-Lung Su, En-Shih Chen, Tzai-Yang Chen, and I-Feng Chen. 2024. "Simplified Assessment of Radioiodine Biokinetics for Thyroid Cancer Patients: A Practical Approach Using Continuous External Radiation Monitoring" Diagnostics 14, no. 10: 1010. https://doi.org/10.3390/diagnostics14101010





