Selective Recovery of Gold from E-Waste Recycling Plants’ Waste Fractions: Waste-to-Resource Transition
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Experimental Procedures
2.2.1. Acid Attack
2.2.2. Leaching Test
2.3. Experimental Design and Data Analysis
3. Results and Discussion
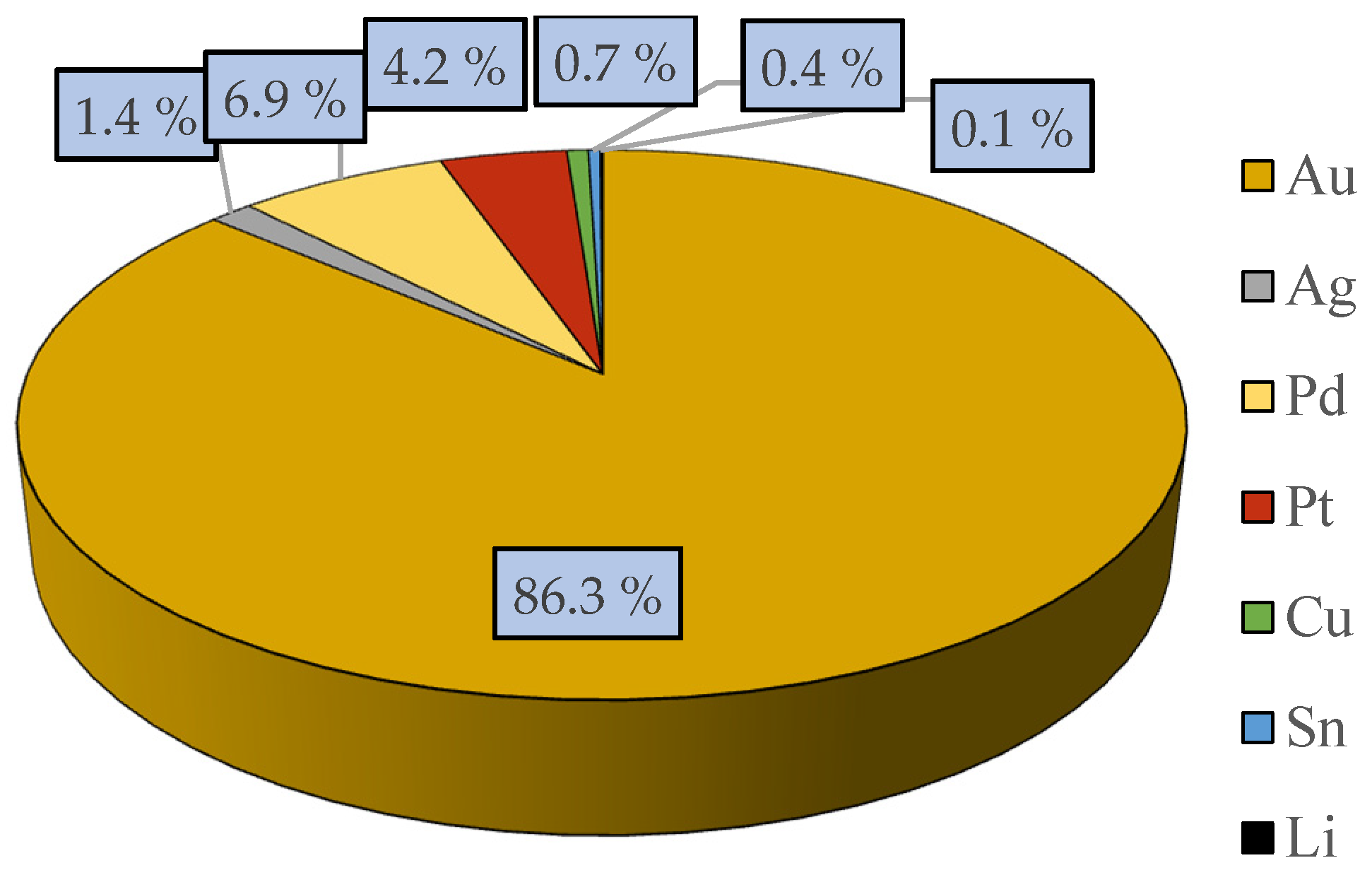
3.1. Sample Characterization
- F1 (filter): 4494 EUR/t;
- C1 (cyclone): 3517 EUR/t;
- F2 (filter output cables): 2243 EUR/t;
- C2 (cyclone output cables): 2429 EUR/t.
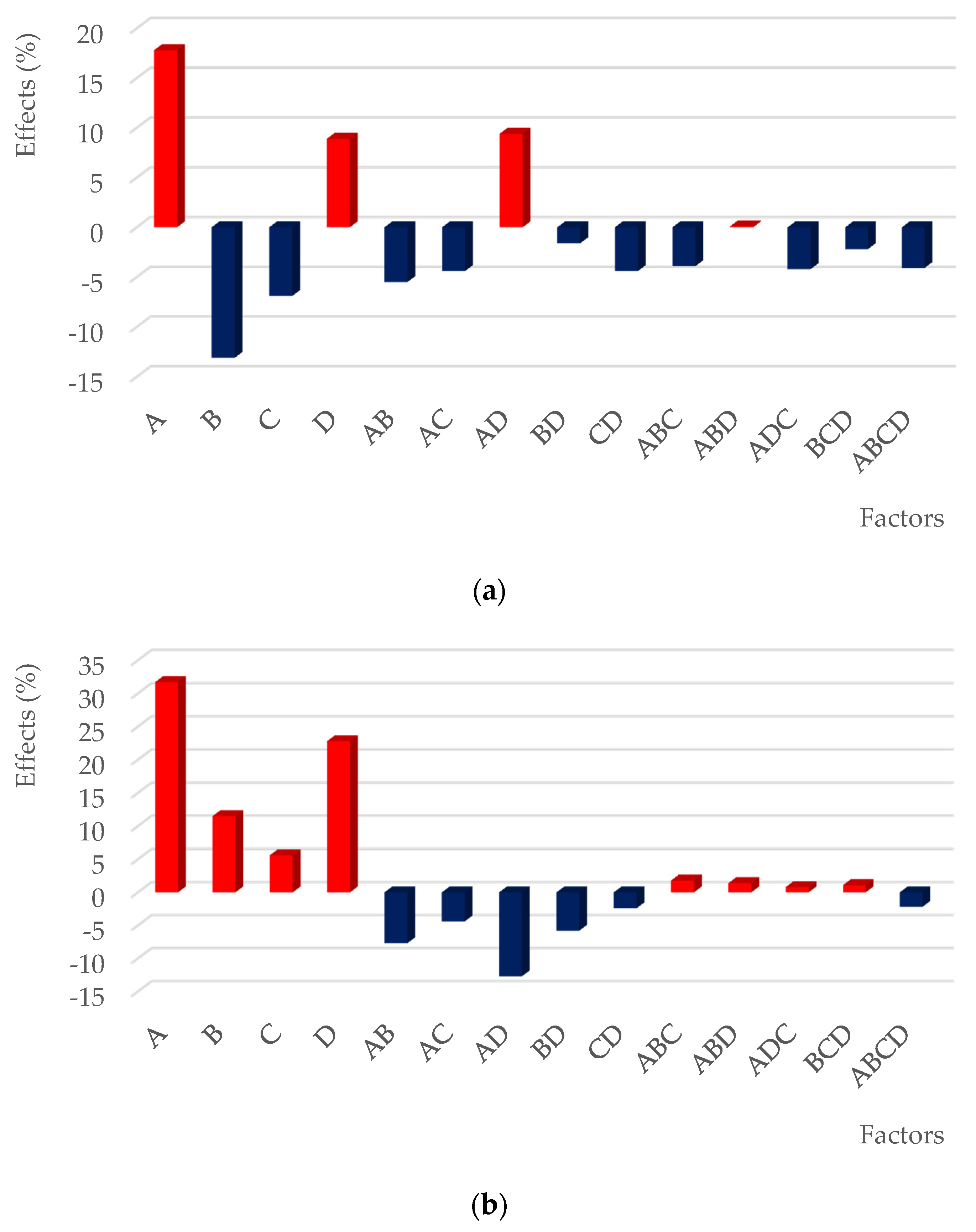
3.2. Optimizing the Leaching of Gold Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuncuk, A.; Stazi, V.; Akcil, A. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; UNU/UNITAR SCYCLE, ITU, ISWA: Geneva, Switzerland, 2020; 120p. [Google Scholar]
- Hazra, A.; Das, S.; Ganguly, A. Plasma arc technology: A potential solution toward waste to energy conversion and of GHGs mitigation. In Waste Valorisation and Recycling, Proceedings of the 7th IconSWM—ISWMAW 2017; Springer: Singapore, 2019; pp. 203–217. [Google Scholar]
- Zulkernain, N.H.; Basant, N.; Ng, C.C. Recovery of precious metals from e-wastes through conventional and phytoremediation treatment methods: A review and prediction. J. Mater. Cycles Waste Manag. 2023, 25, 2726–2752. [Google Scholar] [CrossRef]
- Critical Raw Materials. Available online: https://ec.europa.eu/growth/sectors/raw-materials/areas-specific-interest/critical-raw-materials/ (accessed on 20 February 2024).
- Directive (EU) 2024/884 Amending Directive 2012/19/EU on Waste Electrical and Electronic Equipment (WEEE). Available online: https://www.europeansources.info/record/directive-eu-2024-884-amending-directive-2012-19-eu-on-waste-electrical-and-electronic-equipment-weee/ (accessed on 14 May 2024).
- WEEE: Illegal Trade of Electronic Waste Must be Stopped to Achieve EU Goals. Available online: https://www.renewablematter.eu/articles/article/weee-illegal-trade-of-electronic-waste-must-be-stopped-to-achieve-eu-goals/ (accessed on 13 April 2024).
- European Commission to fight shell entities. Available online: https://www.dpc.bg/insights/european-commission-to-fight-shell-entities/ (accessed on 13 April 2024).
- Tuncuk, A. Lab scale optimization and two-step sequential bench scale reactor leaching tests for the chemical dissolution of Cu, Au & Ag from waste electrical and electronic equipment (WEEE). Waste Manag. 2019, 95, 636–643. [Google Scholar]
- Rene, E.R.; Sethurajan, M.; Ponnusamy, V.K.; Kumar, G.; Dung, T.N.B.; Brindhadevi, K.; Pugazhendhi, A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef]
- Neto, I.F.F.; Soares, H.M.V.M. Simple and near-zero-waste processing for recycling gold at a high purity level from waste printed circuit boards. Waste Manag. 2021, 135, 90–97. [Google Scholar] [CrossRef]
- Mori de Oliveira, C.; Bellopede, R.; Tori, A.; Marini, P. Study of Metal Recovery from Printed Circuit Boards by Physical-Mechanical Treatment Processes. Mater. Proc. 2021, 5, 121. [Google Scholar]
- Ahirwar, R.; Tripathi, A.K. E-waste management: A review of recycling process, environmental and occupational health hazards, and potential solutions. Environmental Nanotechnology. Monit. Manag. 2021, 15, 100409. [Google Scholar] [CrossRef]
- Anuar, W.; Ardani, M.; Baharun, N.; Ismail, S.; Rahman, S. Optimization Process of the Refractory Gold Ore Extraction by Hypochlorite Oxidative Leaching. Min. Metall. Explor. 2021, 38, 1191–1201. [Google Scholar] [CrossRef]
- Veit, H.M.; de Pereira, C.C.; Bernardes, A.M. Using mechanical processing in recycling printed wiring boards. JOM 2002, 54, 45–47. [Google Scholar] [CrossRef]
- Gurgul, A.; Szczepaniak, W.; Zabłocka-Malicka, M. Incineration and pyrolysis vs. steam gasification of electronic waste. Sci. Total Environ. 2018, 624, 1119–1124. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A comprehensive review on pyrolysis from the circular economy point of view and its environmental and social effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Udayakumar, S.; Razak, M.I.B.A.; Ismail, S. Recovering valuable metals from Waste Printed Circuit Boards (WPCB): A short review Mater. Today Proc. 2022, 66, 3062–3070. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Passadoro, M.; Ferella, F.; Pellei, G.; Veglio, F. Recovery of Metals from Printed Circuit Boards by Gold-REC 1 Hydrometallurgical. Sustainability 2023, 15, 7348. [Google Scholar] [CrossRef]
- Do, M.H.; Nguyen, G.T.; Thach, U.D.; Lee, Y.; Bui, T.H. Advances in hydrometallurgical approaches for gold recovery from E-waste: A comprehensive review and perspectives. Miner. Eng. 2023, 191, 107977. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources. A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Sousa, R.; Regufe, M.J.; Fiúza, A.; Leite, M.M.; Futuro, A. A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extr. Ind. Soc. 2022, 9, 101018. [Google Scholar] [CrossRef]
- Ray, D.A.; Baniasadi, M.; Graves, J.E.; Greenwood, A.; Farnaud, S. Thiourea Leaching: An Update on a Sustainable Approach for Gold Recovery from E-waste. J. Sustain. Metall. 2022, 8, 597–612. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Tiburcio-Munive, G.; Salazar-Campoy, M.M.; Valenzuela-García, J.L. Dissolution of Silver and Gold with Sodium Hypochlorite and Hydrochloric Acid in Refractory Minerals (Mangano-Argentiferous). Min. Metall. Explor. 2020, 37, 1213–1220. [Google Scholar] [CrossRef]
- De Carvalho, F.A.; Resende, A.; Leão, V.A. Gold Leaching by Sodium Chloride and Calcium Hypochlorite Solutions Extraction. In Extraction 2018, Proceedings of the First Global Conference on Extractive Metallurgy; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Wang, R.; Deng, Y.; Li, S.; Yu, K.; Liu, Y.; Shang, M.; Wang, J.; Shu, J.; Sun, Z.; Chen, M. Waste Electrical and Electronic Equipment Reutilization in China. Sustainability 2021, 13, 11433. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; Springer: New York, NY, USA, 1997; ISBN 0-471-31649-0. [Google Scholar]
- Kremer, N. Teoriya Veroyatnostei i Matematicheskaya Statistika (Probability Theory and Mathematical Statistics); YuNITI DANA: Moscow, Russia, 2009. [Google Scholar]
- Romano, P.; Ippolito, N.M.; Veglio, F. Chemical Characterization of an ARDUINO® Board and Its Surface Mount Devices for the Evaluation of Their Intrinsic Economic Value. Processes 2023, 11, 1911. [Google Scholar] [CrossRef]
- New in JMP® 16 and JMP Pro® 16. Available online: https://www.jmp.com/en_is/events/mastering/topics/new-in-jmp16-and-jmp-pro16.html (accessed on 13 April 2024).


| Acronym | Source | Granulometry Distribution, % | Sample Images | |
|---|---|---|---|---|
| <2 mm | >2 mm | |||
| F1 | Filter | 93.1 | 6.9 |  |
| C1 | Cyclone | 89.3 | 10.7 |  |
| F2 | Filter output cables | 84.8 | 15.2 |  |
| C2 | Cyclone output cables | 85.1 | 14.9 |  |
| Factors | Levels | |||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| A | HCl, mol/L | 1 | 2.5 | 4 |
| B | NaClO, % | 5 | 10 | 15 |
| C | NaCl, % | 0 | 1 | 2 |
| D | Temperature, °C | 20 | 40 | 60 |
| Factors | Levels | |||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | HCl, mol/L | 1 | 2,5 | 4 |
| X2 | NaClO, % | 0 | 2,5 | 5 |
| Au, g/t | Ag, g/t | Pd, g/t | Pt, g/t | Li, g/t | Sn, g/t | Cu, % | |
|---|---|---|---|---|---|---|---|
| F1 | 71.6 ± 16.7 | 84.6 ± 20.0 | 4.7 ± 2.0 | 6.9 ± 4.8 | 257.4 ± 69.3 | 623.3 ± 138.4 | 0.40 ± 0.17 |
| C1 | 35.4 ± 14.6 | 85.3 ± 49.9 | 16.8 ± 13.2 | <5 | 37.7 ± 17.1 | 1529 ± 994 | 4.82 ± 3.53 |
| F2 | <3 | 28.0 ± 23.5 | 22.8 ± 10.1 | <3 | 11.8 ± 5.2 | 1076 ± 572 | 8.65 ± 5.84 |
| C2 | <3 | 59.2 ± 106.7 | 25.3 ± 17.2 | <3 | 5.6 ± 1.3 | 661.9 ± 267.2 | 8.77 ± 4.98 |
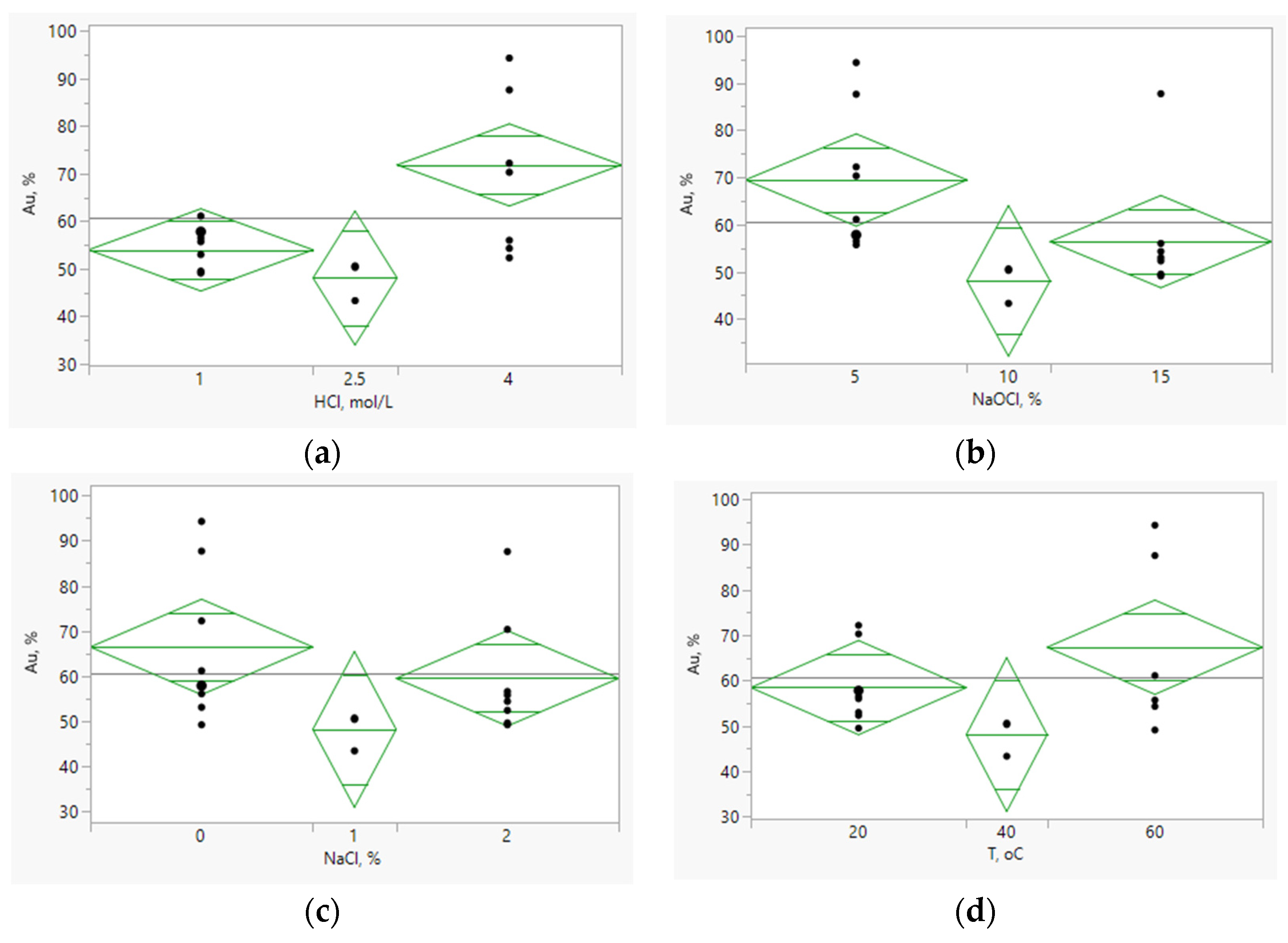
| Test n. | Factors with Actual Levels | Me Recovery, % | Weight Loss, % | |||||
|---|---|---|---|---|---|---|---|---|
| A HCl, mol/L | B NaClO, % | C NaCl, % | D t, °C | Au | Ag | Cu | ||
| 1 | 1 | 5 | 0 | 20 | 57.78 | 17.59 | 66.02 | 21.6 |
| 2 | 4 | 5 | 0 | 20 | 72.17 | 80.41 | 69.40 | 21.6 |
| 3 | 1 | 15 | 0 | 20 | 52.99 | 49.60 | 59.63 | 21.6 |
| 4 | 4 | 15 | 0 | 20 | 55.96 | 86.21 | 81.36 | 23.2 |
| 5 | 1 | 5 | 2 | 20 | 56.50 | 36.46 | 47.99 | 20.6 |
| 6 | 4 | 5 | 2 | 20 | 70.29 | 80.79 | 67.41 | 22.4 |
| 7 | 1 | 15 | 2 | 20 | 49.52 | 57.24 | 66.39 | 21.0 |
| 8 | 4 | 15 | 2 | 20 | 52.29 | 91.42 | 66.02 | 20.4 |
| 9 | 1 | 5 | 0 | 60 | 61.08 | 66.78 | 50.98 | 26.4 |
| 10 | 4 | 5 | 0 | 60 | 94.32 | 95.35 | 87.96 | 32.6 |
| 11 | 1 | 15 | 0 | 60 | 49.07 | 77.88 | 84.94 | 22.6 |
| 12 | 4 | 15 | 0 | 60 | 87.73 | 94.56 | 83.83 | 30.0 |
| 13 | 1 | 5 | 2 | 60 | 55.68 | 72.68 | 68.63 | 23.0 |
| 14 | 4 | 5 | 2 | 60 | 87.63 | 94.75 | 90.45 | 34.8 |
| 15 | 1 | 15 | 2 | 60 | 49.08 | 85.65 | 83.71 | 23.8 |
| 16 | 4 | 15 | 2 | 60 | 54.30 | 94.33 | 90.97 | 26.8 |
| 17 | 2,5 | 10 | 1 | 40 | 43.32 | 77.64 | 73.83 | 24.0 |
| 18 | 2,5 | 10 | 1 | 40 | 50.58 | 77.34 | 78.90 | 23.6 |
| 19 | 2,5 | 10 | 1 | 40 | 50.27 | 74.78 | 71.05 | 26.4 |
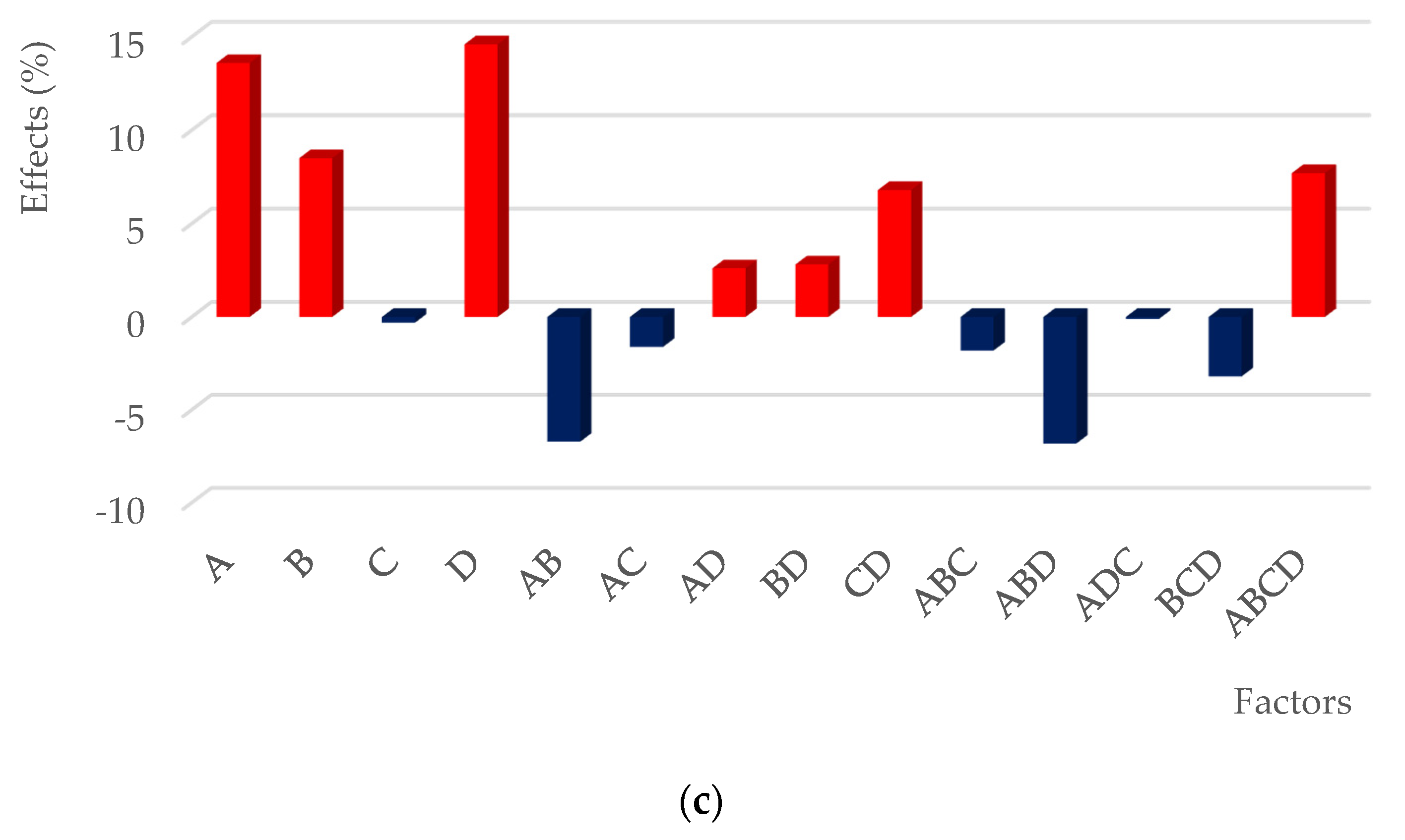
| Terms | Effect | SS = MS | F-Value | P-Value | Significance % |
|---|---|---|---|---|---|
| intercept | 63.59 | - | - | - | - |
| A | 16.50 | 1088.8 | 64.6 | 0.015 | 98.49 |
| B | −14.44 | 833.9 | 49.5 | 0.020 | 98.04 |
| AB | −4.09 | 67.0 | 4.0 | 0.184 | 81.57 |
| C | −5.60 | 125.5 | 7.4 | 0.112 | 88.79 |
| AC | −5.82 | 135.3 | 8.0 | 0.105 | 89.48 |
| BC | −4.54 | 82.4 | 4.9 | 0.158 | 84.25 |
| ABC | −2.59 | 26.9 | 1.6 | 0.334 | 66.63 |
| D | 10.30 | 424.3 | 25.2 | 0.037 | 96.25 |
| AD | 8.02 | 257.2 | 15.3 | 0.060 | 94.03 |
| BD | −2.94 | 34.7 | 2.1 | 0.288 | 71.21 |
| ABD | 1.52 | 9.2 | 0.5 | 0.537 | 46.30 |
| CD | −3.03 | 36.6 | 2.2 | 0.278 | 72.17 |
| ACD | −5.62 | 126.2 | 7.5 | 0.112 | 88.84 |
| BCD | −3.54 | 50.2 | 3.0 | 0.226 | 77.36 |
| ABCD | −2.69 | 29.0 | 1.7 | 0.320 | 68.02 |
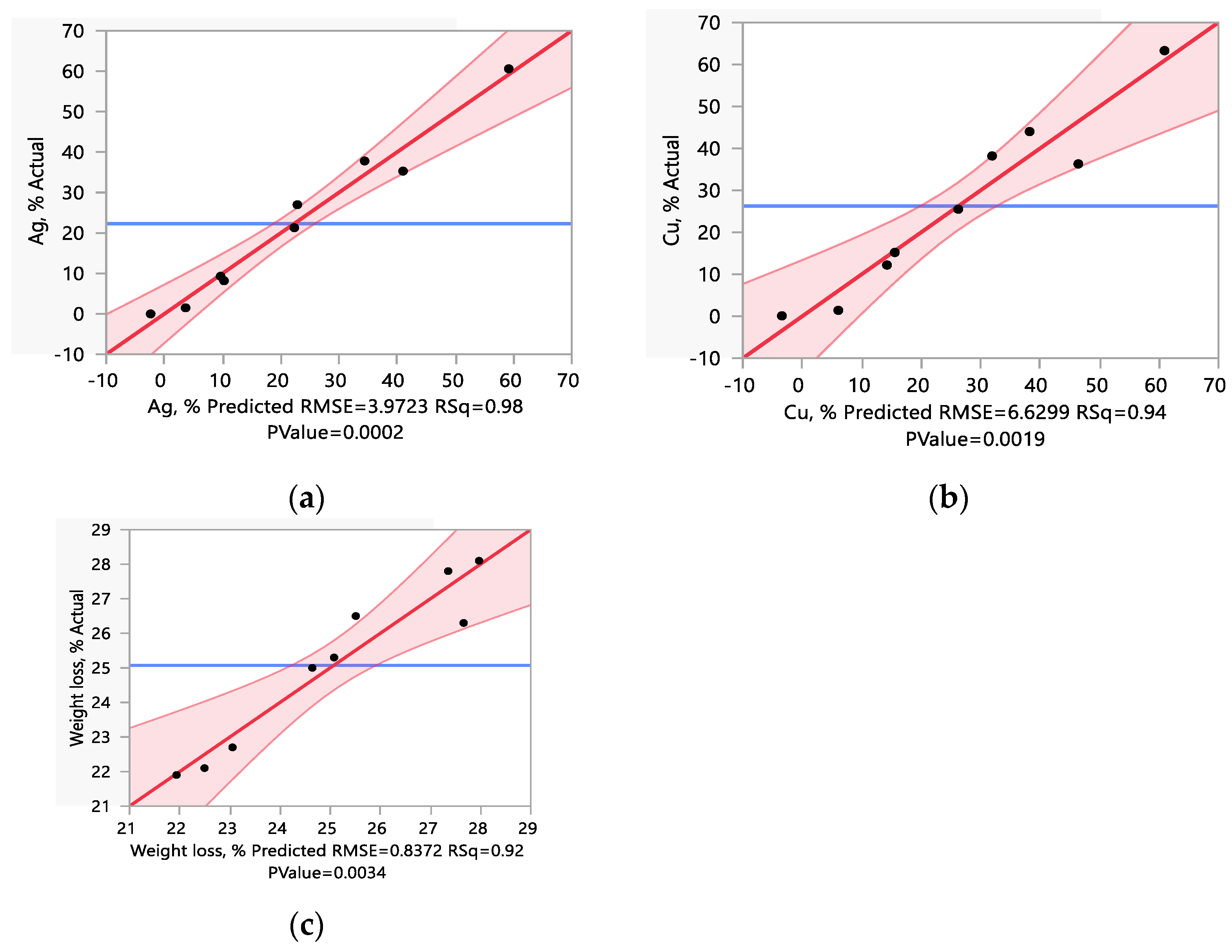
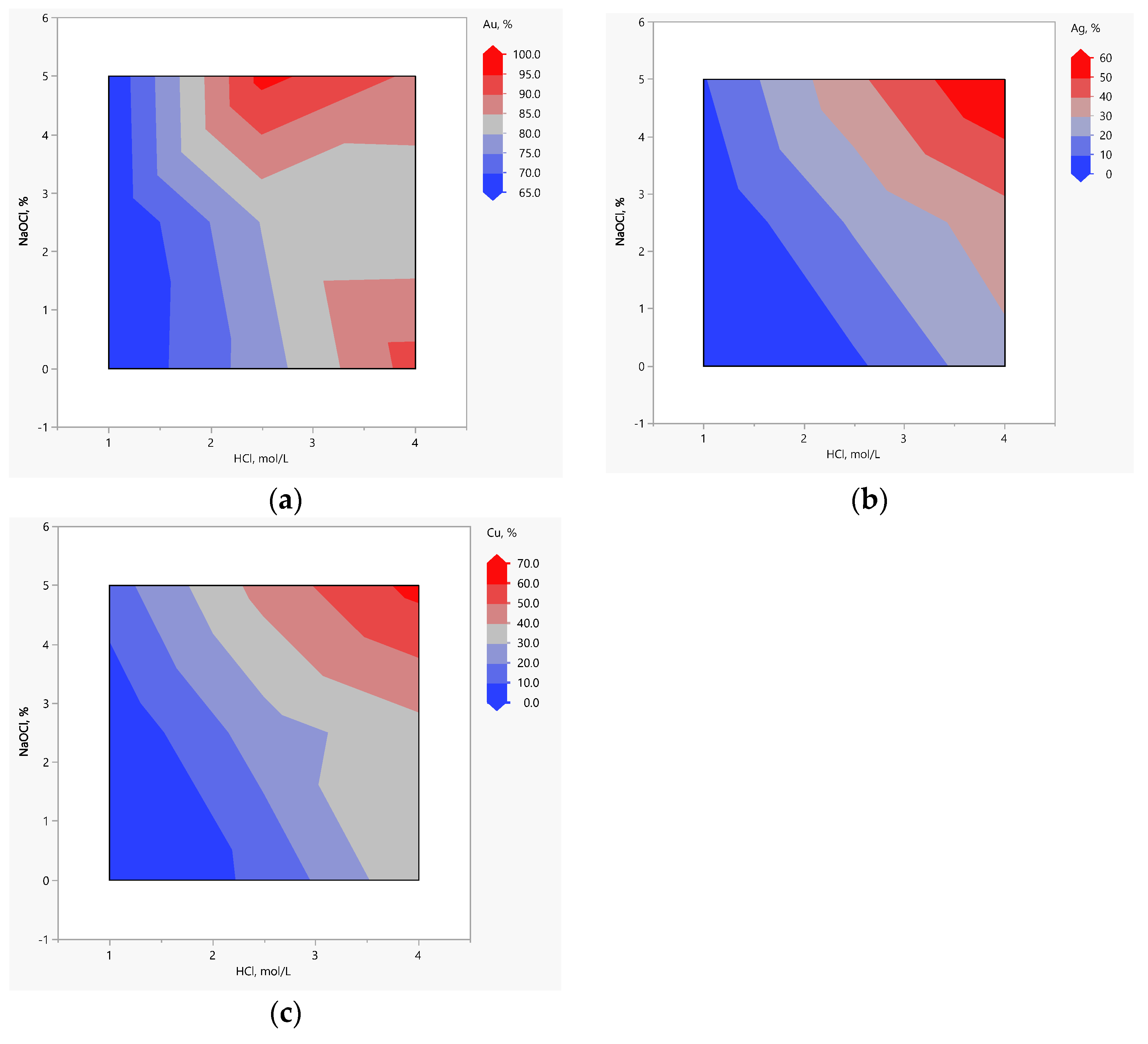
| Test n. | Factors with Actual Levels | Me Recovery, % | Weight Loss % | |||
|---|---|---|---|---|---|---|
| A HCl, mol/L | B NaClO, % | Au | Ag | Cu | ||
| 1 | 1 | 0 | 67.4 | 0.0 | 1.0 | 22.1 |
| 2 | 2.5 | 0 | 77.5 | 8.2 | 12.2 | 25.0 |
| 3 | 4 | 0 | 92.1 | 27.0 | 38.2 | 27.8 |
| 4 | 1 | 2.5 | 64.8 | 1.5 | 1.4 | 22.1 |
| 5 | 2.5 | 2.5 | 80.2 | 21.3 | 25.5 | 25.3 |
| 6 | 4 | 2.5 | 80.5 | 35.3 | 36.3 | 26.3 |
| 7 | 1 | 5 | 65.5 | 9.3 | 15.2 | 22.7 |
| 8 | 2.5 | 5 | 91.6 | 37.8 | 44.0 | 28.1 |
| 9 | 4 | 5 | 90.9 | 60.1 | 61.0 | 28.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zueva, S.; Ippolito, N.M.; Passadoro, M.; Romano, P.; Ferella, F.; Vegliò, F. Selective Recovery of Gold from E-Waste Recycling Plants’ Waste Fractions: Waste-to-Resource Transition. Minerals 2024, 14, 518. https://doi.org/10.3390/min14050518
Zueva S, Ippolito NM, Passadoro M, Romano P, Ferella F, Vegliò F. Selective Recovery of Gold from E-Waste Recycling Plants’ Waste Fractions: Waste-to-Resource Transition. Minerals. 2024; 14(5):518. https://doi.org/10.3390/min14050518
Chicago/Turabian StyleZueva, Svetlana, Nicolò Maria Ippolito, Marco Passadoro, Pietro Romano, Francesco Ferella, and Francesco Vegliò. 2024. "Selective Recovery of Gold from E-Waste Recycling Plants’ Waste Fractions: Waste-to-Resource Transition" Minerals 14, no. 5: 518. https://doi.org/10.3390/min14050518









