Effect of Salt Solution Tracer Dosage on the Transport and Mixing of Tracer in a Water Model of Asymmetrical Gas-Stirred Ladle with a Moderate Gas Flowrate
Abstract
:1. Introduction
2. Method of Experiment
2.1. Principle of Experiment
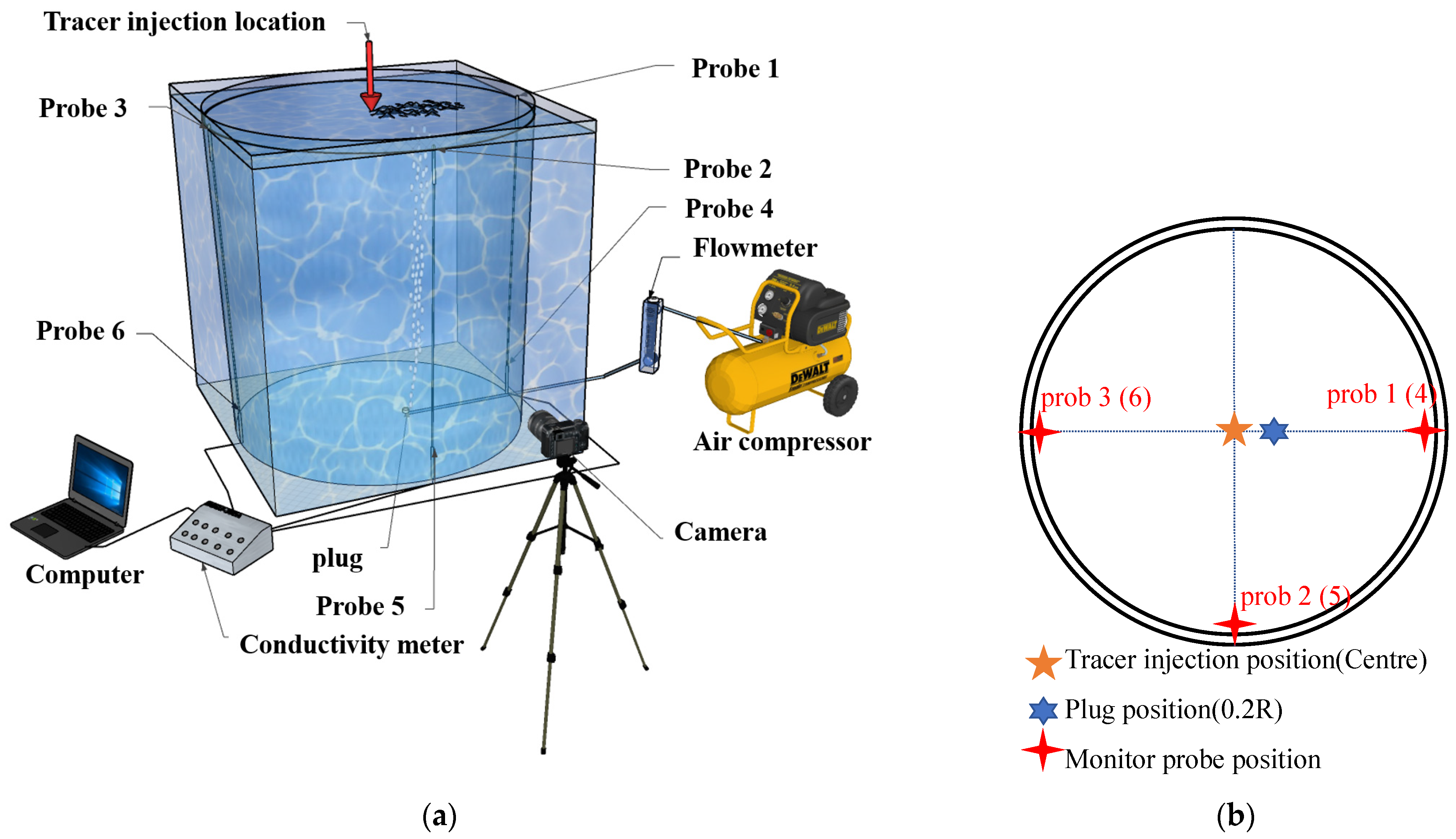
2.2. Experimental Facility
2.3. Experimental Scheme
3. Results
3.1. Experimental Error Analysis
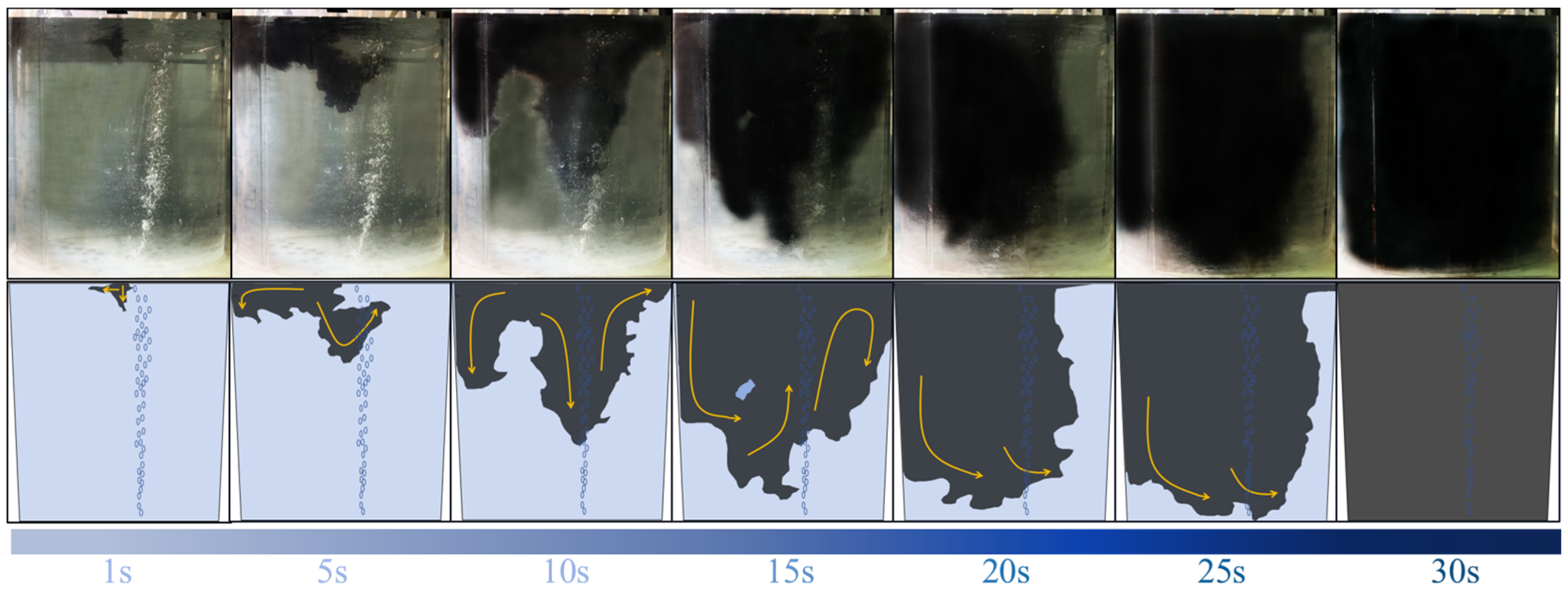
3.2. Transport Path of Tracer
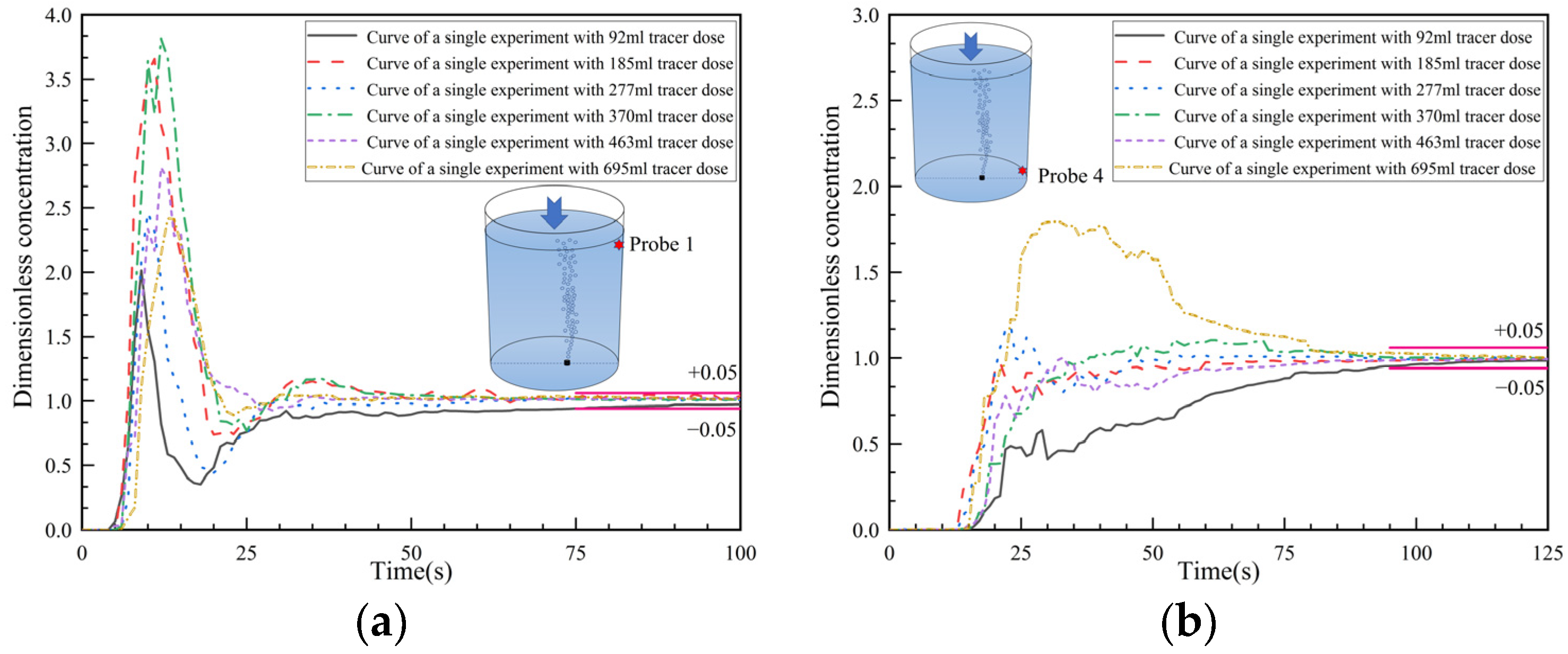
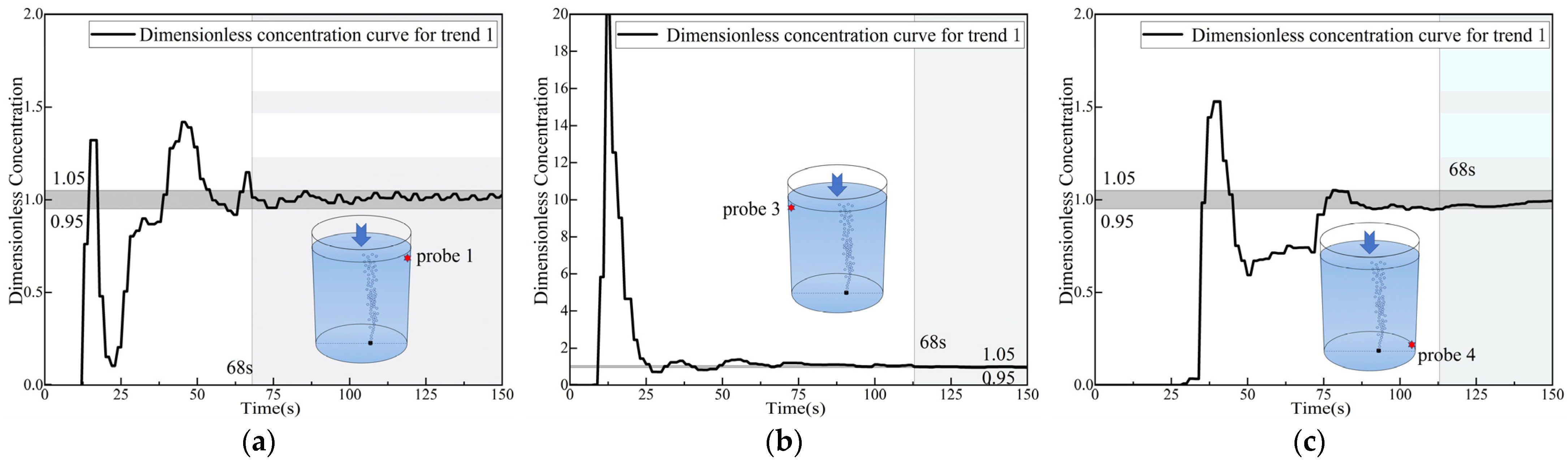
3.3. Analysis of Dimensionless Concentration Curves at Monitoring Points 1 and 4
3.4. Analysis of Dimensionless Concentration Curves at Monitoring Points 2 and 5
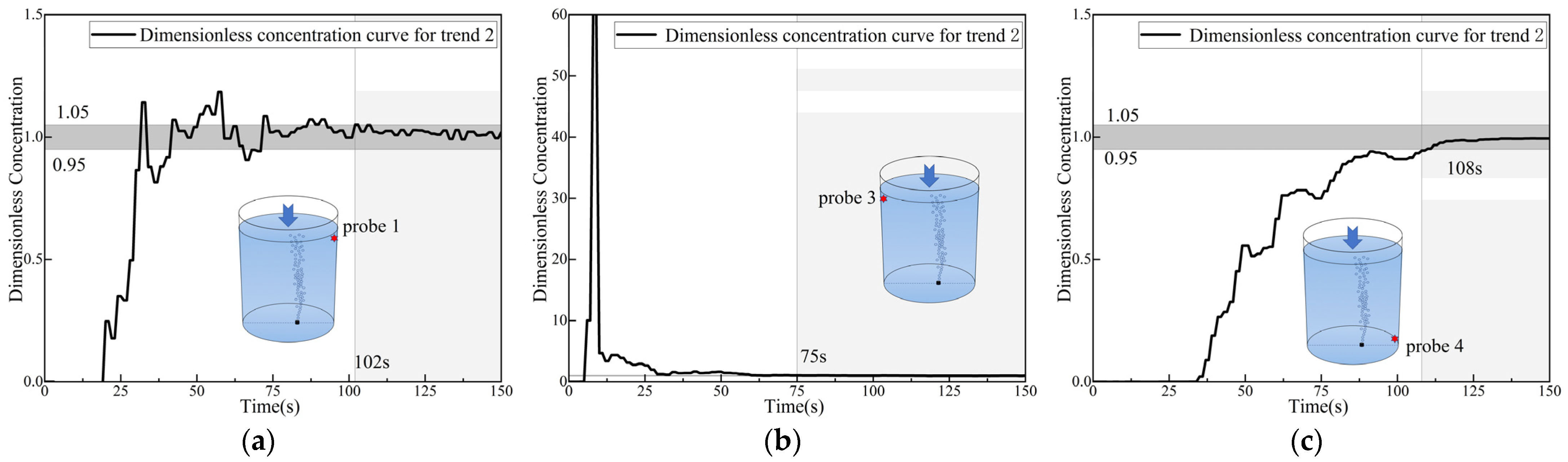
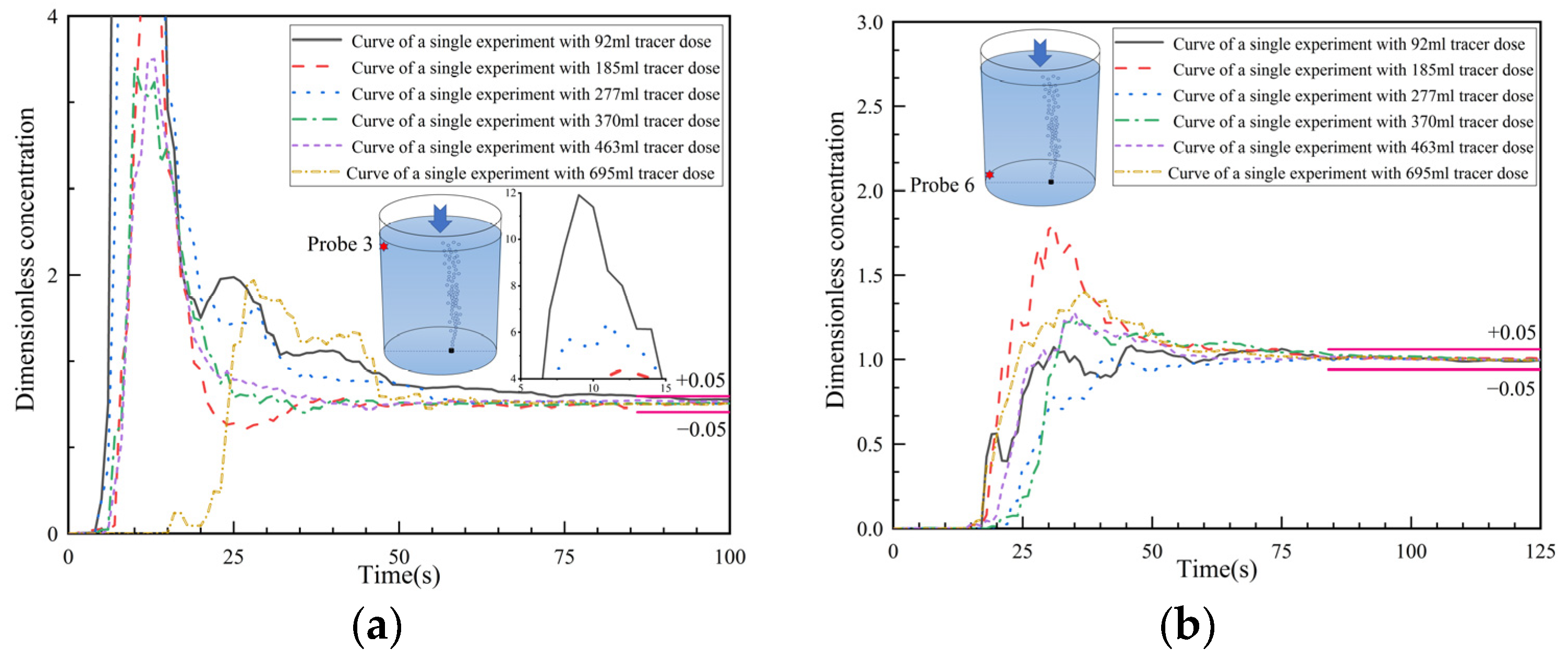
3.5. Analysis of Dimensionless Concentration Curves at Monitoring Points 3 and 6
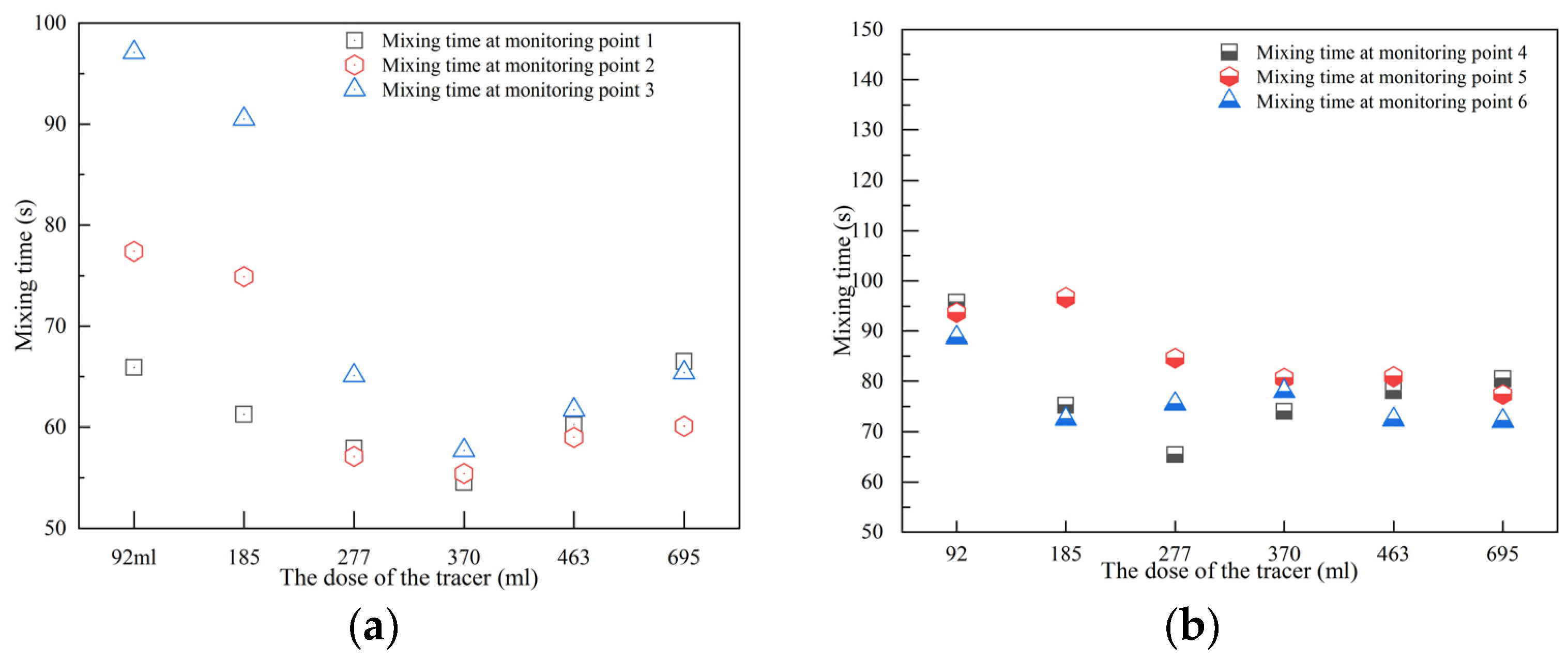
3.6. Mixing Time
4. Discussions
5. Concluding Remarks
- An uncertainty analysis for the experimental data was carried out. The ratio of the standard deviations of the repeated trials to the averaged mixing times ranged within 15%.
- For the dimensionless concentration curves, sinusoidal-type curves, which represent rapid mixing, are observed at the top monitoring points. Meanwhile, parabolic-type curves, which represent slow mixing by diffusion, are observed at the bottom monitoring points. An exception is the monitoring point at the bottom right side (close to the asymmetric gas nozzle area), where two distinct trends, both sinusoidal and parabolic, were observed at this point.
- After a well-designed visualization experiment involving the mixture of salt solution and ink tracers, two trends of asymmetrical transport of the tracer were observed. In the first trend, the injection of the tracer occurred during the oscillation of the gas plume. The injected salt solution tracer was transported both by the main circulation to the left sidewall and by downward flow towards the gas column. The downward flow was accelerated and became a major flow pattern when the tracer volume increased. For the second trend, which can also be observed in the lighter-density ink tracer scheme, the injected salt solution tracer was asymmetrically transported towards the left sidewall of the ladle by the main circulation.
- The response time, peak time, peak concentrations, second/third peaks, and the overall concentration curve types are described in the text. The details of the curves and the ink visualizations can be used to illustrate the transport of salt solution tracers with different dosages/volume. Overall, the curves at the top surface monitoring points are less influenced by the tracer volume, except for the monitoring point at the upper left side. In this area, the portion of the transported salt solution varies in the two trends, i.e., the transported tracer portion is significant in trend 1 but minor in trend 2. The curves at the bottom monitoring points are notably influenced by the tracer volume, since the downward flow intensity varied across different schemes. As a result, the salt concentration is deposited at the bottom with a slow subsequent diffusion.
- For the overall mixing time, a trend of decreasing and then increasing as the tracer volume increases was found at the top monitoring points. The transition tracer volume is 370 mL. Meanwhile, the mixing times at the bottom monitoring points decrease with the increase in tracer volume.
- It is noted that the results are based on moderate gas flow rate conditions. Further studies on the gas flow rate conditions are required, which we have completed and our results will be available soon.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szekely, J.; Carlsson, G.; Helle, L. Ladle Metallurgy; Springer: Berlin/Heidelberg, Germany, 1989; pp. 27–71. [Google Scholar]
- Mazumdar, D.; Guthrie, R.L. The physical and mathematical modelling of gas stirred ladle systems. ISIJ Int. 1995, 35, 1–20. [Google Scholar] [CrossRef]
- Jönsson, P.; Jonsson, L.I. The Use of Fundamental Process Models in Studying Ladle Refining Operations. ISIJ Int. 2001, 41, 1289–1302. [Google Scholar] [CrossRef]
- Mazumdar, D.; Evans, J.W. Macroscopic Models for Gas Stirred Ladles. ISIJ Int. 2004, 44, 447–461. [Google Scholar] [CrossRef]
- Xin, Z.; Zhang, J.; Peng, K.; Zhang, J.; Zhang, C.; Liu, Q. Modeling of LF refining process: A review. J. Iron Steel Res. Int. 2024, 31, 289–317. [Google Scholar] [CrossRef]
- Niu, K.; Feng, W.; Conejo, A.N.; Ramírez-Argáez, M.A.; Yan, H. 3D CFD Model of Ladle Heat Transfer with Gas Injection. Metall. Mater. Trans. B 2023, 54, 2066–2079. [Google Scholar] [CrossRef]
- Conejo, A.N. Physical and Mathematical Modelling of Mass Transfer in Ladles due to Bottom Gas Stirring: A Review. Processes 2020, 8, 750. [Google Scholar] [CrossRef]
- Niu, K.; Feng, W.; Conejo, A.N. Effect of the Nozzle Radial Position and Gas Flow Rate on Mass Transfer during Bottom Gas Injection in Ladles with One Nozzle. Metall. Mater. Trans. B 2022, 53, 1344–1350. [Google Scholar] [CrossRef]
- Ji, S.; Niu, K.; Conejo, A.N. Multiphase modeling of steel-slag mass transfer through distorted interface in bottom-stirred ladle. ISIJ Int. 2024, 64, 52–58. [Google Scholar] [CrossRef]
- Podder, A.; Coley, K.S.; Phillion, A.B. Modeling Study of Steel–Slag–Inclusion Reactions During the Refining of Si–Mn Killed Steel. Steel Res. Int. 2022, 94, 2100831. [Google Scholar] [CrossRef]
- Podder, A.; Coley, K.S.; Phillion, A.B. Simulation of Ladle Refining Reactions in Si–Mn-Killed Steel. Steel Res. Int. 2024. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/srin.202300330 (accessed on 26 March 2024). [CrossRef]
- Duan, H.; Zhang, L.; Thomas, B.; Conejo, A.N. Fluid Flow, Dissolution, and Mixing Phenomena in Argon-Stirred Steel Ladles. Metall. Mater. Trans. B 2018, 49, 2722–2743. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, L.; Thomas, B.G. Effect of melt superheat and alloy size on the mixing phenomena in argon-stirred steel ladles. Steel Res. Int. 2019, 90, 1800288. [Google Scholar] [CrossRef]
- Liu, C.; Peng, K.Y.; Wang, Q.; Li, G.Q.; Wang, J.J.; Zhang, L.F. CFD Investigation of Melting Behaviors of Two Alloy Particles During Multiphase Vacuum Refining Process. Metall. Mater. Trans. B 2023, 54, 2174–2187. [Google Scholar] [CrossRef]
- Yang, R.; Chen, C.; Lin, Y.; Zhao, Y.; Zhao, J.; Zhu, J.; Yang, S. Water model experiment on motion and melting of scarp in gas stirred reactors. Chin. J. Process Eng. 2022, 22, 954–962. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Tian, J.; Wang, D.; Qu, T.; Hou, D.; Hu, S.; Wu, G. Investigation on the Alloy Mixing and Inclusion Removement through Using a New Slot-Porous Matched Tuyeres. Metals 2023, 13, 667. [Google Scholar] [CrossRef]
- Wen, X.; Ren, Y.; Zhang, L. Effect of CaF2 Contents in Slag on Inclusion Absorption in a Bearing Steel. Steel Res. Int. 2022, 94, 2200218. [Google Scholar] [CrossRef]
- Kim, T.S.; Yang, J.; Park, J.H. Effect of Physicochemical Properties of Slag on the Removal Rate of Alumina Inclusions in the Ruhrstahl–Heraeus (RH) Refining Conditions. Metall. Mater. Trans. B 2022, 53, 2523–2533. [Google Scholar]
- Li, X.; Hu, S.; Wang, D.; Qu, T.; Wu, G.; Zhang, P.; Quan, Q.; Zhou, X.; Zhang, Z. Inclusion Removements in a Bottom-Stirring Ladle with Novel Slot-Porous Matched Dual Plugs. Metals 2022, 12, 162. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, H.; Zhang, L. A boundary layer model for capture of inclusions by steel–slag interface in a turbulent flow. J. Iron Steel Res. Int. 2023, 30, 1101–1108. [Google Scholar] [CrossRef]
- Huang, C.; Duan, H.; Zhang, L. Modeling of Motion of Inclusions in Argon-Stirred Steel Ladles. Steel Res. Int. 2024, 96, 2300537. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/srin.202300537 (accessed on 26 March 2024). [CrossRef]
- Qiao, T.; Cheng, G.; Huang, Y.; Li, Y.; Zhang, Y.; Li, Z. Formation and Removal Mechanism of Nonmetallic Inclusions in 42CrMo4 Steel during the Steelmaking Process. Metals 2022, 12, 1505. [Google Scholar] [CrossRef]
- Zhong, H.; Jiang, M.; Wang, Z.; Zhen, X.; Zhao, H.; Li, T.; Wang, X. Formation and Evolution of Inclusions in AH36 Steel During LF–RH–CC Process: The Influences of Ca-Treatment, Reoxidation, and Solidification. Metall. Mater. Trans. B 2023, 54, 593–601. [Google Scholar] [CrossRef]
- Ocampo Vaca, F.A.; Hernández Bocanegra, C.A.; Ramos Banderas, J.Á.; Herrera-Ortega, M.; López Granados, N.M.; Solorio Díaz, G. Effect of Ladle Shroud Blockage on Flow Dynamics and Cleanliness of Steel in Coupled Ladle–Shroud–Tundish System. Steel Res. Int. 2024. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/srin.202300616 (accessed on 13 March 2024). [CrossRef]
- Conejo, A.N.; Kitamura, S.; Maruoka, N.; Kim, S.J. Effects of Top Layer, Nozzle Arrangement, and Gas Flow Rate on Mixing Time in Agitated Ladles by Bottom Gas Injection. Metall. Mater. Trans. B 2013, 44, 914–923. [Google Scholar] [CrossRef]
- Terrazas, M.S.C.; Conejo, A.N. Effect of Nozzle Diameter on Mixing Time During Bottom-Gas Injection in Metallurgical Ladles. Metall. Mater. Trans. B 2014, 45, 711–718. [Google Scholar] [CrossRef]
- Feng, W.; Conejo, A.N. Physical and Numerical Simulation of the Optimum Nozzle Radial Position in Ladles with One Nozzle and Bottom Gas Injection. ISIJ Int. 2022, 62, 1211–1221. [Google Scholar] [CrossRef]
- Conejo, A.N.; Feng, W. Ladle Eye Formation Due to Bottom Gas Injection: A Reassessment of Experimental Data. Metall. Mater. Trans. B 2022, 53, 999–1017. [Google Scholar] [CrossRef]
- Wondrak, T.; Timmel, K.; Bruch, C.; Gardin, P.; Hackl, G.; Lachmund, H.; Lüngen, H.B.; Odenthal, H.J.; Eckert, S. Large-Scale Test Facility for Modeling Bubble Behavior and Liquid Metal Two-Phase Flows in a Steel Ladle. Metall. Mater. Trans. B 2022, 53, 1703–1720. [Google Scholar] [CrossRef]
- Wang, R.; Jin, Y.; Cui, H. The Flow Behavior of Molten Steel in an RH Degasser Under Different Ladle Bottom Stirring Processes. Metall. Mater. Trans. B 2022, 53, 342–351. [Google Scholar] [CrossRef]
- Jojo-Cunningham, Y.; Guo, X.; Zhou, C.; Liu, Y. Volumetric Flow Field inside a Gas Stirred Cylindrical Water Tank. Fluids 2024, 9, 11. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G.G.; Yang, H.K.; Hou, Z.B. Physical Modeling of Fluid Flow Characteristics in a Delta Shaped, Four-Strand Continuous Casting Tundish with Different Flow Control Devices. Adv. Mater. Res. 2011, 284, 1071–1079. [Google Scholar]
- Schwarz, M.P.; Koh, P.T.L. Numerical Modelling of Bath Mixing by Swirled Gas Injection. In Proceedings of the SCANINJECT IV-4th International Conference on Injection Metallurgy, MEFOS, Luleå, Sweden, 11–13 July 1986. Part I, paper 6. [Google Scholar]
- Schwarz, M.P.; Turner, W.J. Applicability of the standard k-ϵ turbulence model to gas-stirred baths. Appl. Math. Model. 1988, 12, 273–279. [Google Scholar] [CrossRef]
- Nick, R.S.; Teng, L.; Yang, H.; Tilliander, A.; Glaser, B.; Sheng, D.Y.; Jönsson, P.G.; Björkvall, J. Mathematical modelling of novel combined stirring method during the final stage of ladle refining. Ironmak. Steelmak. 2023, 50, 721–733. [Google Scholar] [CrossRef]
- Wang, J.; Ni, P.; Chen, C.; Ersson, M.; Li, Y. Effect of gas blowing nozzle angle on multiphase flow and mass transfer during RH refining process. Int. J. Miner. Metall. Mater. 2023, 30, 844–856. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Tian, J.; Wang, H.; Qu, T.; Hou, D.; Hu, S.; Zhang, Z.; Zhou, X.; Wu, G. Modeling of Bubble Transportation, Expansion, as Well as Adhesion of Inclusions in a Ladle With Different Tuyeres. Metall. Mater. Trans. B 2024, 55, 14–31. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; He, Q.; Ni, P.; Yue, Q.; Ersson, M. Novel Evaluation Method to Determine the Mixing Time in a Ladle Refining Process. Metall. Mater. Trans. B 2022, 53, 4114–4123. [Google Scholar] [CrossRef]
- Hua, C.; Bao, Y.; Wang, M. Multiphysics numerical simulation model and hydraulic model experiments in the argon-stirred ladle. Processes 2022, 10, 1563. [Google Scholar] [CrossRef]
- Tiwari, R.; Girard, B.; Labrecque, C.; Isac, M.M.; Guthrie, R.I.L. CFD Predictions for Mixing Times in an Elliptical Ladle Using Single- and Dual-Plug Configurations. Processes 2023, 11, 1665. [Google Scholar] [CrossRef]
- Schwarz, M.P. Buoyancy and expansion power in gas-agitated baths. ISIJ Int. 1991, 31, 947–951. [Google Scholar] [CrossRef]
- Schwarz, M.P.; Dang, P. Simulation of Blowthrough in Smelting Baths with Bottom Gas Injection. In Proceedings of the 13th Process Technology Conference Proceedings, ISS, Nashville, TN, USA, 2–5 April 1995; pp. 415–421. [Google Scholar]
- Ramírez-López, A. Analysis of Mixing Efficiency in a Stirred Reactor Using Computational Fluid Dynamics. Symmetry 2024, 16, 237. [Google Scholar] [CrossRef]
- Ramírez-López, A.A. Analysis of the Hydrodynamics Behavior Inside a Stirred Reactor for Lead Recycling. Fluids 2023, 8, 268. [Google Scholar] [CrossRef]
- Jauhiainen, A.; Jonsson, L.; Sheng, D. Modelling of alloy mixing into steel: The influence of porous plug placement in the ladle bottom on the mixing of alloys into steel in a gas-stirred ladle. A comparison made by numerical simulation. Scand. J. Metall. 2001, 30, 242–253. [Google Scholar] [CrossRef]
- Ganguly, S.; Chakraborty, S. Numerical modelling studies of flow and mixing phenomena in gas stirred steel ladles. Ironmak. Steelmak. 2008, 35, 524–530. [Google Scholar] [CrossRef]
- Taylor, I.F.; Dang, P.; Schwarz, M.P.; Wright, J.K. An Improved Method for the Experimental Validation of Numerical Mixing Time Predictions. In Proceedings of the 72nd Steelmaking Proceedings, ISS, Chicago, IL, USA, 2–5 April 1989; pp. 505–516. [Google Scholar]
- Pan, S.; Chiang, J.D.; Hwang, W.S. Effects of gas injection condition on mixing efficiency in the ladle refining process. J. Mater. Eng. Perform 1997, 6, 113–117. [Google Scholar] [CrossRef]
- Wen, D.; Li, J.; Xie, C.; Tang, H.; Zhang, L.; Wang, Z. Physical model of 150 t ladle refining process. J. Univ. Sci. Technol. Beijing 2007, 29, 101–104. [Google Scholar] [CrossRef]
- Ek, M.; Wu, L.; Valentin, P.; Sichen, D. Effect of Inert Gas Flow Rate on Homogenization and Inclusion Removal in a Gas Stirred Ladle. ISIJ Int. 2010, 81, 1056–1063. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, H.; Liu, H.; Ersson, M.; Jönsson, P.G.; Gan, Y. Physical and Numerical Modelling on the Mixing Condition in a 50 t Ladle. Metals 2019, 9, 1136. [Google Scholar] [CrossRef]
- Tan, F.; He, Z.; Jin, S.; Pan, L.; Li, Y.; Li, B. Physical Modeling Evaluation on Refining Effects of Ladle with Different Purging Plug Designs. Steel Res. Int. 2020, 91, 1900606. [Google Scholar] [CrossRef]
- Aguilar, G.; Solorio-Diaz, G.; Aguilar-Corona, A.; Ramos-Banderas, J.A.; Hernández, C.A.; Saldaña, F. Study of the Effect of a Plug with Torsion Channels on the Mixing Time in a Continuous Casting Ladle Water Model. Metals 2021, 11, 1942. [Google Scholar] [CrossRef]
- Conejo, A.N.; Zhao, G.; Zhang, L. On the Limits of the Geometric Scale Ratio Using Water Modeling in Ladles. Metall. Mater. Trans. B 2021, 52, 2263–2274. [Google Scholar] [CrossRef]
- Shi, P.; Tian, Y.; Xu, L.; Guo, W.; Qiu, S. Physical simulation of 120 t ladle bottom blown argon gas. China Metall. 2021, 31, 30–36. [Google Scholar] [CrossRef]
- Herrera-Ortega, M.; Ramos-Banderas, J.Á.; Hernández-Bocanegra, C.A.; Montes-Rodríguez, J.J. Effect of the location of tracer addition in a ladle on the mixing time through physical and numerical modeling. ISIJ Int. 2021, 61, 2185–2192. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, L.; Yin, Y.; Zhang, J. Effect of side blowing on fluid flow and mixing phenomenon in gas-stirred ladle. Metals 2021, 11, 369. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, S.; Zhu, M. Optimization of the Structure and Injection Position of Top Submerged Lance in Hot Metal Ladle. ISIJ Int. 2021, 61, 792–801. [Google Scholar] [CrossRef]
- Wu, W.; Dong, J.; Wei, G. Physical Simulation of Bottom Blowing Argon in 150t Ladle. Contin. Cast. 2022, 2, 45–48. [Google Scholar] [CrossRef]
- Li, Z.; Ouyang, W.; Wang, Z.; Zheng, R.; Bao, Y.; Gu, C. Physical Simulation Study on Flow Field Characteristics of Molten Steel in 70t Ladle Bottom Argon Blowing Process. Metals 2023, 13, 639. [Google Scholar] [CrossRef]
- Shan, Q.; Sun, Y.; Duan, H.; Li, Z.; Jia, N.; Chen, W. Water modeling on the transport phenomenon during bottom gas injection of a 210 t ladle. Steelmaking 2023, 39, 35–43. [Google Scholar]
- Li, L.; Chen, C.; Wang, J.; Tao, X.; Liu, T.; Zhao, Y.; Rong, Z. Flow field optimization and analysis on inclusion removal in elliptical ladle. China Metall. 2024. Available online: https://link.cnki.net/urlid/11.3729.TF.20240407.1139.001 (accessed on 12 April 2024). [CrossRef]
- Chen, C.; Cheng, G.; Sun, H.; Hou, Z.; Wang, X.; Zhang, J. Effects of Salt Tracer Amount, Concentration and Kind on the Fluid Flow Behavior in a Hydrodynamic Model of Continuous Casting Tundish. Steel Res. Int. 2012, 83, 1141–1151. [Google Scholar] [CrossRef]
- Chen, C.; Jonsson, L.I.; Tilliander, A.; Cheng, G.; Jönsson, P. A Mathematical Modeling Study of Tracer Mixing in a Continuous Casting Tundish. Metall. Mater. Trans. B 2015, 46, 169–190. [Google Scholar] [CrossRef]
- Chen, C.; Jonsson, L.I.; Tilliander, A.; Cheng, G.; Jönsson, P. A mathematical modeling study of the influence of small amounts of KCl solution tracers on mixing in water and residence time distribution of tracers in a continuous flow reactor-metallurgical tundishc. Chem. Eng. Sci. 2015, 137, 914–937. [Google Scholar] [CrossRef]
- Ding, C.; Lei, H.; Niu, H.; Zhang, H.; Yang, B.; Li, Q. Effects of Tracer Solute Buoyancy on Flow Behavior in a Single-Strand Tundish. Metall. Mater. Trans. B 2021, 52, 3788–3804. [Google Scholar] [CrossRef]
- Ding, C.; Lei, H.; Niu, H.; Zhang, H.; Yang, B.; Zhao, Y. Effects of Salt Tracer Volume and Concentration on Residence Time Distribution Curves for Characterization of Liquid Steel Behavior in Metallurgical Tundish. Metals 2021, 11, 430. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Lin, W.; Yu, Y.; Dianyu, E.; Wang, S. Numerical Simulation of Tracers Transport Process in Water Model of a Vacuum Refining Unit: Single Snorkel Refining Furnace. Steel Res. Int. 2020, 91, 202000022. [Google Scholar] [CrossRef]
- Ouyang, X.; Lin, W.; Luo, Y.; Zhang, Y.; Fan, J.; Chen, C.; Cheng, G. Effect of Salt Tracer Dosages on the Mixing Process in the Water Model of a Single Snorkel Refining Furnace. Metals 2022, 12, 1948. [Google Scholar] [CrossRef]
- Xu, Z.; Ouyang, X.; Chen, C.; Li, Y.; Wang, T.; Ren, R.; Yang, M.; Zhao, Y.; Xue, L.; Wang, J. Numerical Simulation of the Density Effect on the Macroscopic Transport Process of Tracer in the Ruhrstahl–Heraeus (RH) Vacuum Degasser. Sustainability 2024, 16, 3923. [Google Scholar] [CrossRef]
- Chen, C.; Rui, Q.; Cheng, G. Effect of Salt Tracer Amount on the Mixing Time Measurement in a Hydrodynamic Model of Gas-Stirred Ladle System. Steel Res. Int. 2013, 84, 900–907. [Google Scholar] [CrossRef]
- Gómez, A.S.; Conejo, A.N.; Zenit, V.R. Effect of Separation Angle and Nozzle Radial Position on Mixing Time in Ladles with Two Nozzles. J. Appl. Fluid Mec. 2018, 11, 11–20. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Zhang, Y.; Wang, S. Numerical Simulation of Tracer Transport Process in Water Model of Gas-stirred Ladle. J. Taiyuan Univ. Technol. 2020, 51, 50–58. [Google Scholar] [CrossRef]
- Krishnapisharody, K.; Irons, G.A. A critical review of the modified Froude number in Ladle Metallurgy. Metall. Mater. Trans. B 2013, 44, 1486–1498. [Google Scholar] [CrossRef]
- Zhan, Z.; Qiu, S.; Yin, S. Study on Water model for 135t LF ladle bottom blowing argon process. Hot Work. Technol. 2017, 46, 98–101. [Google Scholar] [CrossRef]
- Oeters, F. Metallurgy of Steelmaking; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1994. [Google Scholar]
- Mietz, J.; Oeters, F. Mixing Theories for Gas-Stirred Melts. Steel Res. 1987, 58, 446–453. [Google Scholar] [CrossRef]
- Mietz, J.; Oeters, F. Model Experiments on Mixing Phenomena in Gas-stirred Melts. Steel Res. 1988, 59, 52–59. [Google Scholar] [CrossRef]
- Mietz, J.; Oeters, F. Flow Field and Mixing with Eccentric Gas Stirring. Steel Res. 1989, 60, 387–394. [Google Scholar] [CrossRef]
- Mietz, J.; Oeters, F. Model Studies of Mixing Phenomena in Stirred Melts. Can. Metall. Quart. 1989, 28, 19–27. [Google Scholar] [CrossRef]
- Becker, J.; Oeters, F. Model Experiments of Mixing in Steel Ladles with Continuous Addition of The Substance to be Mixed. Steel Res. 1998, 69, 8–16. [Google Scholar] [CrossRef]
- Krishna-Murthy, G.G.; Mehrotra, S.P.; Ghosh, A. Experimental investigation of mixing phenomena in a gas stirred liquid bath. Metall. Trans. B 1988, 19, 839–850. [Google Scholar] [CrossRef]
- Oymo, D.; Guthrie, R.I.L. Mixing Times in Combination Blowing Processes. In Proceedings of the 4th Process Technology Conference Proceedings, ISS, Chicago, IL, USA, 3–4 April 1984; pp. 45–52. [Google Scholar]






| Researchers | Year | Tracer | Water Volume [L] | Tracer/Water | ||
|---|---|---|---|---|---|---|
| Kind | Concentration | Amount [mL] | ||||
| Pan et al. [48] | 1997 | NaCl | - | 15 | 395.9 | 0.038 × 10−3 |
| Wen et al. [49] | 2007 | KCl | Saturated | 200 | 360.3 | 0.56 × 10−3 |
| Ek et al. [50] | 2010 | NaCl | - | 400 | 90.47 | 4.42 × 10−3 |
| Liu et al. [51] | 2019 | NaCl | Saturated | 50 | 46 | 1.09 × 10−3 |
| Tan et al. [52] | 2020 | NaCl | Saturated | 150 | 156 | 0.96 × 10−3 |
| Aguilar et al. [53] | 2021 | KCl | 3.35 mol/L | 20 | 49.3 | 0.41 × 10−3 |
| Conejo et al. [54] | 2021 | KCl | Saturated | 100/20/10 | 333/66.7/33.3 | 0.3× 10−3 |
| Shi et al. [55] | 2021 | NaCl | Saturated | 500 | 519 | 0.96 × 10−3 |
| Ortega et al. [56] | 2021 | KCl | Saturated | 35 | 85.4 | 0.41 × 10−3 |
| Cheng et al. [57] | 2021 | KCl | Saturated | 500 | 428.7 | 1.17 × 10−3 |
| Wang et al. [58] | 2021 | NaCl | Saturated | 24 | 217 | 0.11 × 10−3 |
| Wu et al. [59] | 2022 | KCl | Saturated | 100 | 374 | 0.267 × 10−3 |
| Zhou et al. [38] | 2022 | NaCl | Saturated | 200 | 930 | 0.215 × 10−3 |
| Li et al. [60] | 2023 | KCl | Saturated | 200 | 384 | 0.52 × 10−3 |
| Shan et al. [61] | 2023 | KCl | Saturated | 100 | 366 | 0.27 × 10−3 |
| Li et al. [62] | 2024 | NaCl | Saturated | 20 | 90.8 | 0.22 × 10−3 |
| Parameters | Industrial Prototype | Water Model |
|---|---|---|
| Inner diameter of ladle top (mm) | 2925 | 975 |
| Inner diameter of ladle bottom (mm) | 2690 | 897 |
| Ladle height (mm) | 3150 | 1050 |
| Liquid level height (mm) | 3000 | 1000 |
| Number of nozzles | 1 | 1 |
| Bottom injection gas flow rate (L·min−1) | 730 | 8.3 |
| Nozzle radial position (r/R) | 0.2 | 0.2 |
| Dosage of Saturated NaCl Solution (mL) | 92 | 185 | 277 | 370 | 463 | 695 |
|---|---|---|---|---|---|---|
| Volume ratio of saturated NaCl solution to water | 0.13 × 10−3 | 0.26 × 10−3 | 0.39 × 10−3 | 0.52 × 10−3 | 0.65 × 10−3 | 0.97 × 10−3 |
| Order | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | AVG | S.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time(s) | ||||||||||||
| Monitor | ||||||||||||
| 1 | 44 | 50 | 65 | 45 | 76 | 73 | 62 | 47 | 58 | 76 | 59.60 | 12.75 |
| 2 | 65 | 72 | 44 | 56 | 44 | 73 | 68 | 54 | 54 | 50 | 58.00 | 10.86 |
| 3 | 103 | 87 | 85 | 102 | 93 | 82 | 91 | 72 | 87 | 87 | 88.90 | 9.13 |
| 4 | 84 | 108 | 116 | 89 | 92 | 89 | 102 | 92 | 92 | 93 | 95.70 | 9.88 |
| 5 | 103 | 80 | 113 | 86 | 85 | 109 | 107 | 92 | 75 | 116 | 96.60 | 14.75 |
| 6 | 87 | 87 | 105 | 98 | 68 | 110 | 72 | 86 | 82 | 66 | 86.10 | 14.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, C.; Tao, X.; Qi, H.; Liu, T.; Yan, Q.; Deng, F.; Allayev, A.; Lin, W.; Wang, J. Effect of Salt Solution Tracer Dosage on the Transport and Mixing of Tracer in a Water Model of Asymmetrical Gas-Stirred Ladle with a Moderate Gas Flowrate. Symmetry 2024, 16, 619. https://doi.org/10.3390/sym16050619
Li L, Chen C, Tao X, Qi H, Liu T, Yan Q, Deng F, Allayev A, Lin W, Wang J. Effect of Salt Solution Tracer Dosage on the Transport and Mixing of Tracer in a Water Model of Asymmetrical Gas-Stirred Ladle with a Moderate Gas Flowrate. Symmetry. 2024; 16(5):619. https://doi.org/10.3390/sym16050619
Chicago/Turabian StyleLi, Linbo, Chao Chen, Xin Tao, Hongyu Qi, Tao Liu, Qiji Yan, Feng Deng, Arslan Allayev, Wanming Lin, and Jia Wang. 2024. "Effect of Salt Solution Tracer Dosage on the Transport and Mixing of Tracer in a Water Model of Asymmetrical Gas-Stirred Ladle with a Moderate Gas Flowrate" Symmetry 16, no. 5: 619. https://doi.org/10.3390/sym16050619







