DNA Microarray and Bioinformatic Analysis Reveals the Potential of Whale Oil in Enhancing Hair Growth in a C57BL/6 Mice Dorsal Skin Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Whale Oil (WO)
2.2. MTT Assay
2.3. Confirmation of Gene Expression Involved in Hair Growth
2.4. Evaluation of Hair Growth Using the Mouse Dorsal Skin Model
2.5. Histological Observations
2.6. Whole-Genome DNA Microarray Analysis
3. Results
3.1. Proliferative Effects of HFDPC by MTT Assay
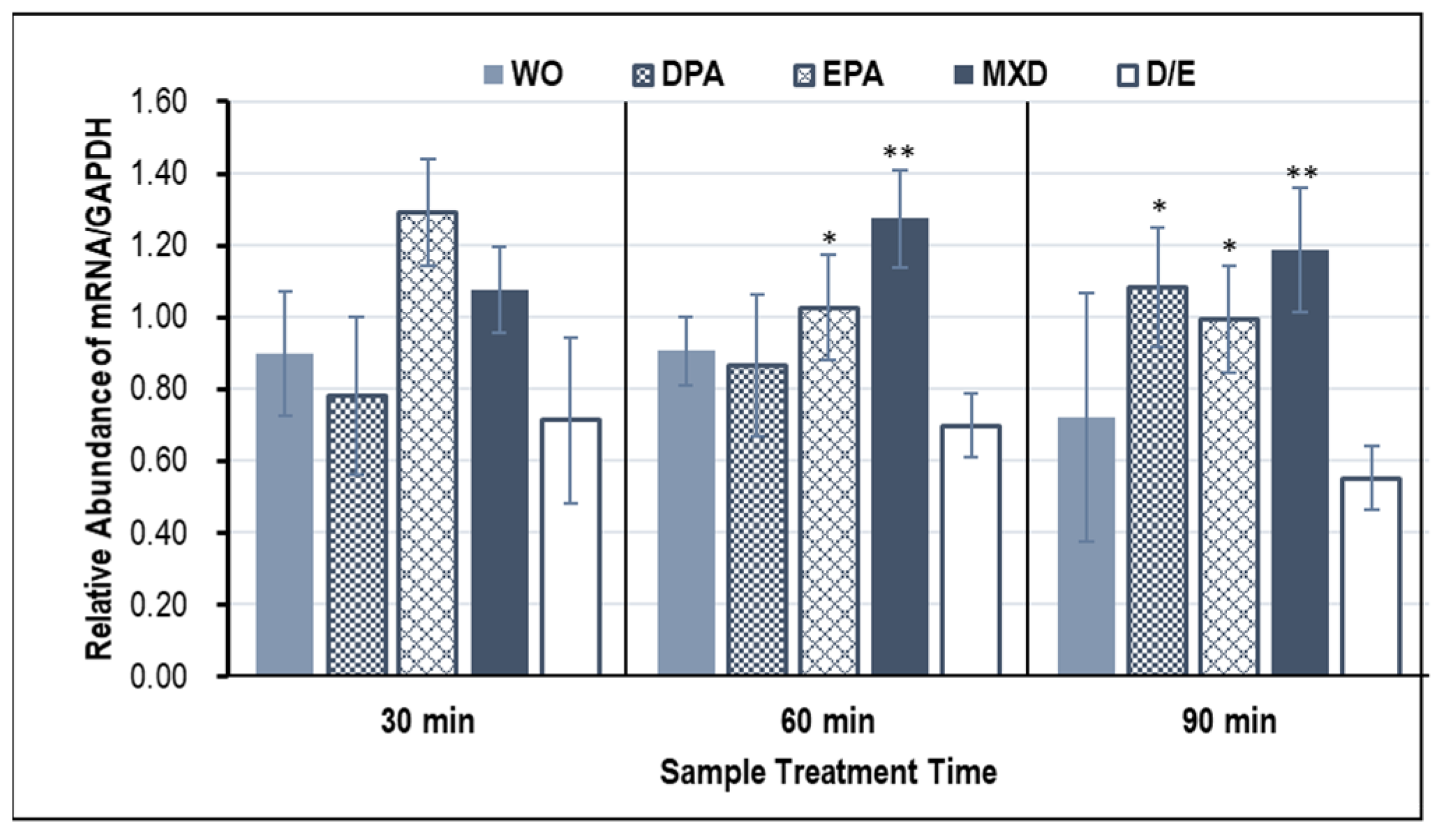
3.2. Hair Growth Marker Gene Expression Results
3.3. Progression of Hair Growth
3.4. Optical Microscopic Observation with H&E Images
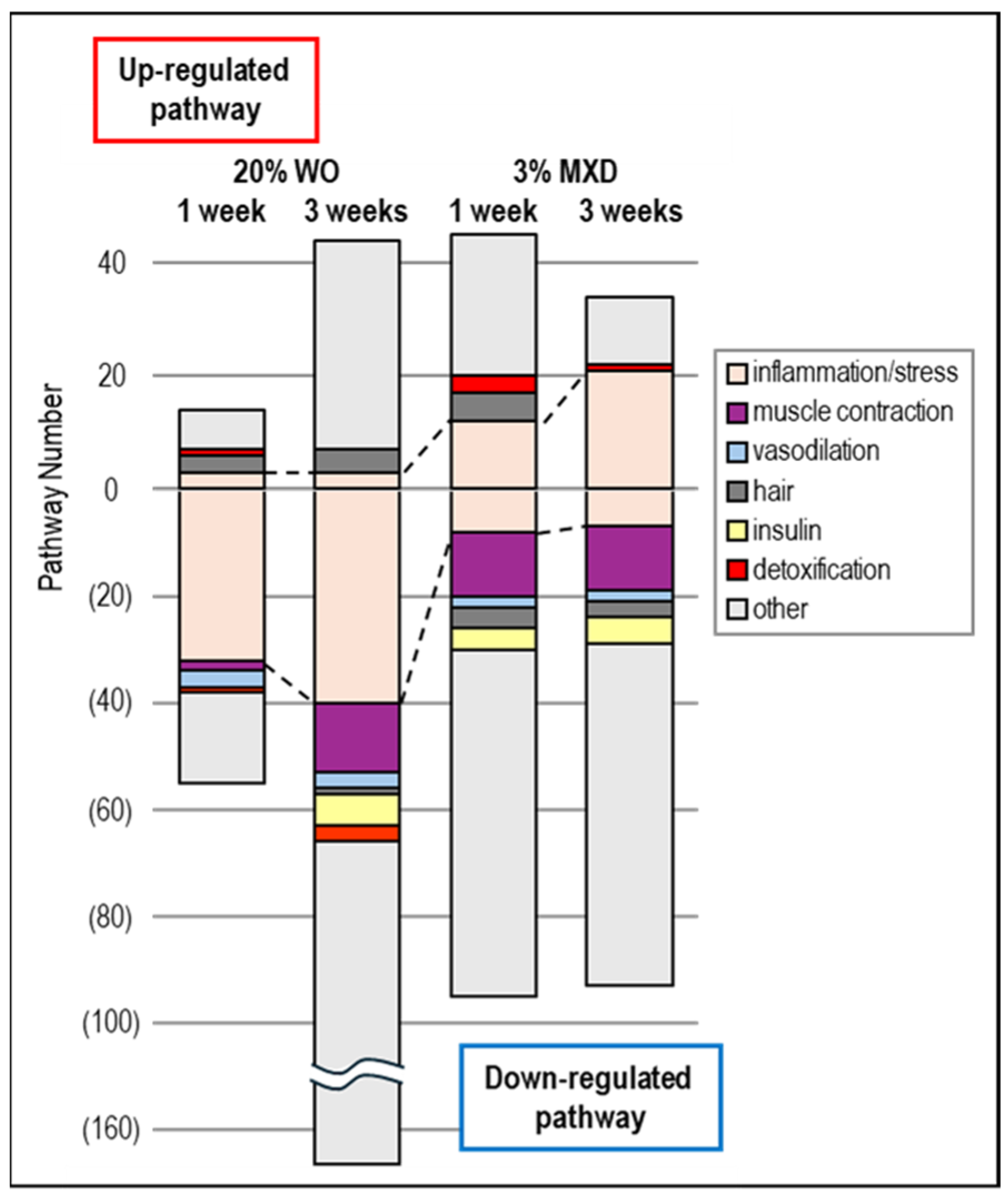
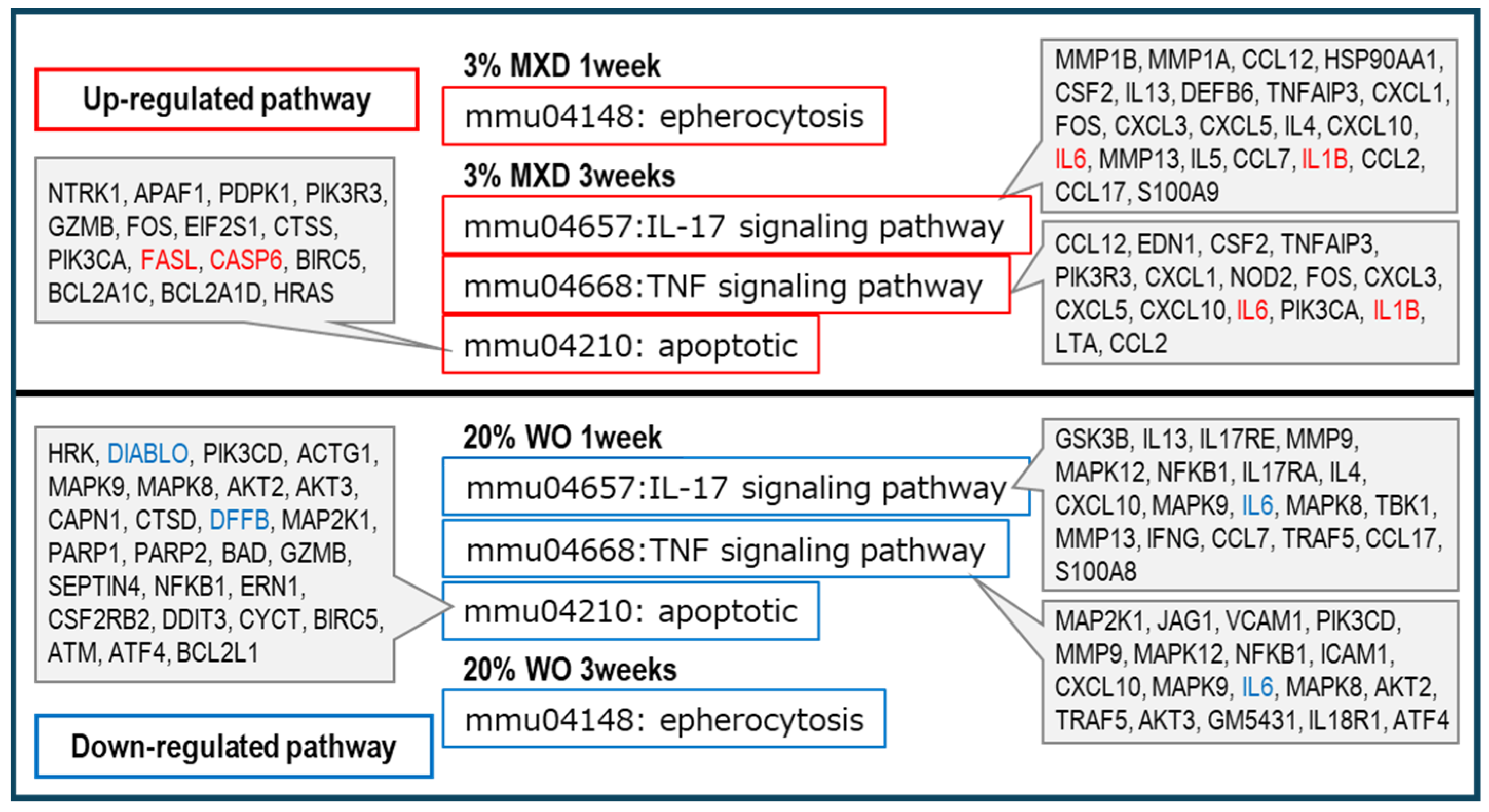
3.5. Transcriptomic Analysis Results
3.5.1. Whale Oil (WO) at 1 Week
3.5.2. Whale Oil (WO) at 3 Weeks
3.5.3. Minoxidil at 1 Week
3.5.4. Minoxidil at 3 Weeks
3.6. Biological Functional Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meisheri, K.D.; Cipkus, L.A.; Taylor, C.J. Mechanism of action of minoxidil sulfate-induced vasodilation: A role for increased K+ permeability. J. Pharmacol. Exp. Ther. 1988, 245, 751–760. [Google Scholar]
- Buhl, A.E.; Waldon, D.J.; Conrad, S.J.; Mulholland, M.J.; Shull, K.L.; Kubicek, M.F.; Johnson, G.A.; Brunden, M.N.; Stefanski, K.J.; Stehle, R.G.; et al. Potassium channel conductance: A mechanism affecting hair growth both in vitro and in vivo. J. Investig. Dermatol. 1992, 98, 315–319. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1988, 138, 407–411. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Mysore, V.; Shashikumar, B.M. Guidelines on the use of finasteride in androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 128–134. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Caruso, D.; Abbiati, F.; Giatti, S.; Calabrese, D.; Piazza, F.; Cavaletti, G. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J. Sex. Med. 2013, 10, 2598–2603. [Google Scholar] [CrossRef]
- Kakali, D.; Anu, T.S.; Ashok, M.; Beena, B.; Ramesh, B.; Anand, C.B. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar]
- Madden, J.; Brunner, A.; Dastur, N.D.; Tan, R.M.; Nash, G.B.; Rainger, G.E.; Shearman, C.P.; Caldr, P.C.; Grimble, R.F. Fish oil induced increase in walking distance, but not ankle brachial pressure index, in peripheral arterial disease is dependent on both body mass index and inflammatory genotype. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 331–340. [Google Scholar] [CrossRef]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.G.; Mocharla, P.; Luscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary alpha-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1772–1780. [Google Scholar] [CrossRef]
- Drake, L.; Reyes-Hadsall, S.; Martinez, J.; Heinrich, C.; Huang, K.; Mostaghimi, A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. 2023, 159, 79–86. [Google Scholar] [CrossRef]
- Nichols, A.J.; Hughes, O.B.; Canazza, A.; Zaiac, M.N. An Open-Label Evaluator Blinded Study of the Efficacy and Safety of a New Nutritional Supplement in Androgenetic Alopecia: A Pilot Study. J. Clin. Aesthet. Dermatol. 2017, 10, 52–56. [Google Scholar]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef]
- Rompolas, P.; Deschene, E.; Zito, G.; Gonzalez, D.G.; Saotome, I.; Haberman, A.M.; Greco, V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487, 496–499. [Google Scholar] [CrossRef]
- Kong, J.; Qiang, W.; Jiang, J.; Hu, X.; Chen, Y.; Guo, Y.; Liu, H.; Sun, S.; Gao, H.; Zhang, Y.; et al. Safflower oil body nanoparticles deliver hFGF10 to hair follicles and reduce microinflammation to accelerate hair regeneration in androgenetic alopecia. Int. J. Pharm. 2022, 25, 121537. [Google Scholar] [CrossRef]
- Munkhbayar, S.; Jang, S.; Cho, A.R.; Choi, S.J.; Shin, C.Y.; Eun, H.C.; Kim, K.H.; Kwon, O. Role of arachidonic acid in promoting hair growth. Ann. Dermatol. 2016, 28, 55–64. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Walquist, M.J.; Stormo, S.K.; Østerud, B.; Elvevoll, E.O.; Eilertsen, K.E. Cold-pressed minke whale oil reduces circulating LDL/VLDL-cholesterol, lipid oxidation and atherogenesis in apolipoprotein E-deficient mice fed a Western-type diet for 13 weeks. Nutr. Metab. 2018, 15, 35. [Google Scholar] [CrossRef]
- Walquist, M.J.; Stormo, S.K.; Jensen, I.J.; Østerud, B.; Eilertsen, K.E. Antioxidant and anti-inflammatory activities in extracts from Minke whale (Balaenoptera acutorostrata) blubber. Mediat. Inflamm. 2017, 2017, 3835851. [Google Scholar] [CrossRef]
- Ogawa, T.; Rakwal, R.; Shibato, J.; Sawa, C.; Saito, T.; Murayama, A.; Kuwagata, M.; Kageyama, H.; Yagi, M.; Satoh, K.; et al. Seeking gene candidates responsible for developmental origins of health and disease. Congenit. Anom. 2011, 51, 110–125. [Google Scholar] [CrossRef]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Nakamura, K.; Wada, Y.; Tsuchikawa, D.; Yoshikawa, A.; Tamaki, K.; Shioda, S. Unraveling the ischemic brain transcriptome in a permanent middle cerebral artery occlusion mouse model by DNA microarray analysis. Dis. Models Mech. 2012, 5, 270–283. [Google Scholar] [CrossRef]
- Pérez-Mora, S.; Ocampo-López, J.; Gómez-García, M.D.C.; Pérez-Ishiwara, D.G. BFNB enhances hair growth in C57BL/6 mice through the induction of EGF and FGF7 factors and the PI3K-AKT-β-Catenin pathway. Int. J. Mol. Sci. 2023, 24, 12110. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; McLean, W.H.; et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, H. Importance of stratum corneum acidification to restore skin barrier function in eczematous diseases. Ann. Dermatol. 2024, 36, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhang, X.; Li, X.; Bai, Y.; Ao, Y.; Hexig, B.; Guo, X.; Liu, D. Fgf21 knockout mice generated using CRISPR/Cas9 reveal genetic alterations that may affect hair growth. Gene 2020, 733, 144242. [Google Scholar] [CrossRef]
- Sundberg, J.P.; Awgulewitsch, A.; Pruett, N.D.; Potter, C.S.; Silva, K.A.; Stearns, T.M.; Sundberg, B.A.; Muñoz, M.W.; Cuasnicu, P.S.; King, L.E., Jr.; et al. Crisp1 and alopecia areata in C3H/HeJ mice. Exp. Mol. Pathol. 2014, 97, 525–528. [Google Scholar] [CrossRef][Green Version]
- Méchin, M.C.; Simon, M. Deimination in epidermal barrier and hair formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220245. [Google Scholar] [CrossRef]
- Takahashi, T.; Mamada, A.; Kizawa, K.; Suzuki, R. Age-dependent damage of hair cuticle: Contribution of S100A3 protein and its citrullination. J. Cosmet. Dermatol. 2016, 15, 211–218. [Google Scholar] [CrossRef]
- Guan, W.; Deng, Q.; Yu, X.L.; Yuan, Y.S.; Gao, J.; Li, J.J.; Zhou, L.; Xia, P.; Han, G.Y.; Han, W.; et al. Blockade of S100A3 activity inhibits murine hair growth. Genet. Mol. Res. 2015, 14, 13532–13544. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Chermnykh, E.S.; Alpeeva, E.V.; Vorotelyak, E.A. Transglutaminase 3: The involvement in epithelial differentiation and cancer. Cells 2020, 9, 1996. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Morita, H.; Naganuma, T.; Miyamoto, M.; Jojima, K.; Nojiri, K.; Matsuda, J.; Kihara, A. Bifunctional DEGS2 has higher hydroxylase activity toward substrates with very-long-chain fatty acids in the production of phytosphingosine ceramides. J. Biol. Chem. 2023, 299, 104603. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. MinoxH^idil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Nessel, T.A.; Kumar, D.D. Minoxidil. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Boskabadi, S.J.; Ramezaninejad, S.; Sohrab, M.; Farhadi, R. Diazoxide-induced hypertrichosis in a neonate with transient hyperinsulinism. Clin. Med. Insights Case Rep. 2023, 16, 11795476231151330. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.C.; Thornton, M.J.; Jenner, T.J.; Chen, Y.J.; Hansen, J.B.; Carr, R.D.; Randall, V.A. Novel and established potassium channel openers stimulate hair growth in vitro: Implications for their modes of action in hair follicles. J. Investig. Dermatol. 2005, 124, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Guo, Z.; Zeng, K.; Tang, H.; Tan, H.; Min, R.; Huang, C. IL-1beta knockdown inhibits cigarette smoke extract-induced inflammation and apoptosis in vascular smooth muscle cells. PLoS ONE 2023, 18, e0277719. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Heit, B. Cellular responses to the efferocytosis of apoptotic cells. Front. Immunol. 2021, 12, 631714. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Long, Y.; Li, Z.; Li, J. Controlling hair loss by regulating apoptosis in hair follicles: A comprehensive overview. Biomolecules 2023, 14, 20. [Google Scholar] [CrossRef]
- Hu, X.M.; Li, Z.X.; Zhang, D.Y.; Yang, Y.C.; Fu, S.A.; Zhang, Z.Q.; Yang, R.H.; Xiong, K. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res. Ther. 2021, 12, 453. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, F.; Fan, Z.; Shen, T.; Liu, B.; Chen, R.; Qu, Q.; Wang, J.; Miao, Y.; Hu, Z. Nanoscale microenvironment engineering for expanding human hair follicle stem cell and revealing their plasticity. J. Nanobiotechnol. 2021, 19, 94. [Google Scholar] [CrossRef] [PubMed]





| Forward Primer | Reverse Primer | |||||
|---|---|---|---|---|---|---|
| Accession (Gene) | Primer Name | Nucleotide Sequence (5′-3′) | Primer Name | Nucleotide Sequence (5′-3′) | Product Size (bp) | Gene Name |
| NM_002009 | hH005 | cacagataggaagaggtcaatga | hH006 | taacttcttgtgtgtcgctcag | 262 | Fgf7 |
| AY047581 | hH007 | ctgaggagtccaacatcaccat | hH008 | ttcgtttaactcaagctgcctc | 254 | Vegf |
| NM_002046 | hH009 | acagtcagccgcatcttctt | hH010 | atgggatttccattgatgaca | 276 | Gapdh |
| 20% WO 1 week | 20% WO 3 weeks | ||||
| Gene name | Fold | Function | Gene name | Fold | Function |
| Mug2 | 12.94 | Anti-inflammatory, Anti-degranulation | Fgf21 | 7.39 | Hair Growth, Barrier Function |
| Scgb1b27 | 7.37 | Anti-inflammatory | Crisp1 | 7.42 | Alopecia |
| Scgb1b26-ps | 6.76 | Anti-inflammatory | Idi2 | 7.79 | Moisturizing |
| Mug1 | 5.82 | Anti-inflammatory, anti-degranulation | Serpina1d | 7.13 | Anti-inflammatory |
| Flg | 5.26 | Anti-inflammatory, Moisturizing, Barrier function | S100a7a | 6.27 | Skin Protection |
| Idi2 | 4.82 | Moisturizing | Tchh | 4.71 | Moisturizing, Barrier function |
| Stfa1 | 4.73 | Anticoagulant | Padi3 | 4.52 | Hair Growth, Barrier Function |
| Scgb1b30 | 4.72 | Anti-inflammatory | S100a3 | 4.00 | Cuticle |
| Slc16a6 (MCT7) | 0.18 | Inflammation | Pon1 | 0.08 | Antioxidant |
| Cndp2 | 0.20 | Oxidation | Myh3 | 0.11 | Muscle contraction |
| Diablo | 0.23 | Apoptosis | Sln | 0.11 | Muscle contraction |
| Acod1 | 0.27 | Oxidation/Inflammation | Pvalb | 0.13 | Muscle contraction |
| Pyy | 0.27 | Vasodilation | Mylpf | 0.13 | Muscle contraction |
| Slc5a3 | 0.28 | Oxidation/Inflammation | Neb | 0.14 | Muscle contraction |
| Renbp | 0.29 | Vasoconstriction | Ttn | 0.14 | Muscle contraction |
| Slc5a3 (SMIT1) | 0.31 | Vasoconstriction | Ptgs1(COX-1) | 0.14 | Vasoconstriction |
| 3% MXD 1 week | 3% MXD 3 weeks | ||||
| Gene name | Fold | Function | Gene name | Fold | Function |
| Oca2 | 54.35 | Melanin synthesis | Sprr2i | 10.10 | Barrier function |
| Calhm4 | 45.11 | Barrier function | Mug2 | 9.32 | Anti-inflammatory, Anti-degranulation |
| S100a7a | 33.73 | Skin Protection | Sprr2a3 | 6.94 | Barrier function |
| Trpm1 | 34.02 | Melanin synthesis | Sprr2j-ps | 5.73 | Barrier function |
| Padi3 | 30.89 | Hair Growth, Barrier Function | Tff1 | 4.76 | Barrier function |
| Shh | 28.83 | Hair growth | Nppb | 4.71 | Vasorelaxation |
| S100a3 | 15.34 | Cuticle | Sprr2e | 4.39 | Barrier function |
| Tgm3 | 4.68 | Hair Growth, Barrier Function | Degs2 | 4.35 | Barrier function |
| Leap2 | 0.24 | Antibacterial | Mgst3 | 0.08 | Detoxification |
| Serpinb12 | 0.26 | Skin Protection | Slc25a15 | 0.14 | Detoxification |
| Hamp2 | 0.27 | Antimicrobial | Slc22a4 | 0.14 | Detoxification |
| Myh4 | 0.31 | Muscle contraction | Ptx3 | 0.19 | Anti-inflammatory |
| Ttn | 0.33 | Muscle contraction | Prdx3 | 0.21 | Antioxidant |
| Myh8 | 0.33 | Muscle contraction | Mylpf | 0.23 | Muscle contraction |
| Myh2 | 0.35 | Muscle contraction | Myh8 | 0.24 | Muscle contraction |
| Myh1 | 0.40 | Muscle contraction | Des | 0.26 | Muscle contraction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibato, J.; Takenoya, F.; Kimura, A.; Yamashita, M.; Hirako, S.; Rakwal, R.; Shioda, S. DNA Microarray and Bioinformatic Analysis Reveals the Potential of Whale Oil in Enhancing Hair Growth in a C57BL/6 Mice Dorsal Skin Model. Genes 2024, 15, 627. https://doi.org/10.3390/genes15050627
Shibato J, Takenoya F, Kimura A, Yamashita M, Hirako S, Rakwal R, Shioda S. DNA Microarray and Bioinformatic Analysis Reveals the Potential of Whale Oil in Enhancing Hair Growth in a C57BL/6 Mice Dorsal Skin Model. Genes. 2024; 15(5):627. https://doi.org/10.3390/genes15050627
Chicago/Turabian StyleShibato, Junko, Fumiko Takenoya, Ai Kimura, Michio Yamashita, Satoshi Hirako, Randeep Rakwal, and Seiji Shioda. 2024. "DNA Microarray and Bioinformatic Analysis Reveals the Potential of Whale Oil in Enhancing Hair Growth in a C57BL/6 Mice Dorsal Skin Model" Genes 15, no. 5: 627. https://doi.org/10.3390/genes15050627
APA StyleShibato, J., Takenoya, F., Kimura, A., Yamashita, M., Hirako, S., Rakwal, R., & Shioda, S. (2024). DNA Microarray and Bioinformatic Analysis Reveals the Potential of Whale Oil in Enhancing Hair Growth in a C57BL/6 Mice Dorsal Skin Model. Genes, 15(5), 627. https://doi.org/10.3390/genes15050627






