Stem Cells Collection and Mobilization in Adult Autologous/Allogeneic Transplantation: Critical Points and Future Challenges
Abstract
:1. Introduction
1.1. Allogeneic Mobilization
1.2. Stem Cell Mobilization in Healthy Donors
1.3. Adverse Events and Long-Term Complications
1.4. Autologous PBSCS Mobilization Strategies
1.5. Chemotherapy Mobilization
1.6. Steady-State Mobilization (Chemotherapy-Free)
1.7. Poor Mobilizer
1.8. Old and New Generation of CXCR4 Antagonists
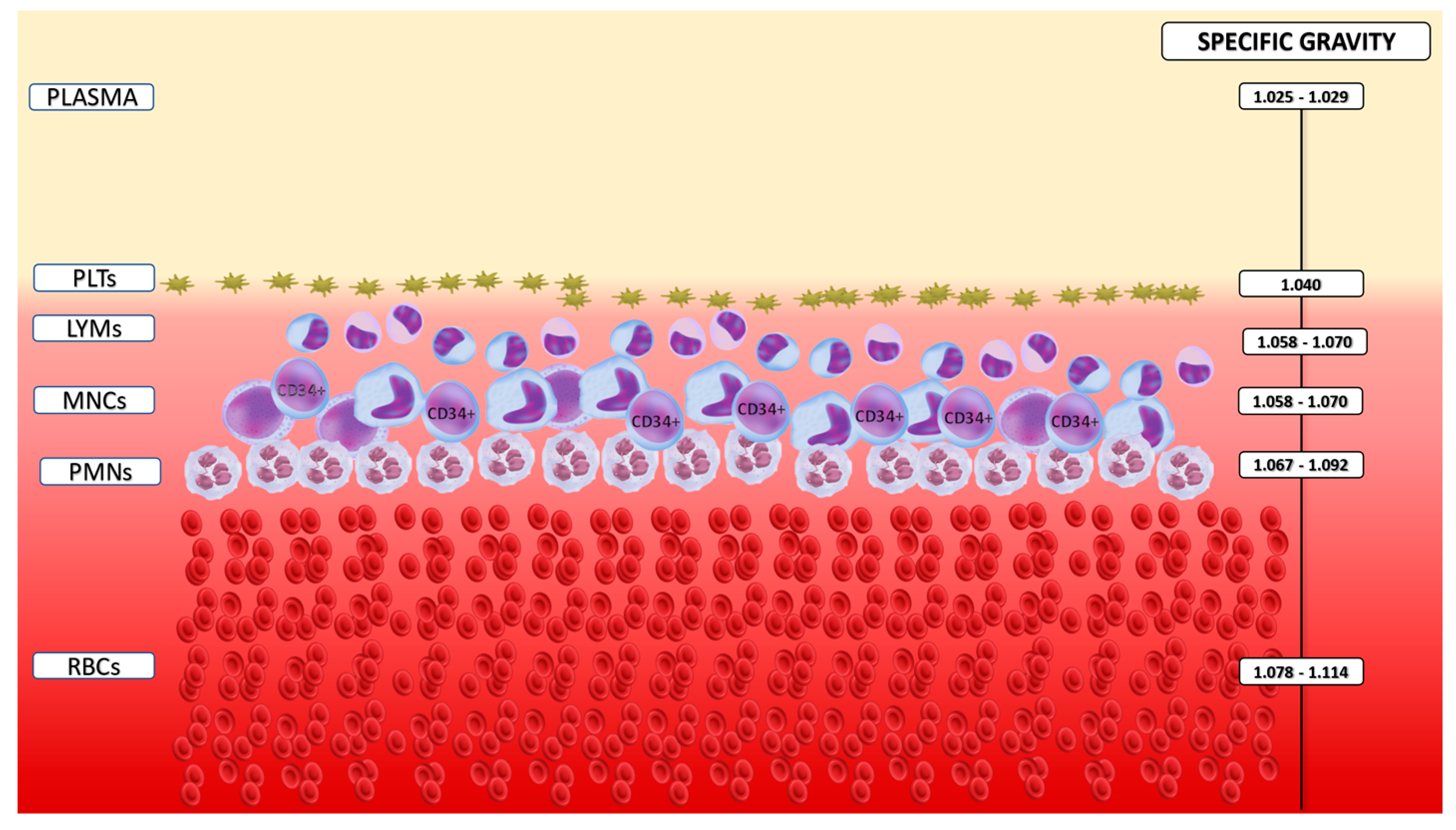
1.9. Current Technologies in Stem Cell Collection
1.10. Timing and Tools for Stem Cell Collection Prediction
1.11. Management Strategies for Intra- and Peri-Procedural Adverse Events in Stem Cell Collection
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moog, R. Management strategies for poor peripheral blood stem cell mobilization. Transfus. Apher. Sci. 2008, 38, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Russell, N.H.; Hunter, A.; Rogers, S.; Hanley, J.; Anderson, D. Peripheral blood stem cells as an alternative to marrow for allogeneic transplantation. Lancet 1993, 341, 1482. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; de la Cámara, R.; Corbacioglu, S.; Dolstra, H.; Duarte, R.; Glass, B.; Greco, R.; et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: Monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021, 56, 1651–1664. [Google Scholar] [CrossRef]
- Gluckman, E.; Devergié, A.; Bourdeau-Esperou, H.; Thierry, D.; Traineau, R.; Auerbach, A.; Broxmeyer, H.E. Transplantation of umbilical cord blood in Fanconi’s anemia. Nouv. Rev. Fr. Hematol. 1990, 32, 423–425. [Google Scholar]
- Laporte, J.P.; Gorin, N.C.; Rubinstein, P.; Lesage, S.; Portnoi, M.F.; Barbu, V.; Lopez, M.; Douay, L.; Najman, A. Cord-blood transplantation from an unrelated donor in an adult with chronic myelogenous leukemia. N. Engl. J. Med. 1996, 335, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Sauter, C.; Barker, J.N. Unrelated donor umbilical cord blood transplantation for the treatment of hematologic malignancies. Curr. Opin. Hematol. 2008, 15, 568–575. [Google Scholar] [CrossRef]
- Kessinger, A.; Smith, D.M.; Strandjord, S.E.; Landmark, J.D.; Dooley, D.C.; Law, P.; Coccia, P.F.; Warkentin, P.I.; Weisenburger, D.D.; Armitage, J.O. Allogeneic transplantation of blood-derived, T cell-depleted hemopoietic stem cells after myeloablative treatment in a patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 1989, 4, 643–646. [Google Scholar]
- Dreger, P.; Suttorp, M.; Haferlach, T.; Löffler, H.; Schmitz, N.; Schroyens, W. Allogeneic granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for treatment of engraftment failure after bone marrow transplantation. Blood 1993, 81, 1404–1407. [Google Scholar] [CrossRef]
- Cashen, A.F.; Lazarus, H.M.; Devine, S.M. Mobilizing stem cells from normal donors: Is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007, 39, 577–588. [Google Scholar] [CrossRef]
- Cottler-Fox, M.H.; Lapidotm, T.; Petitm, I.; Kollet, O.; DiPersio, J.F.; Link, D.; Devine, S. Stem cell mobilization. Am. Soc. Hematol. Educ. Program 2003, 419–437. [Google Scholar] [CrossRef]
- Winkler, I.G.; Lévesque, J.P. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails the cells that make blood and bone. Exp. Hematol. 2006, 34, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Kollet, O.; Dar, A.; Shivtiel, S.; Kalinkovich, A.; Lapid, K.; Sztainberg, Y.; Tesio, M.; Samstein, R.M.; Goichberg, P.; Spiegel, A.; et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006, 12, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Melve, G.K.; Ersvaer, E.; Eide, G.E.; Kristoffersen, E.K.; Bruserud, Ø. Peripheral Blood Stem Cell Mobilization in Healthy Donors by Granulocyte Colony-Stimulating Factor Causes Preferential Mobilization of Lymphocyte Subsets. Front. Immunol. 2018, 9, 845. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sakamaki, S.; Kohgo, Y.; Ohi, S.; Hirayama, Y.; Niitsu, Y. Recombinant human granulocyte colony-stimulating factor can mobilize sufficient amounts of peripheral blood stem cells in healthy volunteers for allogeneic transplantation. Bone Marrow Transplant. 1993, 11, 103–108. [Google Scholar]
- Schmitz, N.; Dreger, P.; Suttorp, M.; Rohwedder, E.B.; Haferlach, T.; Löffler, H.; Hunter, A.; Russell, N.H. Primary transplantation of allogeneic peripheral blood progenitor cells mobilized by filgrastim (granulocyte colony-stimulating factor). Blood 1995, 85, 1666–1672. [Google Scholar] [CrossRef]
- Russell, J.A.; Luider, J.; Weaver, M.; Brown, C.; Selinger, S.; Railton, C.; Karlsson, L.; Klassen, J. Collection of progenitor cells for allogeneic transplantation from peripheral blood of normal donors. Bone Marrow Transplant. 1995, 15, 111–115. [Google Scholar]
- Körbling, M.; Przepiorka, D.; Huh, Y.O.; Engel, H.; van Besien, K.; Giralt, S.; Andersson, B.; Kleine, H.D.; Seong, D.; Deisseroth, A.B.; et al. Allogeneic blood stem cell transplantation for refractory leukemia and lymphoma: Potential advantage of blood over marrow allografts. Blood 1995, 85, 1659–1665. [Google Scholar] [CrossRef]
- Bensinger, W.I.; Weaver, C.H.; Appelbaum, F.R.; Rowley, S.; Demirer, T.; Sanders, J.; Storb, R.; Buckner, C.D. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood 1995, 85, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Dreger, P.; Haferlach, T.; Eckstein, V.; Jacobs, S.; Suttorp, M.; Löffler, H.; Müller-Ruchholtz, W.; Schmitz, N. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: Safety, kinetics of mobilization, and composition of the graft. Br. J. Haematol. 1994, 87, 609–613. [Google Scholar] [CrossRef]
- Russel, N.; Gratwohl, A.; Schmitz, N. The place of blood stem cells in allogeneic transplantation. Br. J. Haematol. 1996, 93, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Miflin, G.; Charley, C.; Stainer, C.; Anderson, S.; Hunter, A.; Russell, N. Stem cell mobilization in normal donors for allogeneic transplantation: Analysis of safety and factors affecting efficacy. Br. J. Haematol. 1996, 95, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Renges, H.; Sonnenberg, S.; Krüger, W.; Gutensohn, K.; Dielschneider, T.; Cortes-Dericks, L.; Zander, A.R. Stem cell mobilisation with 16 microg/kg vs 10 microg/kg of G-CSF for allogeneic transplantation in healthy donors. Bone Marrow Transplant. 2002, 29, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Majolino, I.; Scimé, R.; Vasta, S.; Cavallaro, A.M.; Fiandaca, T.; Indovina, A.; Catania, P.; Santoro, A. Mobilization and collection of PBSC in healthy donors: Comparison between two schemes of rhG-CSF administration. Eur. J. Haematol. 1996, 57, 214–221. [Google Scholar] [CrossRef]
- Reményi, P.; Gopcsa, L.; Marton, I.; Réti, M.; Mikala, G.; Pető, M.; Barta, A.; Bátai, Á.; Farkas, Z.; Borbényi, Z.; et al. Peripheral blood stem cell mobilization and engraftment after autologous stem cell transplantation with biosimilar rhG-CSF. Adv. Ther. 2014, 31, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Sivgin, S.; Karakus, E.; Keklik, M.; Zararsiz, G.; Solmaz, M.; Kaynar, L.; Eser, B.; Cetin, M.; Unal, A. Evaluation of the efficacy and safety of original filgrastim (Neupogen®), biosimilar filgrastim (Leucostim®) and Lenograstim (Granocyte®) in CD34(+) peripheral hematopoietic stem cell mobilization procedures for allogeneic hematopoietic stem cell transplant donors. Transfus. Apher. Sci. 2016, 54, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Goren Sahin, D.; Arat, M. Peripheral blood stem cell collection for allogeneic hematopoietic stem cell transplantation: Practical implications after 200 consequent transplants. Transfus. Apher. Sci. 2017, 56, 800–803. [Google Scholar] [CrossRef]
- Passeri, C.; Iuliani, O.; Di Ianni, M.; Sorrentino, C.; Giancola, R.; Abbruzzese, L.; Dallavalle, F.M.; Gattillo, S.; Mariano, M.T.; Martino, M.; et al. Comparison between peripheral blood progenitor cell collection on the 4(th) or 5(th) day of granulocyte colony-stimulating factor treatment in allogeneic stem cell donors: Implications for hematopoietic progenitor cell apheresis guidelines. Blood Transfus. 2023, 21, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Mizorogi, F.; Inoue, T.; Sudo, K.; Ohnishi, A. Pharmacokinetics and adverse events following 5-day repeated administration of lenograstim, a recombinant human granulocyte colony-stimulating factor, in healthy subjects. Bone Marrow Transplant. 2000, 26, 939–946. [Google Scholar] [CrossRef]
- Winkler, I.G.; Wiercinska, E.; Barbier, V.; Nowlan, B.; Bonig, H.; Levesque, J.P. Mobilization of hematopoietic stem cells with highest self-renewal by G-CSF precedes clonogenic cell mobilization peak. Exp. Hematol. 2016, 44, 303–314.e1. [Google Scholar] [CrossRef]
- Arbona, C.; Prosper, F.; Benet, I.; Mena, F.; Solano, C.; Garcia-Conde, J. Comparison between once a day vs twice a day G-CSF for mobilization of peripheral blood progenitor cells (PBPC) in normal donors for allogeneic PBPC transplantation. Bone Marrow Transplant. 1998, 22, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Renges, H.; Krüger, W.; Gutensohn, K.; Löliger, C.; Carrero, I.; Cortes, L.; Zander, A.R. A randomized comparison of once versus twice daily recombinant human granulocyte colony-stimulating factor (filgrastim) for stem cell mobilization in healthy donors for allogeneic transplantation. Br. J. Haematol. 2000, 111, 761–765. [Google Scholar] [PubMed]
- Anderlini, P.; Donato, M.; Lauppe, M.J.; Huh, Y.O.; Martin, T.G.; Chan, K.W.; Champlin, R.E.; Körbling, M. A comparative study of once-daily versus twice-daily filgrastim administration for the mobilization and collection of CD34+ peripheral blood progenitor cells in normal donors. Br. J. Haematol. 2000, 109, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Katayama, Y.; Sunami, K.; Deguchi, S.; Nawa, Y.; Hiramatsu, Y.; Nakayama, H.; Arakawa, T.; Ishimaru, F.; Teshima, T.; et al. G-CSF-induced mobilization of peripheral blood stem cells for allografting: Comparative study of daily single versus divided dose of G-CSF. Int. J. Hematol. 1997, 66, 169–178. [Google Scholar] [CrossRef]
- Rutella, S.; Filippini, P.; Bertaina, V.; Li Pira, G.; Altomare, L.; Ceccarelli, S.; Brescia, L.P.; Lucarelli, B.; Girolami, E.; Conflitti, G.; et al. Mobilization of healthy donors with plerixafor affects the cellular composition of T-cell receptor (TCR)-αβ/CD19-depleted haploidentical stem cell grafts. J. Transl. Med. 2014, 12, 240. [Google Scholar] [CrossRef]
- Kean, L.S.; Sen, S.; Onabajo, O.; Singh, K.; Robertson, J.; Stempora, L.; Bonifacino, A.C.; Metzger, M.E.; Promislow, D.E.; Mattapallil, J.J.; et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood 2011, 118, 6580–6590. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Smith, A.L.; Takahashi, Y.; Wong, S.; Bahceci, E.; Cook, L.; Ramos, C.; Tawab, A.; McCoy, J.P., Jr.; Read, E.J.; et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J. Immunol. 2013, 191, 6241–6249. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Vij, R.; Rettig, M.; Todt, L.; McGlauchlen, K.; Fisher, N.; Devine, H.; Link, D.C.; Calandra, G.; Bridger, G.; et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood 2008, 112, 990–998. [Google Scholar] [CrossRef]
- Pantin, J.; Purev, E.; Tian, X.; Cook, L.; Donohue-Jerussi, T.; Cho, E.; Reger, R.; Hsieh, M.; Khuu, H.; Calandra, G.; et al. Effect of high-dose plerixafor on CD34(+) cell mobilization in healthy stem cell donors: Results of a randomized crossover trial. Haematologica 2017, 102, 600–609. [Google Scholar] [CrossRef]
- Hölig, K.; Schmidt, H.; Hütter, G.; Kramer, M.; Teipel, R.; Heidrich, K.; Zimmer, K.; Heidenreich, F.; Blechschmidt, M.; Torosian, T.; et al. Salvage treatment with plerixafor in poor mobilizing allogeneic stem cell donors: Results of a prospective phase II-trial. Bone Marrow Transplant. 2021, 56, 635–645. [Google Scholar] [CrossRef]
- Zhuang, L.; Lauro, D.; Wang, S.; Yuan, S. Addition of plerixafor in poorly mobilized allogeneic stem cell donors. J. Clin. Apher. 2022, 37, 388–394. [Google Scholar] [CrossRef]
- Ciceri, F.; Botti, S.; Cioce, M. Handbook GITMO; GITMO Nurses Group: Italy, 2023; Volume III, p. 622. [Google Scholar]
- Hauge, A.W.; Haastrup, E.K.; Sengeløv, H.; Minulescu, L.; Dickmeiss, E.; Fischer-Nielsen, A. Addition of plerixafor for CD34+ cell mobilization in six healthy stem cell donors ensured satisfactory grafts for transplantation. Transfusion 2014, 54, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Anderlini, P.; Rizzo, J.D.; Nugent, M.L.; Schmitz, N.; Champlin, R.E.; Horowitz, M.M. Peripheral blood stem cell donation: An analysis from the International Bone Marrow Transplant. Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. Bone Marrow Transplant. 2001, 27, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.W.; Shaw, B.E.; Kim, S.; Logan, B.R.; Sees, J.A.; Confer, D.L.; Pulsipher, M.A.; Shah, N.; Switzer, G.E.; Abidi, M.H.; et al. Collection of Peripheral Blood Progenitor Cells in 1 Day Is Associated with Decreased Donor Toxicity Compared to 2 Days in Unrelated Donors. Biol. Blood Marrow Transplant. 2020, 26, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, C.; Savignano, C.; Pasca, S.; Sperotto, A.; Patriarca, F.; Isola, M.; Fanin, R.; De Angelis, V. Efficacy and safety of peripheral blood stem cell mobilization and collection: A single-center experience in 190 allogeneic donors. Transfusion 2012, 52, 2387–2394. [Google Scholar] [CrossRef]
- Pulsipher, M.A.; Chitphakdithai, P.; Miller, J.P.; Logan, B.R.; King, R.J.; Rizzo, J.D.; Leitman, S.F.; Anderlini, P.; Haagenson, M.D.; Kurian, S.; et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: Results of a prospective trial from the National Marrow Donor Program. Blood 2009, 113, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Hölig, K.; Kramer, M.; Kroschinsky, F.; Bornhäuser, M.; Mengling, T.; Schmidt, A.H.; Rutt, C.; Ehninger, G. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood 2009, 114, 3757–3763. [Google Scholar] [CrossRef]
- Abutalib, S.A.; Padmanabhan, A.; Pham, H.P.; Worel, N. Best Practices of Apheresis in Hematopoietic Cell Transplantation; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Lanza, F.; Marchetti, M.; Zannetti, B.A. Overview on novel strategies and current guidelines for hematopoietic stem cell mobilisation and collection. Transfus. Apher. Sci. 2023, 62, 103830. [Google Scholar] [CrossRef]
- Kanate, A.S.; Majhail, N.S.; Savani, B.N.; Bredeson, C.; Champlin, R.E.; Crawford, S.; Giralt, S.A.; LeMaistre, C.F.; Marks, D.I.; Omel, J.L.; et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2020, 26, 1247–1256. [Google Scholar] [CrossRef]
- Liu, W.; Ji, X.; Song, Y.; Wang, X.; Zheng, W.; Lin, N.; Tu, M.; Xie, Y.; Ping, L.; Ying, Z.; et al. Improving survival of 3760 patients with lymphoma: Experience of an academic center over two decades. Cancer Med. 2020, 9, 3765–3774. [Google Scholar] [CrossRef]
- Giralt, S.; Costa, L.; Schriber, J.; Dipersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biol. Blood Marrow Transplant. 2014, 20, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Hübel, K.; Kröger, N.; Aljurf, M.; Apperley, J.; Basak, G.W.; Bazarbachi, A.; Douglas, K.; Gabriel, I.; Garderet, L.; et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: A position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014, 49, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Annibali, O.; Santangelo, S.; Tomarchio, V.; Ferraro, S.; Armiento, D.; Scardocci, A.; Arcese, W.; Antonelli Incalzi, R.; Avvisati, G. Pulmonary complications and survival after autologous stem cell transplantation: Predictive role of pulmonary function and pneumotoxic medications. Eur. Respir. J. 2017, 49, 1601902. [Google Scholar] [CrossRef] [PubMed]
- Annibali, O.; Piccioni, L.; Tomarchio, V.; Circhetta, E.; Sarlo, C.; Franceschini, L.; Cantonetti, M.; Rizzo, E.; Angeletti, S.; Tirindelli, M.C.; et al. Impact of IFN lambda 3/4 single nucleotide polymorphisms on the cytomegalovirus reactivation in autologous stem cell transplant patients. PLoS ONE 2018, 13, e0200221. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Majhail, N.S.; Liu, H. Hematopoietic Progenitor Cell Mobilization for Autologous Stem Cell Transplantation in Multiple Myeloma in Contemporary Era. Clin. Lymphoma Myeloma Leuk. 2019, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Fronteau, C.; Antier, C.; Dupuis, P.; Tessoulin, B.; Gastinne, T.; Mahé, B.; Blin, N.; Dubruille, V.; Lok, A.; et al. Autologous stem-cell collection following VTD or VRD induction therapy in multiple myeloma: A single-center experience. Bone Marrow Transplant. 2021, 56, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Callander, N.; Watts, N.L.; Costa, L.J.; Thapa, B.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; et al. Stem Cell Mobilization Yields with Daratumumab- and Lenalidomide-Containing Quadruplet Induction Therapy in Newly Diagnosed Multiple Myeloma: Findings from the MASTER and GRIFFIN Trials. Transplant. Cell. Ther. 2023, 29, 174.e1–174.e10. [Google Scholar] [CrossRef]
- Mina, R.; Petrucci, M.T.; Bonello, F.; Bongarzoni, V.; Saccardi, R.; Bertuglia, G.; Mengarelli, A.; Spadaro, A.; Lisi, C.; Curci, P.; et al. A prospective, multicenter study on hematopoietic stem-cell mobilization with cyclophosphamide plus granulocyte colony-stimulating factor and ‘on-demand’ plerixafor in multiple myeloma patients treated with novel agents. Haematologica 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lemonakis, K.; Tatting, L.; Lisak, M.; Carlson, K.; Crafoord, J.; Blimark, C.H.; Santamaria, A.I.; Wichert, S.; Lenhoff, S.; Hansson, M. Impact of daratumumab-based induction on stem cell collection parameters in Swedish myeloma patients. Haematologica 2023, 108, 610–614. [Google Scholar] [CrossRef]
- Johnsrud, A.; Ladha, A.; Muffly, L.; Shiraz, P.; Goldstein, G.; Osgood, V.; Shizuru, J.A.; Johnston, L.; Arai, S.; Weng, W.K.; et al. Stem Cell Mobilization in Multiple Myeloma: Comparing Safety and Efficacy of Cyclophosphamide +/- Plerixafor versus Granulocyte Colony-Stimulating Factor +/- Plerixafor in the Lenalidomide Era. Transplant. Cell. Ther. 2021, 27, 590.e1–590.e8. [Google Scholar] [CrossRef]
- Russell, N.; Douglas, K.; Ho, A.D.; Mohty, M.; Carlson, K.; Ossenkoppele, G.J.; Milone, G.; Pareja, M.O.; Shaheen, D.; Willemsen, A.; et al. Plerixafor and granulocyte colony-stimulating factor for first-line steady-state autologous peripheral blood stem cell mobilization in lymphoma and multiple myeloma: Results of the prospective PREDICT trial. Haematologica 2013, 98, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.S.; He, D.H.; Li, Y.; Zhang, Y.; Zheng, G.F.; Zhu, Y.Y.; He, J.S.; Zhang, E.F.; Cai, Z.; Zhao, Y. Efficacy and Safety of Plerixafor Combined with G-CSF for Autologous Peripheral Blood Hematopoietic Stem Cell Mobilization in Lymphoma Patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Thurlapati, A.; Roubal, K.; Davis, J.A.; Shah, S.Z.; Smith, D.; McGann, M.; Gaffney, K.; Cendagorta, A.; Maldonado, A.; Weeda, E.; et al. Stem Cell Mobilization for Multiple Myeloma Patients Receiving Daratumumab-Based Induction Therapy: A Real- World Experience. Transplant. Cell. Ther. 2023, 29, 340.e1–340.e4. [Google Scholar] [CrossRef]
- Wuchter, P.; Ran, D.; Bruckner, T.; Schmitt, T.; Witzens-Harig, M.; Neben, K.; Goldschmidt, H.; Ho, A.D. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol. Blood Marrow Transplant. 2010, 16, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Marchetti, M.; Lemoli, R.; Tarella, C.; Iacone, A.; Lanza, F.; Rambaldi, A.; Bosi, A. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: An analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012, 47, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Alexander, E.T.; Hogan, K.R.; Schaub, C.; Fouts, T.V.; Stuart, R.K. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011, 46, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sukhtankar, D.D.; Fung, J.J.; Kim, M.N.; Cayton, T.; Chiou, V.; Caculitan, N.G.; Zalicki, P.; Kim, S.; Jo, Y.; Lee, J.M.; et al. GPC-100, a novel CXCR4 antagonist, improves in vivo hematopoietic cell mobilization when combined with propranolol. PLoS ONE 2023, 18, e0287863. [Google Scholar] [CrossRef] [PubMed]
- Setia, G.; Hagog, N.; Jalilizeinali, B.; Funkhouser, S.; Pierzchanowski, L.; Lan, F.; Gabig, T.G.; Kiner-Strachan, B.; Kelleher, K.; Hsu, M.-C.; et al. A phase II, open-label pilot study to evaluate the hematopoietic stem cell mobilization of TG-0054 combined with G-CSF in 12 patients with multiple myeloma, non-Hodgkin lymphoma or Hodgkin lymphoma-an interim analysis. Blood 2015, 126, 515. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Wang, Q.; Su, H.; Ding, K.; Shuang, Y.; Gao, S.; Zou, D.; Jing, H.; Chai, Y.; et al. YF-H-2015005, a CXCR4 Antagonist, for the Mobilization of Hematopoietic Stem Cells in Non-Hodgkin Lymphoma Patients: A Randomized, Controlled, Phase 3 Clinical Trial. Front. Med. 2021, 8, 609116. [Google Scholar] [CrossRef]
- Crees, Z.D.; Rettig, M.P.; Jayasinghe, R.G.; Stockerl-Goldstein, K.; Larson, S.M.; Arpad, I.; Milone, G.A.; Martino, M.; Stiff, P.; Sborov, D. Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: A randomized phase 3 trial. Nat. Med. 2023, 29, 869–879. [Google Scholar] [CrossRef]
- Balogun, R.A.; Aqui, N.; Alicia, G.; Pham Huy, P.; Torloni, A.S.; Wrhri, G.; Yamada, C. Principles of Apheresis Technology. Techinical Principles of Apheresis Medicine, 7th ed.; American Society for Apheresis: Vancouver, BC, Canada, 2020. [Google Scholar]
- Maitta, R.W. Current state of apheresis technology and its applications. Transfus. Apher. Sci. 2018, 57, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Sputtek, A.; Schubert, C.; Peine, S.; Rowe, A.W. Safe collection of peripheral blood stem cells in patients and donors using the Amicus (R) separator and the Spectra Optia (R) apheresis system. In Vox Sanguinis; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 294–295. [Google Scholar]
- Burgstaler, E.A.; Porrata, L.F.; Markovic, S.N.; Winters, J.L. Use of various offset settings in the Fenwal Amicus during hematopoietic progenitor cell collection to increase lymphocyte yield and reduce cross-cellular contamination. J. Clin. Apher. 2010, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Panch, S.R.; Yau, Y.Y.; Kang, E.M.; De Ravin, S.S.; Malech, H.L.; Leitman, S.F. Mobilization characteristics and strategies to improve hematopoietic progenitor cell mobilization and collection in patients with chronic granulomatous disease and severe combined immunodeficiency. Transfusion 2015, 55, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Chen, S.H.; Yang, S.H.; Su, Y.C.; Chu, S.C.; Li, D.K. Poor harvest of peripheral blood stem cell in donors with microcytic red blood cells. Transfusion 2013, 53, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Leitman, S.F.; Yau, Y.; Matthews, C.L.; Hopkins, J.A.; Min, K. Optimization of Unstimulated Mononuclear Cell Collections Using the Amicus Continuous-Flow Apheresis Device. In Transfusion; Wiley-Blackwell Publishing, Inc.: Malden, MA, USA, 2010; p. 15A. [Google Scholar]
- Trébéden-Negre, H.; Rosenzwajg, M.; Tanguy, M.L.; Lefrere, F.; Azar, N.; Heshmati, F.; Belhocine, R.; Vernant, J.P.; Klatzmann, D.; Norol, F. Delayed recovery after autologous peripheral hematopoietic cell transplantation: Potential effect of a high number of total nucleated cells in the graft. Transfusion 2010, 50, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, P.; Terruzzi, E.; Dassi, M.; Baldini, V.; Parma, M.; Coluccia, P.; Accorsi, P.; Confalonieri, G.; Tavecchia, L.; Verga, L.; et al. Management of poor peripheral blood stem cell mobilization: Incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus. Apher. Sci. 2009, 41, 33–37. [Google Scholar] [CrossRef]
- Miyazaki, K.; Suzuki, K. Poor mobilizer and its countermeasures. Transfus. Apher. Sci. 2018, 57, 623–627. [Google Scholar] [CrossRef]
- To, L.B.; Levesque, J.P.; Herbert, K.E. How I treat patients who mobilize hematopoietic stem cells poorly. Blood 2011, 118, 4530–4540. [Google Scholar] [CrossRef] [PubMed]
- Ataca Atilla, P.; Bakanay Ozturk, S.M.; Demirer, T. How to manage poor mobilizers for high dose chemotherapy and autologous stem cell transplantation? Transfus. Apher. Sci. 2017, 56, 190–198. [Google Scholar] [CrossRef]
- Seggewiss, R.; Buss, E.C.; Herrmann, D.; Goldschmidt, H.; Ho, A.D.; Fruehauf, S. Kinetics of peripheral blood stem cell mobilization following G-CSF-supported chemotherapy. Stem Cells 2003, 21, 568–574. [Google Scholar] [CrossRef]
- Roberts, A.W.; Foote, S.; Alexander, W.S.; Scott, C.; Robb, L.; Metcalf, D. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood 1997, 89, 2736–2744. [Google Scholar] [CrossRef]
- Soukup, A.A.; Bresnick, E.H. Gata2 noncoding genetic variation as a determinant of hematopoietic stem/progenitor cell mobilization efficiency. Blood Adv. 2023, 7, 7564–7575. [Google Scholar] [CrossRef]
- Pierelli, L.; Perseghin, P.; Marchetti, M.; Accorsi, P.; Fanin, R.; Messina, C.; Olivieri, A.; Risso, M.; Salvaneschi, L.; Bosi, A. Best practice for peripheral blood progenitor cell mobilization and collection in adults and children: Results of a Società Italiana Di Emaferesi e Manipolazione Cellulare (SIDEM) and Gruppo Italiano Trapianto Midollo Osseo (GITMO) consensus process. Transfusion 2011, 52, 893–905. [Google Scholar] [CrossRef]
- Bashey, A.; Donohue, M.; Liu, L.; Medina, B.; Corringham, S.; Ihasz, A.; Carrier, E.; Castro, J.E.; Holman, P.R.; Xu, R.; et al. Peripheral blood progenitor cell mobilization with intermediate-dose cyclophosphamide, sequential granulocyte-macrophage-colony-stimulating factor and granulocyte-colony-stimulating factor, and scheduled commencement of leukapheresis in 225 patients undergoing autologous transplantation. Transfusion 2007, 47, 2153–2160. [Google Scholar] [CrossRef]
- Spoerl, S.; Peter, R.; Wäscher, D.; Götze, K.; Verbeek, M.; Peschel, C.; Krackhardt, A.M. Patients’ outcome after rescue plerixafor administration for autologous stem cell mobilization: A single-center retrospective analysis. Transfusion 2017, 57, 115–121. [Google Scholar] [CrossRef]
- Andritsos, L.A.; Huang, Y.; Abraham, I.; Huff, K.; Scrape, S.R.; Fan, T.; Alkhatib, N.; Hofmeister, C.C.; Drea, E.; McBride, A. Clinical and cost outcomes of pre-emptive plerixafor administration in patients with multiple myeloma undergoing stem cell mobilization. Leuk. Res. 2019, 85, 106215. [Google Scholar] [CrossRef]
- Shi, P.A.; Miller, L.K.; Isola, L.M. Prospective study of mobilization kinetics up to 18 h after late-afternoon dosing of plerixafor. Transfusion 2014, 54, 1263–1268. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S. How do we mobilize and collect autologous peripheral blood stem cells? Transfusion 2017, 57, 13–23. [Google Scholar] [CrossRef]
- Flommersfeld, S.; Sohlbach, K.; Jaques, G.; Bein, G.; Hoffmann, J.; Kostrewa, P.; Sachs, U.J. Collection of peripheral blood progenitor cells on Day 4 is feasible and effective while reducing granulocyte-colony-stimulating factor exposure to healthy donors. Transfusion 2015, 55, 1269–1274. [Google Scholar] [CrossRef]
- Kimura, S.; Ohkawara, H.; Minakawa, K.; Fukatsu, M.; Mori, H.; Takahashi, H.; Harada-Shirado, K.; Ohara, Y.; Takahashi, N.; Mochizuki, K.; et al. Optimal timing of apheresis for the efficient mobilization of peripheral blood progenitor cells recruited by high-dose granulocyte colony-stimulating factor in healthy donors. Transfus. Apher. Sci. 2020, 59, 102737. [Google Scholar] [CrossRef]
- Kaul, E.; Kothari, S.; Setia, R.; Sharma, S.; Suleiman, S.; Khandelwal, V.; Kharya, G.; Sharma, B.; Handoo, A.; Choudhary, D. Peripheral Blood Stem Cell Collection on Day 4 Is Feasible and Safe in a Majority of Allogeneic Stem Cell Transplant Donors. Biol. Blood Marrow Transplant. 2016, 22, S328. [Google Scholar] [CrossRef]
- Humpe, A.; Riggert, J.; Meineke, I.; Kurz, M.; Eil, A.; Storkebaum, B.; Binder, C.; Munzel, U.; Funke, I.; Höcker, P.; et al. A cell-kinetic model of CD34+ cell mobilization and harvest: Development of a predictive algorithm for CD34+ cell yield in PBPC collections. Transfusion 2000, 40, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Delamain, M.T.; Marques, J.F., Jr.; de Souza, C.A.; Lorand-Metze, I.; Metze, K. An algorithm based on peripheral CD34+ cells and hemoglobin concentration provides a better optimization of apheresis than the application of a fixed CD34 threshold. Transfusion 2008, 48, 1133–1137. [Google Scholar] [CrossRef]
- Pierelli, L.; Maresca, M.; Piccirillo, N.; Pupella, S.; Gozzer, M.; Foddai, M.L.; Vacca, M.; Adorno, G.; Coppetelli, U.; Paladini, U. Accurate prediction of autologous stem cell apheresis yields using a double variable-dependent method assures systematic efficiency control of continuous flow collection procedures. Vox Sang. 2006, 91, 126–134. [Google Scholar] [CrossRef]
- Vrielink, H.; Le Poole, K.; Stegmayr, B.; Kielstein, J.; Berlin, G.; Ilhan, O.; Seval, G.C.; Prophet, H.; Aandahl, A.; Deeren, D.; et al. The world apheresis association registry, 2023 update. Transfus Apher. Sci. 2023, 62, 103831. [Google Scholar] [CrossRef]
- Bueno, J.L.; Alegre, A.; López-Villar, O.; Querol, S.; Arroyo, J.L.; Goterris, R.; Sureda, A.; García-Gala, J.M.; Amunarriz, C.; Albo, C.; et al. Agreements and uncertainties in autologous haematopoietic stem cell mobilization and collection. A Spanish consensus document. Bone Marrow Transplant. 2020, 55, 811–817. [Google Scholar] [CrossRef]
- Couzin, C.; Manceau, S.; Diana, J.S.; Joseph, L.; Magnani, A.; Magrin, E.; Amrane, H.; Dupont, E.; Raphalen, J.; Sibon, D.; et al. Vascular access for optimal hematopoietic stem cell collection. J. Clin. Apher. 2021, 36, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, B.; Anderlini, P. Allogeneic peripheral blood stem cell collection as of 2008. Transfus. Apher. Sci. 2008, 38, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hölig, K.; Blechschmidt, M.; Kramer, M.; Zimmer, K.; Kroschinsky, F.; Poppe-Thiede, K.; Bornhäuser, M.; Ehninger, G. Peripheral blood stem cell collection in allogeneic donors: Impact of venous access. Transfusion 2012, 52, 2600–2605. [Google Scholar] [CrossRef]
- Leitner, G.C.; Baumgartner, K.; Kalhs, P.; Biener, D.; Greinix, H.T.; Hoecker, P.; Worel, N. Regeneration, health status and quality of life after rhG-CSF-stimulated stem cell collection in healthy donors: A cross-sectional study. Bone Marrow Transplant. 2009, 43, 357–363. [Google Scholar] [CrossRef]
- Vacca, M.; Perseghin, P.; Accorsi, P.; Pierelli, L. Central venous catheter insertion in peripheral blood hematopoietic stem cell sibling donors: The SIdEM (Italian Society of Hemapheresis and Cell Manipulation) point of view. Transfus. Apher. Sci. 2014, 50, 200–206. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.F.; Dunbar, N.M.; Kim, H.C.; Draper, N.L.; Linenberger, M.; Schwartz, J.; Miller, Y.; Murtaugh, A.; West, F.B.; Fernando, L.P.; et al. Venous access for hematopoietic progenitor cell collection: An international survey by the ASFA HPC donor subcommittee. J. Clin. Apher. 2016, 31, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Caime, A.; Piredda, A.; Lucchetti, B.; Magarò, A.; Zencovich, C.; Clerici, M.; Laszlo, D. Midline catheter as effective device in healthy allogeneic donors and patients without an adequate peripheral venous access for HPC collection by apheresis: Preliminary experience at IEO. Transfus. Apher. Sci. 2020, 59, 102740. [Google Scholar] [CrossRef]
- Casacchia, C.; Lozano, M.; Schomberg, J.; Barrows, J.; Salcedo, T.; Puthenveetil, G. Novel use of a midline catheter for therapeutic and donor apheresis in children and adults. J. Clin. Apher. 2021, 36, 711–718. [Google Scholar] [CrossRef]
| MNC COLLECTION INSTRUMENTS | TYPE OF SYSTEM | NEEDLE SYSTEM (ECV Approx. [50,74]) | KIT AND PROTOCOL |
|---|---|---|---|
| FRESENIUS-KABI | |||
| AMICUS® | CFC | Double needle (163 mL) | MNC Kit (works in cycles, centrifuge) |
| COM.TEC® | CFC | Double needle P1YA, P1Y, (177 mL) * C4Y, RVY (193 mL) * | P1YA, P1Y, C4Y, RVY (works in cycles, centrifuge) |
| HAEMONETICS CORP | |||
| MCS+® 9000 | IFC | Single needle (Variable 125 bowl 380 (38% HCT)-259 (52% HCT)/82–87 mL) * | PROTOCOL PBSC 0971E-00 (works in cycles, centrifuge) |
| TERUMO BCT INC | |||
| SPECTRA OPTIA® | CFC | Double needle MNC (191 mL) CMNC (297 mL) | MNC protocol (works in cycles, centrifuge + elutriation) CMNC protocol (centrifuge) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prisciandaro, M.; Santinelli, E.; Tomarchio, V.; Tafuri, M.A.; Bonchi, C.; Palazzo, G.; Nobile, C.; Marinucci, A.; Mele, M.; Annibali, O.; et al. Stem Cells Collection and Mobilization in Adult Autologous/Allogeneic Transplantation: Critical Points and Future Challenges. Cells 2024, 13, 586. https://doi.org/10.3390/cells13070586
Prisciandaro M, Santinelli E, Tomarchio V, Tafuri MA, Bonchi C, Palazzo G, Nobile C, Marinucci A, Mele M, Annibali O, et al. Stem Cells Collection and Mobilization in Adult Autologous/Allogeneic Transplantation: Critical Points and Future Challenges. Cells. 2024; 13(7):586. https://doi.org/10.3390/cells13070586
Chicago/Turabian StylePrisciandaro, Michele, Enrico Santinelli, Valeria Tomarchio, Maria Antonietta Tafuri, Cecilia Bonchi, Gloria Palazzo, Carolina Nobile, Alessandra Marinucci, Marcella Mele, Ombretta Annibali, and et al. 2024. "Stem Cells Collection and Mobilization in Adult Autologous/Allogeneic Transplantation: Critical Points and Future Challenges" Cells 13, no. 7: 586. https://doi.org/10.3390/cells13070586





