Higher Concentrations of Essential Trace Elements in Women Undergoing IVF May Be Associated with Poor Reproductive Outcomes Following Single Euploid Embryo Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Collection of Follicular Fluid, Whole Blood, and Urine Samples
2.3. Quantification of Essential Trace Elements by ICP-MS
2.4. Clinical Management and Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Essential Trace Element Distribution among Biofluids
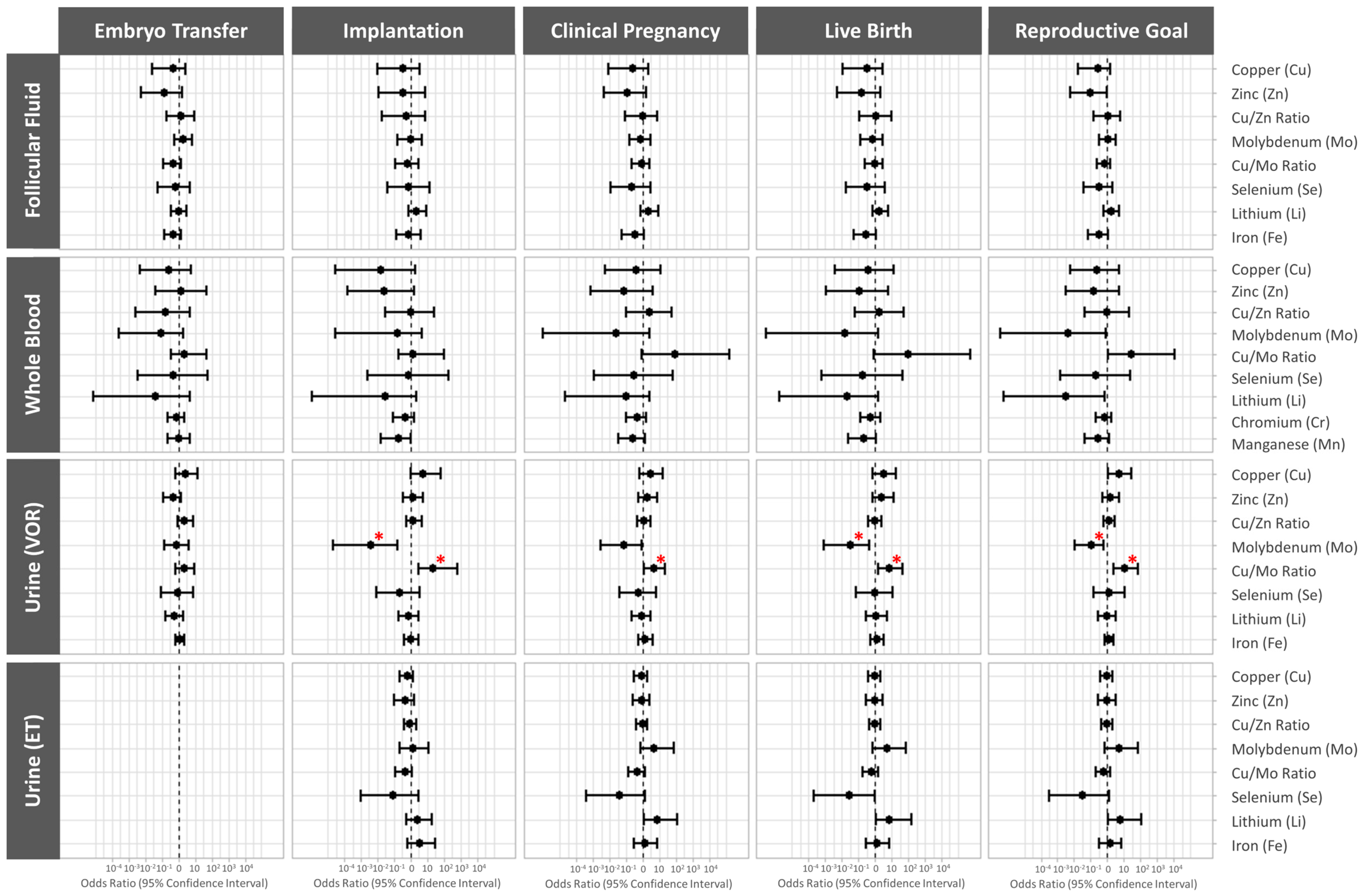
3.3. Associations between Essential Trace Element Concentrations and Ovarian Response Outcomes
3.4. Association between Essential Trace Element Concentrations and Preimplantation IVF Outcomes
3.5. Association between Essential Trace Element Concentrations and Clinical IVF Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Beigi Harchegani, A. An Overview on Role of Some Trace Elements in Human Reproductive Health, Sperm Function and Fertilization Process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.C.; Coelho, L.M.; Acevedo, M.S.M.S.F.; Coelho, N.M.M. The Oligoelements. In Handbook of Mineral Elements in Food; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 109–122. [Google Scholar] [CrossRef]
- Martinez-Morata, I.; Sobel, M.; Tellez-Plaza, M.; Navas-Acien, A.; Howe, C.G.; Sanchez, T.R. A State-of-the-Science Review on Metal Biomarkers; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; Volume 10, ISBN 0123456789. [Google Scholar]
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkin, A.V.; Jankovic-Karasoulos, T.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Skalnaya, M.G.; Tinkov, A.A.; Lobanova, Y.N.; Chang, J.S.; Skalny, A.V. Serum Levels of Copper, Iron, and Manganese in Women with Pregnancy, Miscarriage, and Primary Infertility. J. Trace Elem. Med. Biol. 2019, 56, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Tinkov, A.A.; Voronina, I.; Terekhina, O.; Skalnaya, M.G.; Kovas, Y. Hair Trace Element and Electrolyte Content in Women with Natural and In Vitro Fertilization-Induced Pregnancy. Biol. Trace Elem. Res. 2018, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, M.O.; Nazıroğlu, M.; Barak, C.; Berkkanoglu, M. Effects of Multivitamin/Mineral Supplementation on Trace Element Levels in Serum and Follicular Fluid of Women Undergoing in Vitro Fertilization (IVF). Biol. Trace Elem. Res. 2011, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmalbrock, L.J.; Weiss, G.; Rijntjes, E.; Reinschissler, N.; Sun, Q.; Schenk, M.; Schomburg, L. Pronounced Trace Element Variation in Follicular Fluids of Subfertile Women Undergoing Assisted Reproduction. Nutrients 2021, 13, 4134. [Google Scholar] [CrossRef] [PubMed]
- Ingle, M.E.; Bloom, M.S.; Parsons, P.J.; Steuerwald, A.J.; Kruger, P.; Fujimoto, V.Y. Associations between IVF Outcomes and Essential Trace Elements Measured in Follicular Fluid and Urine: A Pilot Study. J. Assist. Reprod. Genet. 2017, 34, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Wdowiak, E.; Bojar, I. Evaluation of Trace Metals in Follicular Fluid in ICSI-Treated Patients. Ann. Agric. Environ. Med. 2018, 25, 213–218. [Google Scholar] [CrossRef]
- Wang, L.; Liang, R.; Zhang, G.; Ren, M.; Long, M.; Na, J.; Li, Z.; Wang, B.; Zhuang, L.; Lu, Q. Serum Zinc Concentration and Risk of Adverse Outcomes to in Vitro Fertilization and Embryo Transfer: A Prospective Cohort Study in Northern China. Sci. Total Environ. 2021, 792, 148405. [Google Scholar] [CrossRef]
- Gonzalez-Martin, R.; Palomar, A.; Quiñonero, A.; Pellicer, N.; Fernandez-Saavedra, R.; Conde-Vilda, E.; Quejido, A.J.; Whitehead, C.; Scott, R.T.; Dominguez, F. The Impact of Essential Trace Elements on Ovarian Response and Reproductive Outcomes Following Single Euploid Embryo Transfer. Int. J. Mol. Sci. 2023, 24, 10968. [Google Scholar] [CrossRef]
- de los Santos, M.J.; Diez Juan, A.; Mifsud, A.; Mercader, A.; Meseguer, M.; Rubio, C.; Pellicer, A. Variables Associated with Mitochondrial Copy Number in Human Blastocysts: What Can We Learn from Trophectoderm Biopsies? Fertil. Steril. 2018, 109, 110–117. [Google Scholar] [CrossRef]
- Pardiñas, M.L.; Nohales, M.; Labarta, E.; De los Santos, J.M.; Mercader, A.; Remohí, J.; Bosch, E.; De los Santos, M.J. Elevated Serum Progesterone Does Not Impact Euploidy Rates in PGT-A Patients. J. Assist. Reprod. Genet. 2021, 38, 1819–1826. [Google Scholar] [CrossRef]
- Hanson, B.M.; Kim, J.G.; Osman, E.K.; Tiegs, A.W.; Lathi, R.B.; Cheng, P.J.; Scott, R.T.; Franasiak, J.M. Impact of Paternal Age on Embryology and Pregnancy Outcomes in the Setting of a Euploid Single-Embryo Transfer with Ejaculated Sperm: Retrospective Cohort Study. FS Rep. 2020, 1, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Bartel, A. Tableone: Create “Table 1” to Describe Baseline Characteristics with or without Propensity Score Weights. Available online: https://cran.r-project.org/package=tableone (accessed on 18 September 2023).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. Available online: https://github.com/taiyun/corrplot (accessed on 18 September 2023).
- Barnier, J.; Briatte, F.; Larmarange, J. Questionr: Functions to Make Surveys Processing Easier. Available online: https://cran.r-project.org/package=questionr (accessed on 18 September 2023).
- Kim, K.; Steuerwald, A.J.; Parsons, P.J.; Fujimoto, V.Y.; Browne, R.W.; Bloom, M.S. Biomonitoring for Exposure to Multiple Trace Elements via Analysis of Urine from Participants in the Study of Metals and Assisted Reproductive Technologies (SMART). J. Environ. Monit. 2011, 13, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Tolunay, H.E.; Şükür, Y.E.; Ozkavukcu, S.; Seval, M.M.; Ateş, C.; Türksoy, V.A.; Ecemiş, T.; Atabekoʇlu, C.S.; Özmen, B.; Berker, B.; et al. Heavy Metal and Trace Element Concentrations in Blood and Follicular Fluid Affect ART Outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 73–77. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Y.; Niu, M.; Kang, Y.; Du, S.; Zheng, B. Follicular Fluid Concentrations of Zinc and Copper Are Positively Associated with in Vitro Fertilization Outcomes. Int. J. Clin. Exp. Med. 2017, 10, 3547–3553. [Google Scholar]
- Abbood, M.; Burhan, S.; Ani, N. Measurement of zinc concentration in serum and follicular fluid to assess its relation with oocyte and embryo quality in women undergoing intra cytolasmic sperm injection. Int. J. Adv. Res. 2017, 5, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Qazi, I.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Han, H.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.G.; Poston, L. Selenium in Reproductive Health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Cao, Y.; Zhu, D.; Shen, L.; Zhang, Z.; Jiang, T.; Zhang, Z.; Zong, K.; Liu, Y.; et al. The Associations of Serum Metals Concentrations with the Intermediate and Pregnancy Outcomes in Women Undergoing in Vitro Fertilization (IVF). Ecotoxicol. Environ. Saf. 2022, 233, 113309. [Google Scholar] [CrossRef]
- Dickerson, E.H.; Sathyapalan, T.; Knight, R.; Maguiness, S.M.; Killick, S.R.; Robinson, J.; Atkin, S.L. Endocrine Disruptor & Nutritional Effects of Heavy Metals in Ovarian Hyperstimulation. J. Assist. Reprod. Genet. 2011, 28, 1223–1228. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Deng, Y.; Qiu, J.; Zhang, X.; Tan, J. Associations of Toxic and Essential Trace Elements in Serum, Follicular Fluid, and Seminal Plasma with In Vitro Fertilization Outcomes. Ecotoxicol. Environ. Saf. 2020, 204, 110965. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of Long-Term Selenium Supplementation on Mortality: Results from a Multiple-Dose, Randomised Controlled Trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, M.; Niu, Q.-J.; Huang, Y.-X.; Zhao, L.; Lei, X.G.; Sun, L.-H. Both Selenium Deficiency and Excess Impair Male Reproductive System via Inducing Oxidative Stress-Activated PI3K/AKT-Mediated Apoptosis and Cell Proliferation Signaling in Testis of Mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef]
- Shalini, S.; Bansal, M.P. Dietary Selenium Deficiency as Well as Excess Supplementation Induces Multiple Defects in Mouse Epididymal Spermatozoa: Understanding the Role of Selenium in Male Fertility. Int. J. Androl. 2008, 31, 438–449. [Google Scholar] [CrossRef] [PubMed]
- ATSDR, (Agency for toxic substances and Disease). Toxicological Profile for Molybdenum; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020. [Google Scholar]
- Fungwe, T.V.; Buddingh, F.; Demick, D.S.; Lox, C.D.; Yang, M.T.; Yang, S.P. The Role of Dietary Molybdenum on Estrous Activity, Fertility, Reproduction and Molybdenum and Copper Enzyme Activities of Female Rats. Nutr. Res. 1990, 10, 515–524. [Google Scholar] [CrossRef]
- Vyskočil, A.; Viau, C. Assessment of Molybdenum Toxicity in Humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Lyubimov, A.V.; Smith, J.A.; Rousselle, S.D.; Mercieca, M.D.; Tomaszewski, J.E.; Smith, A.C.; Levine, B.S. The Effects of Tetrathiomolybdate (TTM, NSC-714598) and Copper Supplementation on Fertility and Early Embryonic Development in Rats. Reprod. Toxicol. 2004, 19, 223–233. [Google Scholar] [CrossRef]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, Lead, and Other Metals in Relation to Semen Quality: Human Evidence for Molybdenum as a Male Reproductive Toxicant. Environ. Health Perspect. 2008, 116, 1473–1479. [Google Scholar] [CrossRef]
- Roney, N.; Osier, M.; Paikoff, S.J.; Smith, C.V.; Williams, M.; De Rosa, C.T. ATSDR Evaluation of the Health Effects of Zinc and Relevance to Public Health. Toxicol. Ind. Health 2006, 22, 423–493. [Google Scholar] [CrossRef]
- Thaker, R.; Oza, H.; Shaikh, I.; Kumar, S. Correlation Copper and Zinc in Spontaneous Abortions? Int. J. Fertil. Steril. 2019, 13, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, s3. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum Copper to Zinc Ratio: Relationship with Aging and Health Status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Szklarska, D.; Rzymski, P. Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification. Biol. Trace Elem. Res. 2019, 189, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Enderle, J.; Klink, U.; Di Giuseppe, R.; Koch, M.; Seidel, U.; Weber, K.; Birringer, M.; Ratjen, I.; Rimbach, G.; Lieb, W. Plasma Lithium Levels in a General Population: A Cross-Sectional Analysis of Metabolic and Dietary Correlates. Nutrients 2020, 12, 2489. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Lithium: Occurrence, Dietary Intakes, Nutritional Essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chang, H.M.; Cheng, J.C.; Chu, G.; Leung, P.C.K.; Yang, G. Lithium Chloride Inhibits StAR and Progesterone Production through GSK-3β and ERK1/2 Signaling Pathways in Human Granulosa-Lutein Cells. Mol. Cell. Endocrinol. 2018, 461, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Mirakhori, F.; Zeynali, B.; Tafreshi, A.P.; Shirmohammadian, A. Lithium Induces Follicular Atresia in Rat Ovary through a GSK-3β/β-Catenin Dependent Mechanism. Mol. Reprod. Dev. 2013, 80, 286–296. [Google Scholar] [CrossRef]
- Harari, F.; Langeén, M.; Casimiro, E.; Bottai, M.; Palm, B.; Nordqvist, H.; Vahter, M. Environmental Exposure to Lithium during Pregnancy and Fetal Size: A Longitudinal Study in the Argentinean Andes. Environ. Int. 2015, 77, 48–54. [Google Scholar] [CrossRef]
- Poels, E.M.P.; Kamperman, A.M.; Vreeker, A.; Gilden, J.; Boks, M.P.; Kahn, R.S.; Ophoff, R.A.; Bergink, V. Lithium Use during Pregnancy and the Risk of Miscarriage. J. Clin. Med. 2020, 9, 1819. [Google Scholar] [CrossRef]
- Neri, C.; De Luca, C.; D’oria, L.; Licameli, A.; Nucci, M.; Pellegrino, M.; Caruso, A.; De Santis, M. Managing Fertile Women under Lithium Treatment: The Challenge of a Teratology Information Service. Minerva Ginecol. 2018, 70, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, R.; Blanes-Zamora, R.; Paz-Montelongo, S.; Gómez-Rodríguez, J.; Fiestas, S.R.; González-Weller, D.; Gutiérrez, Á.J.; Rubio, C.; Hardisson, A.; Niebla-Canelo, D.; et al. The Influence of Follicular Fluid Metals on Assisted Reproduction Outcome. Biol. Trace Elem. Res. 2023, 201, 5069–5082. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Y.; Wang, D.; Yang, T.; Qi, J.; Zhang, Y.; Jiang, H.; Zhang, J.; Sun, B.; Liang, S. Iron Overload-Induced Ferroptosis Impairs Porcine Oocyte Maturation and Subsequent Embryonic Developmental Competence in Vitro. Front. Cell Dev. Biol. 2021, 9, 673291. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte Quality and Aging. JBRA Assist. Reprod. 2021, 26, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, A.; Kochman, K. Involvement of Copper in Female Reproduction. Reprod. Biol. 2007, 7, 193–205. [Google Scholar]
- Peacey, L.; Elphick, M.R.; Jones, C.E. Roles of Copper in Neurokinin B and Gonadotropin-Releasing Hormone Structure and Function and the Endocrinology of Reproduction. Gen. Comp. Endocrinol. 2020, 287, 113342. [Google Scholar] [CrossRef]
- Choi, H.; Lee, J.; Yoon, J.D.; Hwang, S.U.; Cai, L.; Kim, M.; Kim, G.; Oh, D.; Kim, E.; Hyun, S.H. The Effect of Copper Supplementation on in Vitro Maturation of Porcine Cumulus-Oocyte Complexes and Subsequent Developmental Competence after Parthenogenetic Activation. Theriogenology 2021, 164, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yi, J.; Zhang, M.; Xiong, J.; Geng, L.; Mu, C.; Yang, L. Effects of Iron and Copper in Culture Medium on Bovine Oocyte Maturation, Preimplantation Embryo Development, and Apoptosis of Blastocysts in Vitro. J. Reprod. Dev. 2007, 53, 777–784. [Google Scholar] [CrossRef]
- Kim, K.; Wactawski-Wende, J.; Michels, K.A.; Schliep, K.C.; Plowden, T.C.; Chaljub, E.N.; Mumford, S.L. Dietary Minerals, Reproductive Hormone Levels and Sporadic Anovulation: Associations in Healthy Women with Regular Menstrual Cycles. Br. J. Nutr. 2018, 120, 81–89. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, K.; Li, M.; Rong, C.; Zheng, J.; Li, J. Metal Elements Associate with in Vitro Fertilization (IVF) Outcomes in 195 Couples. J. Trace Elem. Med. Biol. 2021, 68, 126810. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Larroya, M.; Crispi, F.; Estruch, R.; Nakaki, A.; Paules, C.; Ruiz-León, A.M.; Sacanella, E.; Freitas, T.; Youssef, L.; et al. Diet Quality and Nutrient Density in Pregnant Women According to Adherence to Mediterranean Diet. Front. Public Health 2023, 11, 1144942. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Nutritional Supplements and IVF: An Evidence-Based Approach. Reprod. Biomed. Online 2024, 48, 103770. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, L.; Chen, L.; Zhang, J.; Zhang, X.; Kang, Q.; Jin, F.; Ye, Y. Parental Plasma Concentrations of Perfluoroalkyl Substances and In Vitro Fertilization Outcomes. Environ. Pollut. 2021, 269, 116159. [Google Scholar] [CrossRef] [PubMed]
- Carignan, C.C.; Mínguez-Alarcón, L.; Williams, P.L.; Meeker, J.D.; Stapleton, H.M.; Butt, C.M.; Toth, T.L.; Ford, J.B.; Hauser, R. Paternal Urinary Concentrations of Organophosphate Flame Retardant Metabolites, Fertility Measures, and Pregnancy Outcomes among Couples Undergoing in Vitro Fertilization. Environ. Int. 2018, 111, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Tielemans, E.; Van Kooij, R.; Looman, C.; Burdorf, A.; Velde, E.T.; Heederik, D. Paternal Occupational Exposures and Embryo Implantation Rates after IVF. Fertil. Steril. 2000, 74, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Dodge, L.E.; Williams, P.L.; Williams, M.A.; Missmer, S.A.; Toth, T.L.; Calafat, A.M.; Hauser, R. Paternal Urinary Concentrations of Parabens and Other Phenols in Relation to Reproductive Outcomes among Couples from a Fertility Clinic. Environ. Health Perspect. 2015, 123, 665–671. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Bellavia, A.; Gaskins, A.J.; Chavarro, J.E.; Ford, J.B.; Souter, I.; Calafat, A.M.; Hauser, R.; Williams, P.L. Paternal Mixtures of Urinary Concentrations of Phthalate Metabolites, Bisphenol A and Parabens in Relation to Pregnancy Outcomes among Couples Attending a Fertility Center. Environ. Int. 2021, 146, 106171. [Google Scholar] [CrossRef]


| Demographic Characteristics | |
| Age (years), median [IQR] | 39.00 [38.00, 41.00] |
| Body mass index (kg/m2), median [IQR] | 22.97 [20.63, 25.12] |
| Race/ethnic group, n (%) | |
| 48 (94.1%) |
| 1 (2.0%) |
| 1 (2.0%) |
| 1 (2.0%) |
| Education, n (%) | |
| 3 (6.5%) |
| 5 (10.9%) |
| 5 (10.9%) |
| 33 (71.7%) |
| Smoking, n (%) | |
| 23 (50.0%) |
| 13 (28.3%) |
| 9 (19.6%) |
| 1 (2.2%) |
| Reproductive Characteristics | |
| Total FSH + hMG dose during COS (IU), median [IQR] | 3300.00 [2437.50, 3925.00] |
| Trigger day E2 (pg/mL), median [IQR] | 2083.00 [1622.50, 4045.50] |
| Number of retrieved oocytes, median [IQR] | 12.00 [7.00, 16.00] |
| Maturation rate, % mean ± SD | 80 ± 17% |
| Fertilization rate, % mean ± SD | 75 ± 25% |
| Blastulation rate, % mean ± SD | 54 ± 28% |
| Euploid rate, % mean ± SD | 42 ± 34% |
| Transfer rate, n (%) | 36 (70.6%) |
| Implantation (positive hCG) rate, n (%) | 23 (63.9%) |
| Clinical pregnancy rate, n (%) | 19 (52.8%) |
| Live birth rate, n (%) | 17 (47.2%) |
| Goal rate, n (%) | 17 (33.3%) |
| LOD | Samples below the LOD, n (%) | GM (SD) | 20% | 50% | 80% | |
|---|---|---|---|---|---|---|
| Follicular Fluid (n = 29) | ||||||
| Copper (Cu) (ng/mL) | NA | 0 (0%) | 841.63 (300.01) | 733.80 | 922.00 | 1120.92 |
| Zinc (Zn) (ng/mL) | NA | 0 (0%) | 343.26 (115.35) | 253.80 | 372.00 | 472.88 |
| Copper/Zinc Ratio (Cu/Zn) | 2.45 (0.89) | 2.07 | 2.55 | 3.01 | ||
| Molybdenum (Mo) (ng/mL) | 1 | 2 (5%) | 2.16 (1.74) | 1.26 | 2.10 | 5.00 |
| Copper/Molybdenum Ratio (Cu/Mo) | 390.45 (314.53) | 180.64 | 491.43 | 737.97 | ||
| Selenium (Se) (ng/mL) | NA | 0 (0%) | 44.49 (15.44) | 32.60 | 50.00 | 61.00 |
| Lithium (Li) (ng/mL) | 1 | 13 (33%) | 1.33 (1.69) | 0.50 | 1.40 | 3.62 |
| Iron (Fe) (ng/mL) | NA | 0 (0%) | 694 (1661.39) | 383.00 | 626.00 | 957.20 |
| Whole Blood (n = 40) | ||||||
| Copper (Cu) (ng/mL) | NA | 0 (0%) | 1039.42 (211.52) | 893.44 | 1091.39 | 1240.97 |
| Zinc (Zn) (ng/mL) | NA | 0 (0%) | 3614.45 (731.91) | 3060.96 | 3640.56 | 4377.56 |
| Copper/Zinc Ratio (Cu/Zn) | 0.29 (0.07) | 0.25 | 0.29 | 0.35 | ||
| Molybdenum (Mo) (ng/mL) | NA | 0 (0%) | 3.84 (2.03) | 3.38 | 3.95 | 4.44 |
| Copper/Molybdenum Ratio (Cu/Mo) | 272.76 (345.13) | 218.51 | 276.12 | 320.32 | ||
| Selenium (Se) (ng/mL) | NA | 0 (0%) | 91.09 (13.46) | 80.94 | 90.85 | 99.37 |
| Lithium (Li) (ng/mL) | NA | 0 (0%) | 6.56 (1.36) | 6.16 | 6.77 | 7.43 |
| Chromium (Cr) (ng/mL) | 1.5 | 7 (15%) | 2.74 (2.44) | 1.75 | 3.04 | 4.86 |
| Manganese (Mn) (ng/mL) | NA | 0 (0%) | 10.62 (6.67) | 7.29 | 9.99 | 16.88 |
| Urine, VOR (n = 50) | ||||||
| Copper (Cu) (ng/mL) | NA | 0 (0%) | 11.67 (7.21) | 7.27 | 12.34 | 19.55 |
| Creatinine-corrected Copper (µg/g CR) | 2.89 (1.81) | 2.02 | 2.62 | 4.42 | ||
| Zinc (Zn) (ng/mL) | NA | 0 (0%) | 310.1 (216.03) | 186.67 | 356.93 | 532.58 |
| Creatinine Corrected Zinc (µg/g CR) | 75.35 (55.09) | 42.36 | 82.82 | 130.14 | ||
| Copper/Zinc Ratio (Cu/Zn) | 0.04 (0.08) | 0.02 | 0.03 | 0.07 | ||
| Molybdenum (Mo) (ng/mL) | NA | 0 (0%) | 41.93 (34.77) | 25.37 | 38.99 | 68.70 |
| Creatinine Corrected Molybdenum (µg/g CR) | 10.1 (4.93) | 6.86 | 10.41 | 14.86 | ||
| Copper/Molybdenum Ratio (Cu/Mo) | 0.29 (0.28) | 0.17 | 0.26 | 0.57 | ||
| Selenium (Se) (ng/mL) | NA | 0 (0%) | 31.95 (10.49) | 26.08 | 32.92 | 40.56 |
| Creatinine Corrected Selenium (µg/g CR) | 7.9 (2.86) | 6.29 | 7.71 | 10.14 | ||
| Lithium (Li) (ng/mL) | NA | 0 (0%) | 39.12 (31.75) | 21.52 | 38.84 | 68.92 |
| Creatinine Corrected Lithium (µg/g CR) | 9.56 (9.19) | 5.63 | 9.40 | 14.53 | ||
| Iron (Fe) (ng/mL) | 1 | 1 (2%) | 7.87 (98.56) | 5.00 | 5.00 | 13.88 |
| Creatinine Corrected Iron (µg/g CR) | 1.96 (38.71) | 0.89 | 1.77 | 3.47 | ||
| Chromium (Cr) (ng/mL) | 0.5 | 28 (56%) | 0.49 (1.06) | 0.25 | 0.25 | 1.45 |
| Creatinine Corrected Chromium (µg/g CR) | 0.12 (0.33) | 0.05 | 0.10 | 0.42 | ||
| Urine, Embryo Transfer Day (n = 27) | ||||||
| Copper (Cu) (ng/mL) | NA | 0 (0%) | 9.16 (67.83) | 5.27 | 8.70 | 14.13 |
| Creatinine Corrected Copper (µg/g CR) | 2.83 (27.45) | 1.36 | 2.40 | 3.68 | ||
| Zinc (Zn) (ng/mL) | NA | 0 (0%) | 164.07 (188.63) | 82.02 | 172.60 | 327.46 |
| Creatinine Corrected Zinc (µg/g CR) | 50.65 (68.6) | 30.46 | 45.78 | 91.93 | ||
| Copper/Zinc Ratio (Cu/Zn) | 0.06 (0.44) | 0.03 | 0.04 | 0.09 | ||
| Molybdenum (Mo) (ng/mL) | NA | 0 (0%) | 33.18 (30.13) | 19.54 | 28.43 | 57.12 |
| Creatinine Corrected Molybdenum (µg/g CR) | 10.24 (7.89) | 6.72 | 10.25 | 15.54 | ||
| Copper/Molybdenum Ratio (Cu/Mo) | 0.28 (0.64) | 0.13 | 0.28 | 0.46 | ||
| Selenium (Se) (ng/mL) | NA | 0 (0%) | 26.08 (13.26) | 17.67 | 22.66 | 43.01 |
| Creatinine Corrected Selenium (µg/g CR) | 8.05 (2.51) | 6.31 | 7.76 | 10.41 | ||
| Lithium (Li) (ng/mL) | NA | 0 (0%) | 29.12 (23.16) | 15.00 | 29.15 | 54.80 |
| Creatinine Corrected Lithium (µg/g CR) | 8.99 (8.08) | 6.15 | 9.41 | 13.90 | ||
| Iron (Fe) (ng/mL) | 5 | 5 (18%) | 8.15 (10.45) | 4.37 | 5.36 | 21.84 |
| Creatinine Corrected Iron (µg/g CR) | 2.51 (3.75) | 1.20 | 2.34 | 4.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Martin, R.; Palomar, A.; Perez-Deben, S.; Salsano, S.; Quiñonero, A.; Caracena, L.; Fernandez-Saavedra, R.; Fernandez-Martinez, R.; Conde-Vilda, E.; Quejido, A.J.; et al. Higher Concentrations of Essential Trace Elements in Women Undergoing IVF May Be Associated with Poor Reproductive Outcomes Following Single Euploid Embryo Transfer. Cells 2024, 13, 839. https://doi.org/10.3390/cells13100839
Gonzalez-Martin R, Palomar A, Perez-Deben S, Salsano S, Quiñonero A, Caracena L, Fernandez-Saavedra R, Fernandez-Martinez R, Conde-Vilda E, Quejido AJ, et al. Higher Concentrations of Essential Trace Elements in Women Undergoing IVF May Be Associated with Poor Reproductive Outcomes Following Single Euploid Embryo Transfer. Cells. 2024; 13(10):839. https://doi.org/10.3390/cells13100839
Chicago/Turabian StyleGonzalez-Martin, Roberto, Andrea Palomar, Silvia Perez-Deben, Stefania Salsano, Alicia Quiñonero, Laura Caracena, Rocio Fernandez-Saavedra, Rodolfo Fernandez-Martinez, Estefania Conde-Vilda, Alberto J. Quejido, and et al. 2024. "Higher Concentrations of Essential Trace Elements in Women Undergoing IVF May Be Associated with Poor Reproductive Outcomes Following Single Euploid Embryo Transfer" Cells 13, no. 10: 839. https://doi.org/10.3390/cells13100839
APA StyleGonzalez-Martin, R., Palomar, A., Perez-Deben, S., Salsano, S., Quiñonero, A., Caracena, L., Fernandez-Saavedra, R., Fernandez-Martinez, R., Conde-Vilda, E., Quejido, A. J., Giles, J., Vidal, C., Bellver, J., & Dominguez, F. (2024). Higher Concentrations of Essential Trace Elements in Women Undergoing IVF May Be Associated with Poor Reproductive Outcomes Following Single Euploid Embryo Transfer. Cells, 13(10), 839. https://doi.org/10.3390/cells13100839






