Immunoglobulin Superfamily Containing Leucine-Rich Repeat (ISLR) Serves as a Redox Sensor That Modulates Antioxidant Capacity by Suppressing Pyruvate Kinase Isozyme M2 Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Studies
2.3. Plasmids and Transfections
2.4. Cell Viability Assay
2.5. Live Cell Number Measurement
2.6. Intracellular ROS Levels
2.7. Total Glutathione Measurement
2.8. PK Activity Assay
2.9. Cell Extraction, SDS-PAGE, and Western Blotting
2.10. Disuccinimidyl Suberate (DSS) Cross Linking
2.11. Immunoprecipitation Analysis
2.12. Immunofluorescence and Crystal Violet Staining
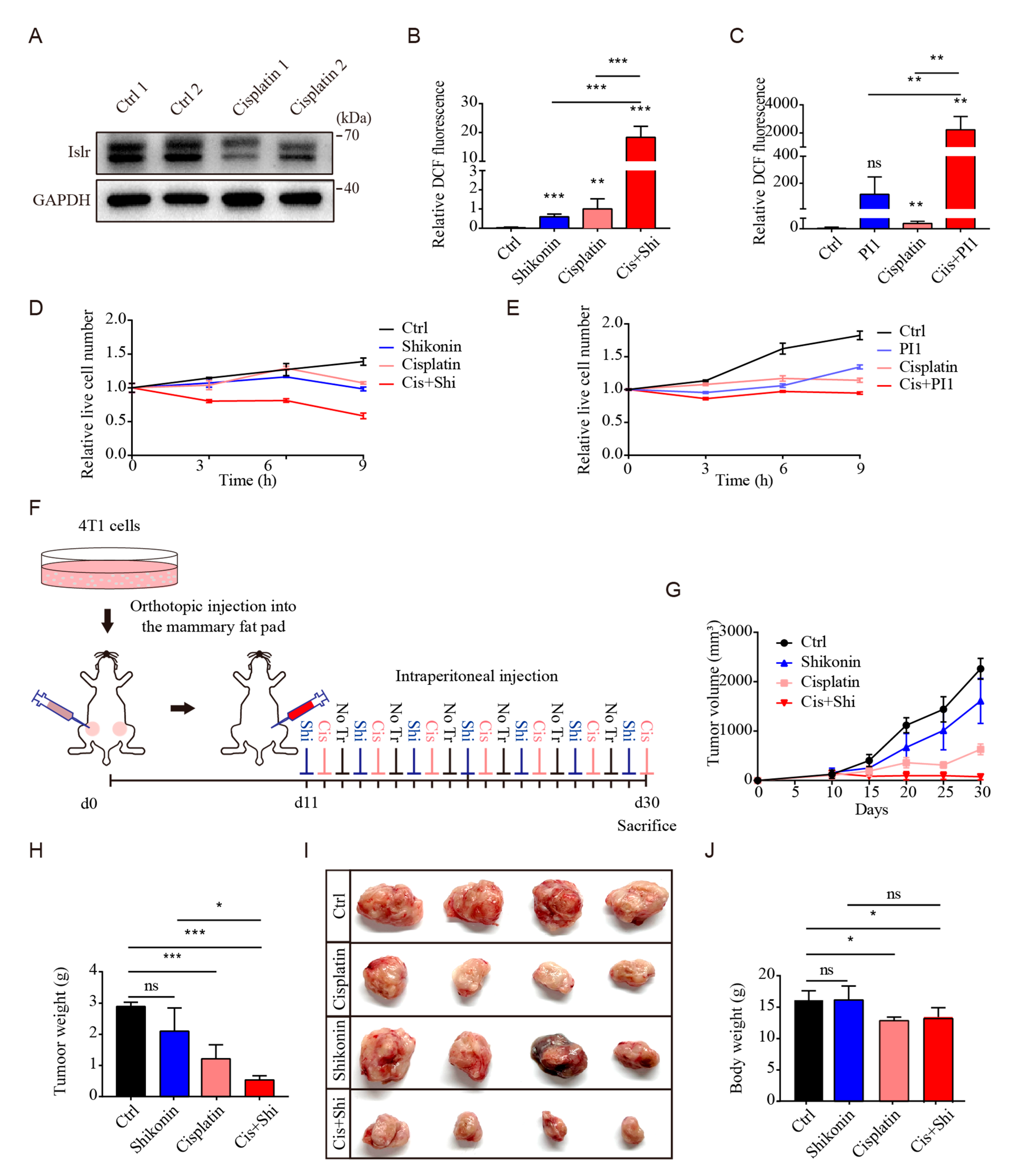
2.13. Triple-Negative Breast Cancer Mouse Model
2.14. Statistical Analysis
3. Results
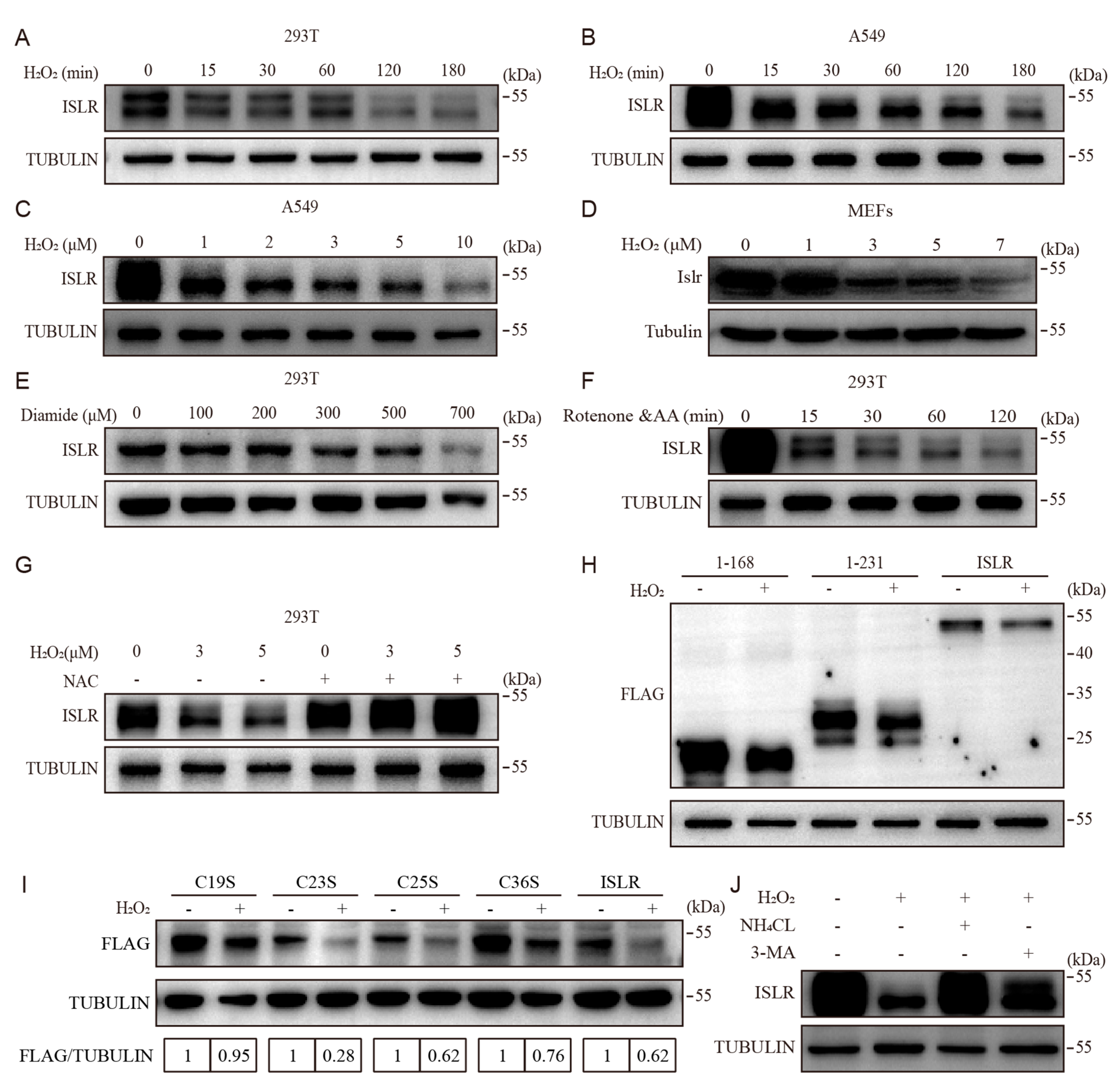
3.1. ISLR Is A Redox Sensor
3.2. Cys19 in ISLR Is Required for Oxidative-Stress-Induced ISLR Degradation
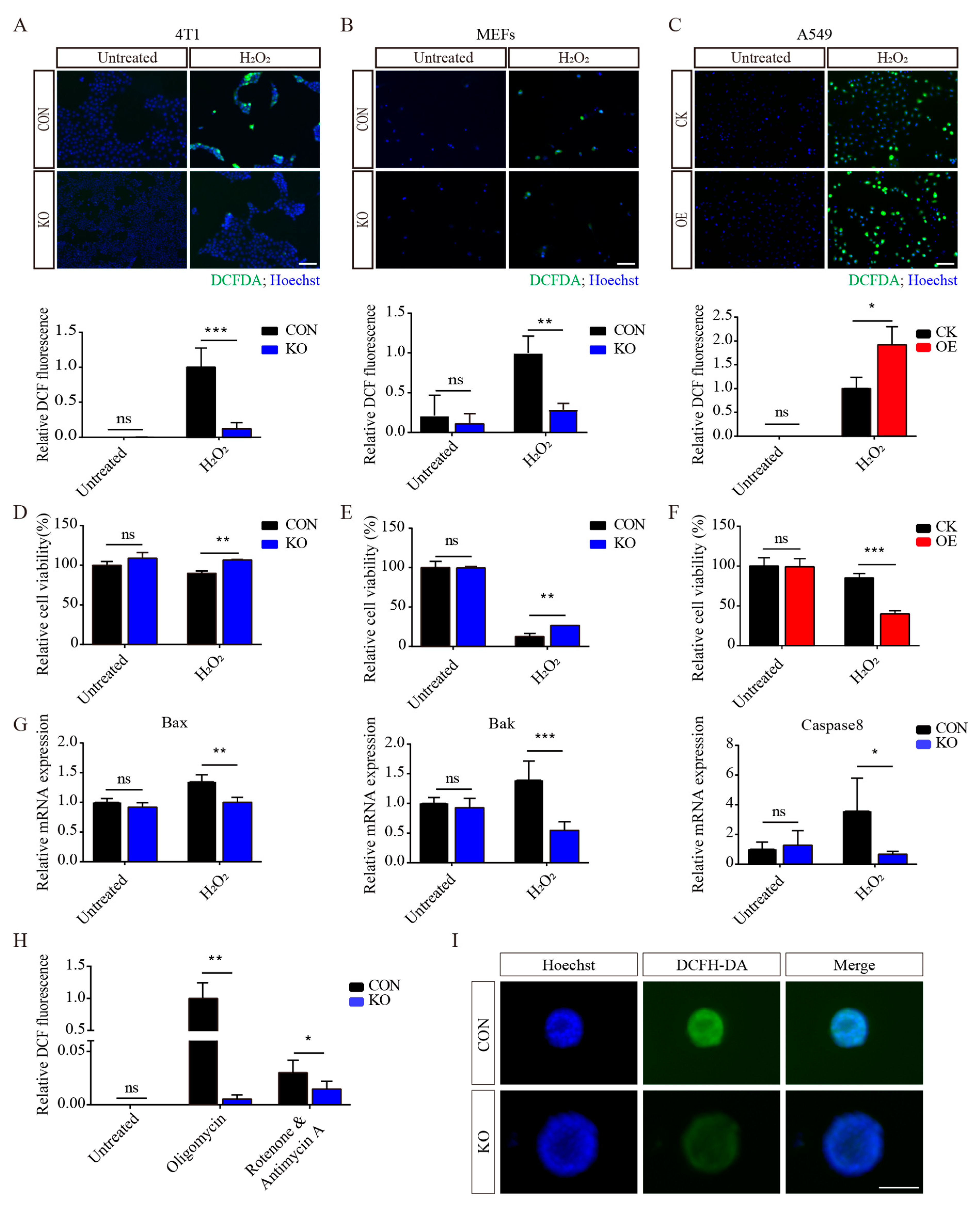
3.3. ISLR Deletion Increases Antioxidative Capacity
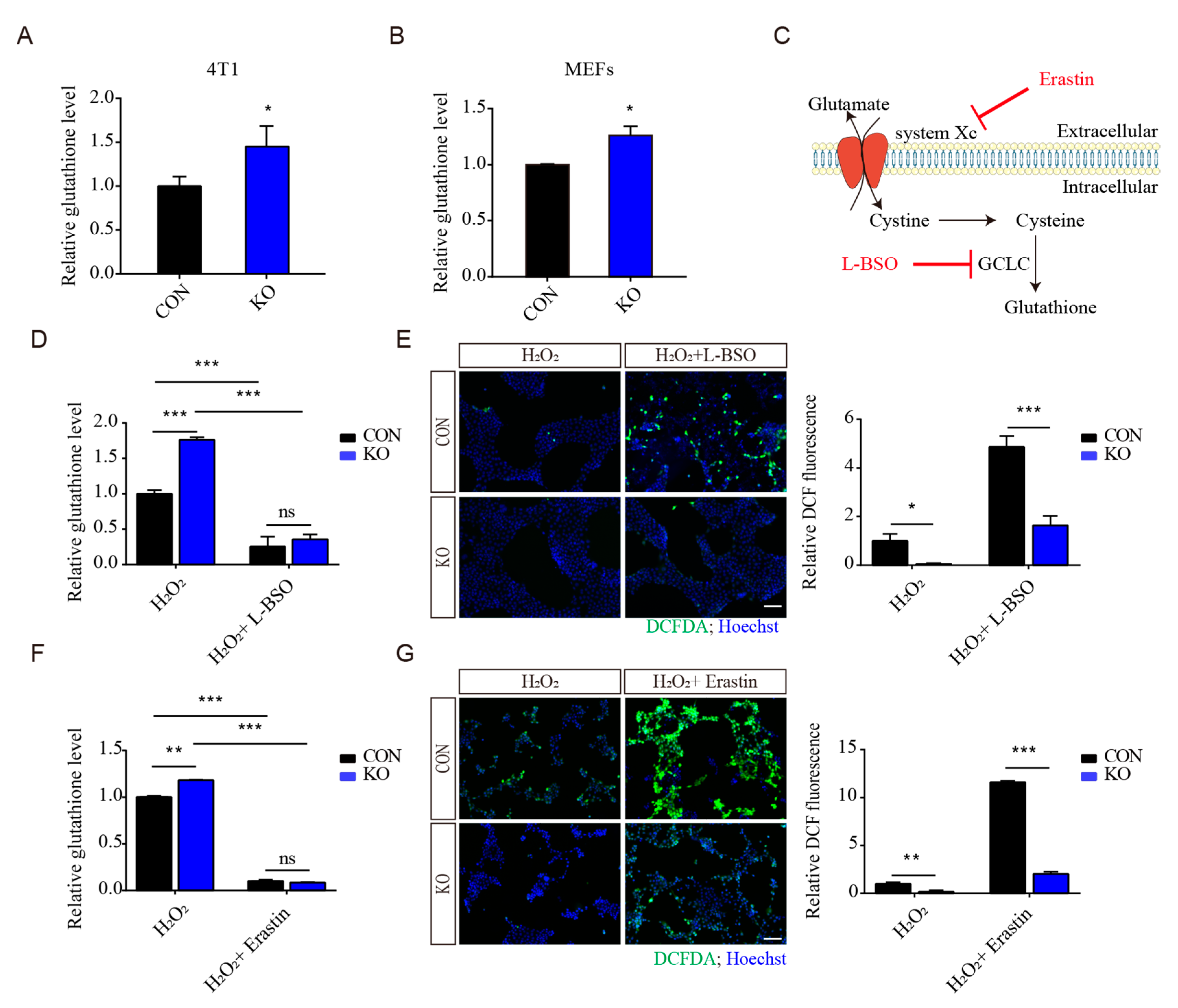
3.4. Regulation of Antioxidant Capacity by ISLR Deletion Is Not Mainly through GSH Production
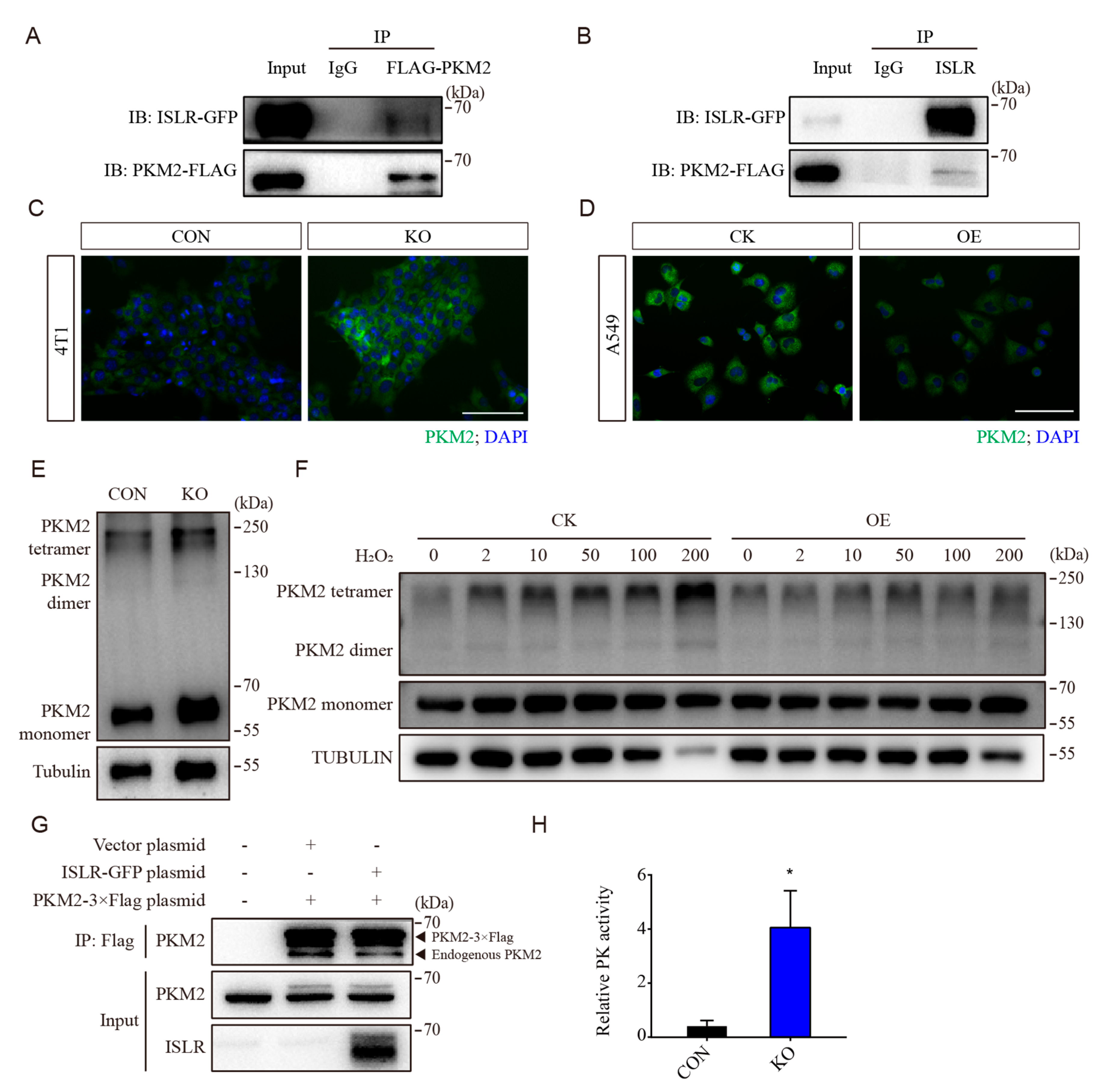
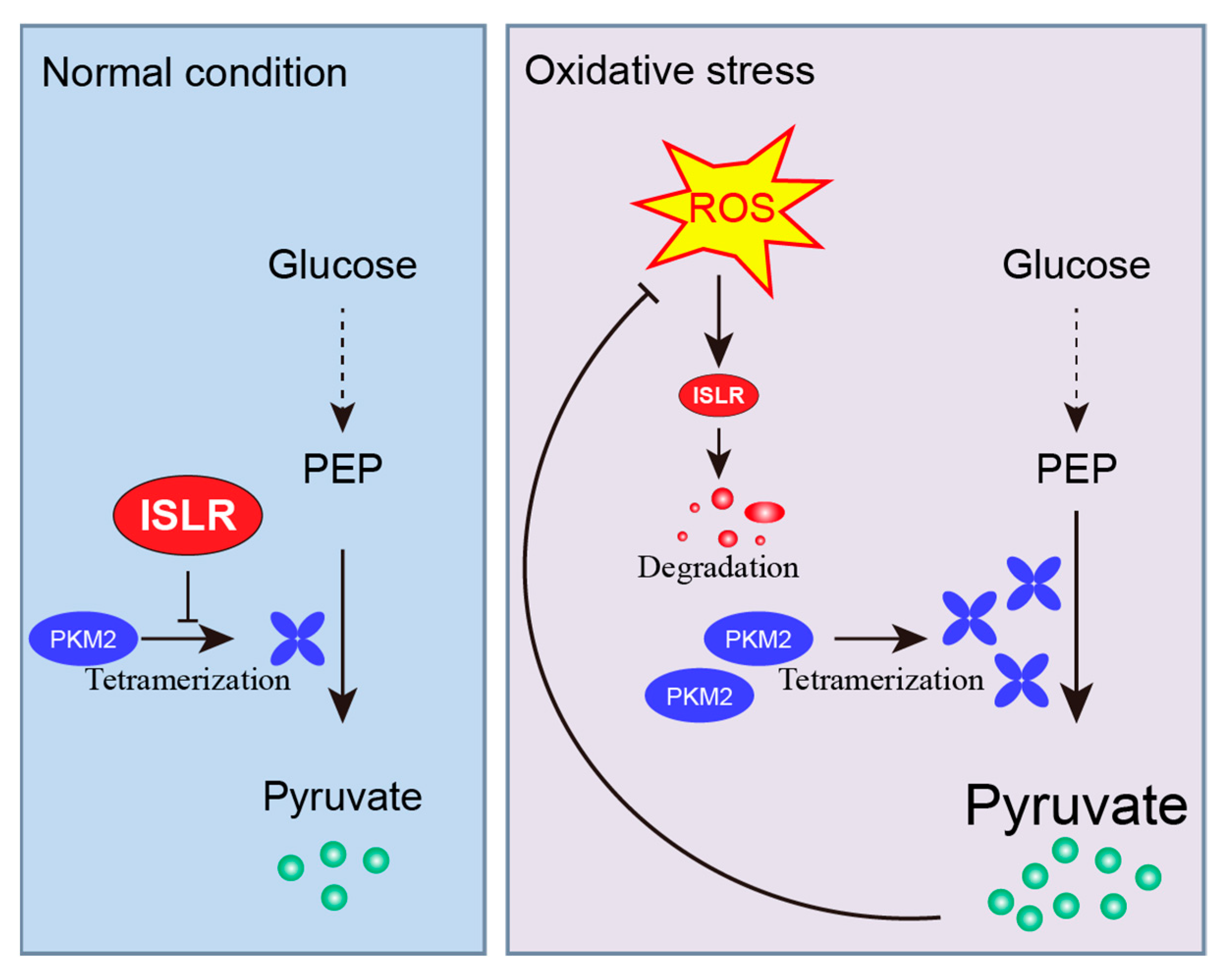
3.5. ISLR Interacted with PKM2 and Downregulates PKM2 Tetramerization
3.6. PKM2 Is Critical to ISLR-Mediated Antioxidative Effect
3.7. PKM2 Inhibition Sensitized Triple-Negative Breast Cancer to Cisplatin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mahar, R.; Merritt, M.E.; Denlinger, D.L.; Hahn, D.A. ROS and hypoxia signaling regulate periodic metabolic arousal during insect dormancy to coordinate glucose, amino acid, and lipid metabolism. Proc. Natl. Acad. Sci. USA 2021, 118, e2017603118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, S.; Liao, L.; Li, Y.; Li, H.; Li, Z.; Lin, L.; Wan, X.; Yang, X.; Chen, L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat. Commun. 2020, 11, 348. [Google Scholar] [CrossRef]
- Nagasawa, A.; Kubota, R.; Imamura, Y.; Nagamine, K.; Wang, Y.M.; Asakawa, S.; Kudoh, J.; Minoshima, S.; Mashima, Y.; Oguchi, Y.; et al. Cloning of the cDNA for a new member of the immunoglobulin superfamily (ISLR) containing leucine-rich repeat (LRR). Genomics 1997, 44, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Kudoh, J.; Noda, S.; Mashima, Y.; Wright, A.; Oguchi, Y.; Shimizu, N. Human and mouse ISLR (immunoglobulin superfamily containing leucine-rich repeat) genes: Genomic structure and tissue expression. Genomics 1999, 61, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Gu, L.; Lan, M.; Liu, C.; Wang, M.; Su, Y.; Ge, M.; Wang, T.; Yu, Y.; et al. Islr regulates canonical Wnt signaling-mediated skeletal muscle regeneration by stabilizing Dishevelled-2 and preventing autophagy. Nat. Commun. 2018, 9, 5129. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Jones, J.M.; McGehee, R.E.; Rando, T.A.; Lecka-Czernik, B.; Lipschitz, D.A.; Peterson, C.A. Activation of an adipogenic program in adult myoblasts with age. Mech. Ageing Dev. 2002, 123, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Wang, T.; Su, Y.; Li, L.; Lan, M.; Yu, Y.; Liu, F.; Xiong, L.; Wang, K.; et al. Immunoglobulin Superfamily Containing Leucine-Rich Repeat (Islr) Participates in IL-6-Mediated Crosstalk between Muscle and Brown Adipose Tissue to Regulate Energy Homeostasis. Int. J. Mol. Sci. 2022, 23, 10008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Z.; Yang, G. Silencing of ISLR inhibits tumour progression and glycolysis by inactivating the IL-6/JAK/STAT3 pathway in non-small cell lung cancer. Int. J. Mol. Med. 2021, 48, 222. [Google Scholar] [CrossRef]
- Maeda, K.; Enomoto, A.; Hara, A.; Asai, N.; Kobayashi, T.; Horinouchi, A.; Maruyama, S.; Ishikawa, Y.; Nishiyama, T.; Kiyoi, H.; et al. Identification of Meflin as a potential marker for mesenchymal stromal Cells. Sci. Rep. 2016, 6, 22288. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.M.; Kleeberger, S.R.; Yamamoto, M.; Kensler, T.W.; Scollick, C.; Biswal, S.; Reddy, S.P. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genom. 2007, 32, 74–81. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kirchner, P.; Bug, M.; Grum, D.; Koerver, L.; Schulze, N.; Poehler, R.; Dressler, A.; Fengler, S.; Arhzaouy, K.; et al. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J. 2017, 36, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Kobayashi, H.; Asai, N.; Saito, S.; Higuchi, T.; Kato, K.; Okumura, T.; Bando, Y.K.; Takefuji, M.; Mizutani, Y.; et al. Roles of the mesenchymal stromal/stem cell marker Meflin in cardiac tissue repair and the development of diastolic dysfunction. Circ. Res. 2019, 125, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Brune, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Nakamura, T.; Moriizumi, H.; Miki, H.; Takekawa, M. Stress-responsive MTK1 SAPKKK serves as a redox sensor that mediates delayed and sustained activation of SAPKs by oxidative stress. Sci. Adv. 2020, 6, 9778. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Xu, C.-J. Astrocytes autophagy in aging and neurodegenerative disorders. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 122, 109691. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.C.; Hung, W.C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From cellular metabolism, transcriptional regulation to extracellular signaling. Mol. Cancer 2018, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Enright, H.; Nutter, L.; Fischereder, M.; Zou, J.N.; Hebbel, R.P. Effect of pyruvate on oxidant injury to isolated and cellular DNA. Kidney Int. 1994, 45, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, N.; Hinoi, E.; Tsuchihashi, Y.; Fujimori, S.; Iemata, M.; Takarada, T.; Yoneda, Y. Cytoprotection by pyruvate through an anti-oxidative mechanism in cultured rat calvarial osteoblasts. Histol. Histopathol. 2006, 21, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Terazawa, R.; Kojima, K.; Nakane, K.; Deguchi, T.; Ando, M.; Tsukamasa, Y.; Ito, M.; Nozawa, Y. Cisplatin induces production of reactive oxygen species via NADPH oxidase activation in human prostate cancer cells. Free Radic. Res. 2011, 45, 1033–1039. [Google Scholar] [CrossRef]
- Liu, V.M.; Howell, A.J.; Hosios, A.M.; Li, Z.; Israelsen, W.J.; Vander Heiden, M.G. Cancer-associated mutations in human pyruvate kinase M2 impair enzyme activity. FEBS Lett. 2020, 594, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Dayton, T.L.; Jacks, T.; Vander Heiden, M.G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016, 17, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Guo, Y.; Zhang, X.; Liu, H.; Yin, M.; Chen, X.; Peng, C. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021, 503, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lu, M.; Mei, M.; Wang, H.; Han, Z.; Chen, M.; Yao, H.; Song, N.; Ding, X.; Ding, J.; et al. Pyridoxine induces glutathione synthesis via PKM2-mediated Nrf2 transactivation and confers neuroprotection. Nat. Commun. 2020, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, C.R.; Li, W.H.; Li, W.X. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008, 18, 879–888. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell-Tormey, J.; Nathan, C.F.; Lanks, K.; DeBoer, C.J.; de la Harpe, J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 1987, 165, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.Y. Reaction rate of pyruvate and hydrogen peroxide: Assessing antioxidant capacity of pyruvate under biological conditions. Sci. Rep 2019, 9, 19568. [Google Scholar] [CrossRef]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A.; et al. Acetate Production from Glucose and Coupling to Mitochondrial Metabolism in Mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Li, J.; Jin, M.; Yang, H.; Huang, G. Shikonin inhibited glycolysis and sensitized cisplatin treatment in non-small cell lung cancer cells via the exosomal pyruvate kinase M2 pathway. Bioengineered 2022, 13, 13906–13918. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Wu, X.-R. Inhibition of Pyruvate Kinase M2 Markedly Reduces Chemoresistance of Advanced Bladder Cancer to Cisplatin. Sci. Rep. 2017, 7, 45983. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, M.; Su, Y.; Zhang, Y.; Liu, C.; Lan, M.; Li, L.; Liu, F.; Li, N.; Yu, Y.; et al. Immunoglobulin Superfamily Containing Leucine-Rich Repeat (ISLR) Serves as a Redox Sensor That Modulates Antioxidant Capacity by Suppressing Pyruvate Kinase Isozyme M2 Activity. Cells 2024, 13, 838. https://doi.org/10.3390/cells13100838
Wang T, Chen M, Su Y, Zhang Y, Liu C, Lan M, Li L, Liu F, Li N, Yu Y, et al. Immunoglobulin Superfamily Containing Leucine-Rich Repeat (ISLR) Serves as a Redox Sensor That Modulates Antioxidant Capacity by Suppressing Pyruvate Kinase Isozyme M2 Activity. Cells. 2024; 13(10):838. https://doi.org/10.3390/cells13100838
Chicago/Turabian StyleWang, Tongtong, Meijing Chen, Yang Su, Yuying Zhang, Chang Liu, Miaomiao Lan, Lei Li, Fan Liu, Na Li, Yingying Yu, and et al. 2024. "Immunoglobulin Superfamily Containing Leucine-Rich Repeat (ISLR) Serves as a Redox Sensor That Modulates Antioxidant Capacity by Suppressing Pyruvate Kinase Isozyme M2 Activity" Cells 13, no. 10: 838. https://doi.org/10.3390/cells13100838





