Protein Quality Control of NKCC2 in Bartter Syndrome and Blood Pressure Regulation
Abstract
:1. Introduction
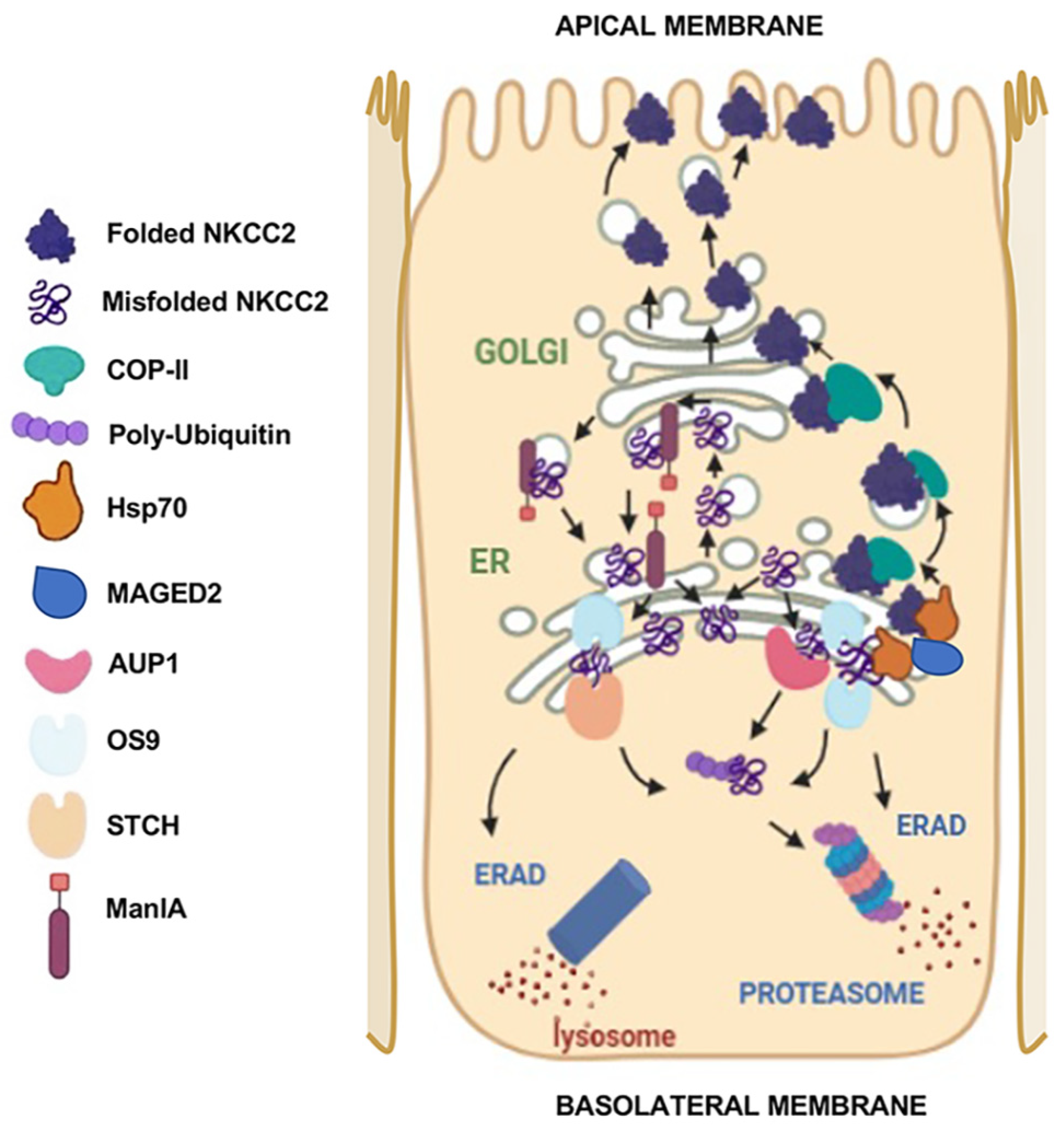
2. NKCC2 Expression in Mammalian Cells: It Is All about Protein Quality Control
3. Molecular Determinants of NKCC2 ER Export
3.1. Di-Leucine-like Motifs
3.2. Di-Acidic Codes
4. ERAD Components of NKCC2
4.1. Interaction with OS9 and AUP1
4.2. Interaction with STCH and Hsp70
4.3. Interaction with Golgi ManIA
5. Plasma Membrane Quality Control of NKCC2
6. Molecular Basis of Bartter Syndrome Type 1
6.1. Molecular Pathogenic Mechanisms of NKCC2 Mutations
6.2. Rescuing NKCC2 Mutants: Potential Strategies
7. Molecular Mechanisms of Bartter Syndrome Type 5
7.1. MAGE-D2: A Key Modulator of NKCC2 Biogenesis
7.2. Molecular Basis of the Transient Nature of BS Type 5
8. Phenotype Variability in Carriers of NKCC2 Mutations
9. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Gamba, G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 2005, 85, 423–493. [Google Scholar] [CrossRef]
- Delpire, E.; Rauchman, M.I.; Beier, D.R.; Hebert, S.C.; Gullans, S.R. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl− cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J. Biol. Chem. 1994, 269, 25677–25683. [Google Scholar] [CrossRef]
- Russell, J. Sodium-potassium-chloride cotransport. Physiol. Rev. 2000, 80, 211–276. [Google Scholar] [CrossRef]
- Mount, D.B. Thick ascending limb of the loop of Henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1974–1986. [Google Scholar] [CrossRef]
- Hennings, J.C.; Andrini, O.; Picard, N.; Paulais, M.; Huebner, A.K.; Cayuqueo, I.K.; Bignon, Y.; Keck, M.; Cornière, N.; Böhm, D.; et al. The ClC-K2 Chloride Channel Is Critical for Salt Handling in the Distal Nephron. J. Am. Soc. Nephrol. 2017, 28, 209–217. [Google Scholar] [CrossRef]
- Sands, J.M.; Layton, H.E. The physiology of urinary concentration: An update. Semin. Nephrol. 2009, 29, 178–195. [Google Scholar] [CrossRef]
- Simon, D.B.; Karet, F.E.; Hamdan, J.M.; DiPietro, A.; Sanjad, S.A.; Lifton, R.P. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Genet. 1996, 13, 183–188. [Google Scholar] [CrossRef]
- Aviv, A.; Hollenberg, N.K.; Weder, A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 2004, 43, 707–713. [Google Scholar] [CrossRef]
- Trudu, M.; Janas, S.; Lanzani, C.; Debaix, H.; Schaeffer, C.; Ikehata, M.; Citterio, L.; Demaretz, S.; Trevisani, F.; Ristagno, G.; et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat. Med. 2013, 19, 1655–1660. [Google Scholar] [CrossRef]
- Gamba, G. Molecular biology of distal nephron sodium transport mechanisms. Kidney Int. 1999, 56, 1606–1622. [Google Scholar] [CrossRef]
- Castrop, H.; Schiessl, I.M. Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2). Am. J. Physiol. Renal Physiol. 2014, 307, F991–F1002. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, I.M.; Castrop, H. Regulation of NKCC2 splicing and phosphorylation. Curr. Opin. Nephrol. Hypertens. 2015, 24, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G. Regulation of NKCC2 activity by SPAK truncated isoforms. Am. J. Physiol. Renal Physiol. 2014, 306, F49–F50. [Google Scholar] [CrossRef] [PubMed]
- Caceres, P.S.; Ortiz, P.A. Molecular regulation of NKCC2 in blood pressure control and hypertension. Curr. Opin. Nephrol. Hypertens. 2019, 28, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R.; Caceres, P.S.; Ortiz, P.A. Molecular regulation of NKCC2 in the thick ascending limb. Am. J. Physiol. Renal Physiol. 2011, 301, F1143–F1159. [Google Scholar] [CrossRef] [PubMed]
- Carmosino, M.; Procino, G.; Svelto, M. Na+-K+-2Cl− cotransporter type 2 trafficking and activity: The role of interacting proteins. Biol. Cell 2012, 104, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hebert, S.C.; Gamba, G. Molecular cloning and characterization of the renal diuretic-sensitive electroneutral sodium-(potassium)-chloride cotransporters. Clin. Investig. 1994, 72, 692–694. [Google Scholar] [CrossRef]
- Payne, J.A.; Forbush, B., 3rd. Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differentially distributed within the rabbit kidney. Proc. Natl. Acad. Sci. USA 1994, 91, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, P.; Vanden Heuvel, G.B.; Payne, J.A.; Forbush, B., 3rd. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am. J. Physiol. 1995, 269, F405–F418. [Google Scholar]
- Marcoux, A.A.; Tremblay, L.E.; Slimani, S.; Fiola, M.J.; Mac-Way, F.; Garneau, A.P.; Isenring, P. Molecular characteristics and physiological roles of Na(+)-K(+)-Cl(−) cotransporter 2. J. Cell Physiol. 2021, 236, 1712–1729. [Google Scholar] [CrossRef]
- Castrop, H.; Schnermann, J. Isoforms of renal Na-K-2Cl cotransporter NKCC2: Expression and functional significance. Am. J. Physiol. Renal Physiol. 2008, 295, F859–F866. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Izumi, Y.; Inoue, T.; Inoue, H.; Nakayama, Y.; Uematsu, T.; Fukuyama, T.; Yamazaki, T.; Yasuoka, Y.; Makino, T.; et al. Expression of three isoforms of Na-K-2Cl cotransporter (NKCC2) in the kidney and regulation by dehydration. Biochem. Biophys. Res. Commun. 2014, 453, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Chernavvsky, D.R.; Gomez, R.A.; Igarashi, P.; Gitelman, H.J.; Smithies, O. Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc. Natl. Acad. Sci. USA 2000, 97, 5434–5439. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, M.; Mizel, D.; Kim, S.M.; Chen, L.; Faulhaber-Walter, R.; Huang, Y.; Li, C.; Deng, C.; Briggs, J.; Schnermann, J.; et al. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J. Am. Soc. Nephrol. 2007, 18, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, M.; Mizel, D.; Huang, G.; Li, C.; Deng, C.; Theilig, F.; Bachmann, S.; Briggs, J.; Schnermann, J.; Castrop, H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J. Am. Soc. Nephrol. 2006, 17, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.B. Membrane trafficking and the regulation of NKCC2. Am. J. Physiol. Renal Physiol. 2006, 290, F606–F607. [Google Scholar] [CrossRef]
- Isenring, P.; Jacoby, S.C.; Forbush, B., 3rd. The role of transmembrane domain 2 in cation transport by the Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. USA 1998, 95, 7179–7184. [Google Scholar] [CrossRef] [PubMed]
- Isenring, P.; Jacoby, S.C.; Payne, J.A.; Forbush, B., 3rd. Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-L-Cl cotransporter. J. Biol. Chem. 1998, 273, 11295–11301. [Google Scholar] [CrossRef] [PubMed]
- Mikalsen, T.; Johannessen, M.; Moens, U. Sequence- and position-dependent tagging protects extracellular-regulated kinase 3 protein from 26S proteasome-mediated degradation. Int. J. Biochem. Cell Biol. 2005, 37, 2513–2520. [Google Scholar] [CrossRef]
- Fajerman, I.; Schwartz, A.L.; Ciechanover, A. Degradation of the Id2 developmental regulator: Targeting via N-terminal ubiquitination. Biochem. Biophys. Res. Commun. 2004, 314, 505–512. [Google Scholar] [CrossRef]
- Breitschopf, K.; Bengal, E.; Ziv, T.; Admon, A.; Ciechanover, A. A novel site for ubiquitination: The N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998, 17, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Benziane, B.; Demaretz, S.; Defontaine, N.; Zaarour, N.; Cheval, L.; Bourgeois, S.; Klein, C.; Froissart, M.; Blanchard, A.; Paillard, M.; et al. NKCC2 surface expression in mammalian cells: Down-regulation by novel interaction with aldolase B. J. Biol. Chem. 2007, 282, 33817–33830. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, N.; Demaretz, S.; Defontaine, N.; Mordasini, D.; Laghmani, K. A highly conserved motif at the COOH terminus dictates endoplasmic reticulum exit and cell surface expression of NKCC2. J. Biol. Chem. 2009, 284, 21752–21764. [Google Scholar] [CrossRef]
- Zaarour, N.; Defontaine, N.; Demaretz, S.; Azroyan, A.; Cheval, L.; Laghmani, K. Secretory carrier membrane protein 2 regulates exocytic insertion of NKCC2 into the cell membrane. J. Biol. Chem. 2011, 286, 9489–9502. [Google Scholar] [CrossRef] [PubMed]
- Delisle, B.P.; Anson, B.D.; Rajamani, S.; January, C.T. Biology of cardiac arrhythmias: Ion channel protein trafficking. Circ. Res. 2004, 94, 1418–1428. [Google Scholar] [CrossRef]
- Ma, D.; Zerangue, N.; Lin, Y.F.; Collins, A.; Yu, M.; Jan, Y.N.; Jan, L.Y. Role of ER export signals in controlling surface potassium channel numbers. Science 2001, 291, 316–319. [Google Scholar] [CrossRef]
- Haardt, M.; Benharouga, M.; Lechardeur, D.; Kartner, N.; Lukacs, G.L. C-terminal Truncations Destabilize the Cystic Fibrosis Transmembrane Conductance Regulator without Impairing Its Biogenesis: A NOVEL CLASS OF MUTATION*. J. Biol. Chem. 1999, 274, 21873–21877. [Google Scholar] [CrossRef] [PubMed]
- Theos, A.C.; Berson, J.F.; Theos, S.C.; Herman, K.E.; Harper, D.C.; Tenza, D.; Sviderskaya, E.V.; Lamoreux, M.L.; Bennett, D.C.; Raposo, G.; et al. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 2006, 17, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, N.; Demaretz, S.; Defontaine, N.; Zhu, Y.; Laghmani, K. Multiple evolutionarily conserved Di-leucine like motifs in the carboxyl terminus control the anterograde trafficking of NKCC2. J. Biol. Chem. 2012, 287, 42642–42653. [Google Scholar] [CrossRef]
- Delpire, E.; Mount, D.B. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 2002, 64, 803–843. [Google Scholar] [CrossRef]
- Delpire, E. Cation-Chloride Cotransporters in Neuronal Communication. News. Physiol. Sci. 2000, 15, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Bakhos-Douaihy, D.; Seaayfan, E.; Demaretz, S.; Komhoff, M.; Laghmani, K. Differential Effects of STCH and Stress-Inducible Hsp70 on the Stability and Maturation of NKCC2. Int. J. Mol. Sci. 2021, 22, 2207. [Google Scholar] [CrossRef] [PubMed]
- Demaretz, S.; Seaayfan, E.; Bakhos-Douaihy, D.; Frachon, N.; Kömhoff, M.; Laghmani, K. Golgi Alpha1,2-Mannosidase IA Promotes Efficient Endoplasmic Reticulum-Associated Degradation of NKCC2. Cells 2022, 11, 101. [Google Scholar] [CrossRef]
- Frachon, N.; Demaretz, S.; Seaayfan, E.; Chelbi, L.; Bakhos-Douaihy, D.; Laghmani, K. AUP1 Regulates the Endoplasmic Reticulum-Associated Degradation and Polyubiquitination of NKCC2. Cells 2024, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Seaayfan, E.; Defontaine, N.; Demaretz, S.; Zaarour, N.; Laghmani, K. OS9 Protein Interacts with Na-K-2Cl Co-transporter (NKCC2) and Targets Its Immature Form for the Endoplasmic Reticulum-associated Degradation Pathway. J. Biol. Chem. 2016, 291, 4487–4502. [Google Scholar] [CrossRef] [PubMed]
- Nezu, A.; Parvin, M.N.; Turner, R.J. A Conserved Hydrophobic Tetrad near the C Terminus of the Secretory Na+-K+-2Cl- Cotransporter (NKCC1) Is Required for Its Correct Intracellular Processing. J. Biol. Chem. 2009, 284, 6869–6876. [Google Scholar] [CrossRef]
- Bakhos-Douaihy, D.; Seaayfan, E.; Frachon, N.; Demaretz, S.; Kömhoff, M.; Laghmani, K. Diacidic Motifs in the Carboxyl Terminus Are Required for ER Exit and Translocation to the Plasma Membrane of NKCC2. Int. J. Mol. Sci. 2022, 23, 12761. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Ruggiano, A.; Foresti, O.; Carvalho, P. Quality control: ER-associated degradation: Protein quality control and beyond. J. Cell Biol. 2014, 204, 869–879. [Google Scholar] [CrossRef]
- Kotler, J.L.M.; Street, T.O. Mechanisms of Protein Quality Control in the Endoplasmic Reticulum by a Coordinated Hsp40-Hsp70-Hsp90 System. Annu. Rev. Biophys. 2023, 52, 509–524. [Google Scholar] [CrossRef]
- Staub, O.; Gautschi, I.; Ishikawa, T.; Breitschopf, K.; Ciechanover, A.; Schild, L.; Rotin, D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997, 16, 6325–6336. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.J.; Loo, M.A.; Pind, S.; Williams, D.B.; Goldberg, A.L.; Riordan, J.R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 1995, 83, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Klemm, E.J.; Spooner, E.; Ploegh, H.L. Dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and endoplasmic reticulum (ER) protein quality control. J. Biol. Chem. 2011, 286, 37602–37614. [Google Scholar] [CrossRef] [PubMed]
- Litovchick, L.; Friedmann, E.; Shaltiel, S. A selective interaction between OS-9 and the carboxyl-terminal tail of meprin beta. J. Biol. Chem. 2002, 277, 34413–34423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Gaiser, S.; Köttgen, M.; Kramer-Zucker, A.; Walz, G.; Wegierski, T. OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J. Biol. Chem. 2007, 282, 36561–36570. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.; Eleveld-Trancikova, D.; Sanecka, A.; van Hout-Kuijer, M.; Hendriks, I.A.; Looman, M.G.; Leusen, J.H.; Adema, G.J. OS9 interacts with DC-STAMP and modulates its intracellular localization in response to TLR ligation. Mol. Immunol. 2009, 46, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Alcock, F.; Swanton, E. Mammalian OS-9 is upregulated in response to endoplasmic reticulum stress and facilitates ubiquitination of misfolded glycoproteins. J. Mol. Biol. 2009, 385, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, A.; Thiele, C. Monotopic topology is required for lipid droplet targeting of ancient ubiquitous protein 1[S]. J. Lipid Res. 2013, 54, 503–513. [Google Scholar] [CrossRef]
- Bernasconi, R.; Pertel, T.; Luban, J.; Molinari, M. A dual task for the Xbp1-responsive OS-9 variants in the mammalian endoplasmic reticulum: Inhibiting secretion of misfolded protein conformers and enhancing their disposal. J. Biol. Chem. 2008, 283, 16446–16454. [Google Scholar] [CrossRef]
- Su, R.; Yin, J.; Ruan, X.; Chen, Y.; Wan, P.; Luo, Z. Featured interactome of homocysteine-inducible endoplasmic reticulum protein uncovers novel binding partners in response to ER stress. Comput. Struct. Biotechnol. J. 2023, 21, 4478–4487. [Google Scholar] [CrossRef]
- Mori, T.; Cowley, A.W., Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 2004, 43, 341–346. [Google Scholar] [CrossRef]
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Lai, G.; Yang, X.; Wang, L.; Zhao, Y. Synergistical effect of 20-HETE and high salt on NKCC2 protein and blood pressure via ubiquitin-proteasome pathway. Hum. Genet. 2012, 132, 179–187. [Google Scholar] [CrossRef]
- Tian, Y.; Riazi, S.; Khan, O.; Klein, J.D.; Sugimura, Y.; Verbalis, J.G.; Ecelbarger, C.A. Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 x Brown Norway rats. Kidney Int. 2006, 69, 304–312. [Google Scholar] [CrossRef]
- Yamazaki, O.; Hirohama, D.; Ishizawa, K.; Shibata, S. Role of the Ubiquitin Proteasome System in the Regulation of Blood Pressure: A Review. Int. J. Mol. Sci. 2020, 21, 5358. [Google Scholar] [CrossRef]
- De Leonibus, C.; Cinque, L.; Settembre, C. Emerging lysosomal pathways for quality control at the endoplasmic reticulum. FEBS Lett. 2019, 593, 2319–2329. [Google Scholar] [CrossRef]
- Fregno, I.; Fasana, E.; Bergmann, T.J.; Raimondi, A.; Loi, M.; Solda, T.; Galli, C.; D’Antuono, R.; Morone, D.; Danieli, A.; et al. ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J. 2018, 37, e99259. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef]
- Donnelly, B.F.; Needham, P.G.; Snyder, A.C.; Roy, A.; Khadem, S.; Brodsky, J.L.; Subramanya, A.R. Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis. J. Biol. Chem. 2013, 288, 13124–13135. [Google Scholar] [CrossRef]
- Kim Chiaw, P.; Hantouche, C.; Wong, M.J.H.; Matthes, E.; Robert, R.; Hanrahan, J.W.; Shrier, A.; Young, J.C. Hsp70 and DNAJA2 limit CFTR levels through degradation. PLoS ONE 2019, 14, e0220984. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, J.H.; Lee, E.J.; Vo, T.T.; Choi, H.; Kim, J.Y.; Jang, J.K.; Wee, H.J.; Lee, H.S.; Jang, S.H.; et al. ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation. Nat. Commun. 2016, 7, 12882. [Google Scholar] [CrossRef]
- Ishizaka, N.; Aizawa, T.; Ohno, M.; Usui Si, S.; Mori, I.; Tang, S.S.; Ingelfinger, J.R.; Kimura, S.; Nagai, R. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension 2002, 39, 122–128. [Google Scholar] [CrossRef]
- Parra, G.; Quiroz, Y.; Salazar, J.; Bravo, Y.; Pons, H.; Chavez, M.; Johnson, R.J.; Rodriguez-Iturbe, B. Experimental induction of salt-sensitive hypertension is associated with lymphocyte proliferative response to HSP70: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008, 74, S55–S59. [Google Scholar] [CrossRef]
- Lins, B.B.; Casare, F.A.M.; Fontenele, F.F.; Gonçalves, G.L.; Oliveira-Souza, M. Long-Term Angiotensin II Infusion Induces Oxidative and Endoplasmic Reticulum Stress and Modulates Na+ Transporters Through the Nephron. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Tempel, W.; Karaveg, K.; Liu, Z.J.; Rose, J.; Wang, B.C.; Moremen, K.W. Structure of mouse Golgi alpha-mannosidase IA reveals the molecular basis for substrate specificity among class 1 (family 47 glycosylhydrolase) alpha1,2-mannosidases. J. Biol. Chem. 2004, 279, 29774–29786. [Google Scholar] [CrossRef]
- Bieberich, E.; Bause, E. Man9-mannosidase from human kidney is expressed in COS cells as a Golgi-resident type II transmembrane N-glycoprotein. Eur. J. Biochem. 1995, 233, 644–649. [Google Scholar] [CrossRef]
- Bieberich, E.; Treml, K.; Volker, C.; Rolfs, A.; Kalz-Fuller, B.; Bause, E. Man9-mannosidase from pig liver is a type-II membrane protein that resides in the endoplasmic reticulum. cDNA cloning and expression of the enzyme in COS 1 cells. Eur. J. Biochem. 1997, 246, 681–689. [Google Scholar] [CrossRef]
- Velasco, A.; Hendricks, L.; Moremen, K.W.; Tulsiani, D.R.; Touster, O.; Farquhar, M.G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J. Cell Biol. 1993, 122, 39–51. [Google Scholar] [CrossRef]
- Sun, Z.; Brodsky, J.L. Protein quality control in the secretory pathway. J. Cell Biol. 2019, 218, 3171–3187. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R. Ubiquitination of NKCC2 by the cullin-RING E3 ubiquitin ligase family in the thick ascending limb of the loop of Henle. Am. J. Physiol. Renal Physiol. 2023, 324, F315–F328. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.R. cGMP induces degradation of NKCC2 in the thick ascending limb via the ubiquitin-proteasomal system. Am. J. Physiol. Renal Physiol. 2019, 316, F838–F846. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.D.S.; Heilberg, I.P. Bartter syndrome: Causes, diagnosis, and treatment. Int. J. Nephrol. Renov. Dis. 2018, 11, 291–301. [Google Scholar] [CrossRef]
- Komhoff, M.; Laghmani, K. Pathophysiology of antenatal Bartter’s syndrome. Curr. Opin. Nephrol. Hypertens. 2017, 26, 419–425. [Google Scholar] [CrossRef]
- Shaukat, I.; Bakhos-Douaihy, D.; Zhu, Y.; Seaayfan, E.; Demaretz, S.; Frachon, N.; Weber, S.; Komhoff, M.; Vargas-Poussou, R.; Laghmani, K. New insights into the role of endoplasmic reticulum-associated degradation in Bartter Syndrome Type 1. Hum. Mutat. 2021, 42, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D.; Farinha, C.M. Rescuing mutant CFTR: A multi-task approach to a better outcome in treating cystic fibrosis. Curr. Pharm. Des. 2013, 19, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Hong-Brown, L.Q.; Biwersi, J.; Verkman, A.S.; Welch, W.J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1996, 1, 117–125. [Google Scholar] [CrossRef]
- Grove, D.E.; Rosser, M.F.; Ren, H.Y.; Naren, A.P.; Cyr, D.M. Mechanisms for rescue of correctable folding defects in CFTRDelta F508. Mol. Biol. Cell 2009, 20, 4059–4069. [Google Scholar] [CrossRef]
- Laghmani, K.; Beck, B.B.; Yang, S.S.; Seaayfan, E.; Wenzel, A.; Reusch, B.; Vitzthum, H.; Priem, D.; Demaretz, S.; Bergmann, K.; et al. Polyhydramnios, Transient Antenatal Bartter’s Syndrome, and MAGED2 Mutations. N. Engl. J. Med. 2016, 374, 1853–1863. [Google Scholar] [CrossRef]
- Komhoff, M.; Laghmani, K. MAGED2: A novel form of antenatal Bartter’s syndrome. Curr. Opin. Nephrol. Hypertens. 2018, 27, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Treard, C.; Roncelin, I.; Dreux, S.; Bertholet-Thomas, A.; Broux, F.; Bruno, D.; Decramer, S.; Deschenes, G.; Djeddi, D.; et al. Prevalence of Novel MAGED2 Mutations in Antenatal Bartter Syndrome. Clin. J. Am. Soc. Nephrol. 2018, 13, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Hu, Y.; Zhang, X.; Gao, X.; Zhao, Y.; Peng, H.; Ouyang, L.; Zhang, C. Identification of a novel intronic mutation of MAGED2 gene in a Chinese family with antenatal Bartter syndrome. BMC Med. Genom. 2024, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, M.; Zhou, Y.; Yao, F.; Wei, M.; Li, Z.; Qiu, Z. A novel MAGED2 variant in a Chinese preterm newborn with transient antenatal Bartter’s syndrome with 4 years follow-up. BMC Nephrol. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, C.; Zheng, W.; Cao, X.; Li, W.; Zhang, D.; Zhu, J.; Zhang, X.; Chen, Y. Proteomic Analysis Revealed the Potential Role of MAGE-D2 in the Therapeutic Targeting of Triple-Negative Breast Cancer. Mol. Cell Proteom. 2024, 23, 100703. [Google Scholar] [CrossRef]
- Ecelbarger, C.A.; Yu, S.; Lee, A.J.; Weinstein, L.S.; Knepper, M.A. Decreased renal Na-K-2Cl cotransporter abundance in mice with heterozygous disruption of the G(s)alpha gene. Am. J. Physiol. 1999, 277, F235–F244. [Google Scholar] [PubMed]
- de Jong, J.C.; Willems, P.H.; van den Heuvel, L.P.; Knoers, N.V.; Bindels, R.J. Functional expression of the human thiazide-sensitive NaCl cotransporter in Madin-Darby canine kidney cells. J. Am. Soc. Nephrol. 2003, 14, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Seaayfan, E.; Nasrah, S.; Quell, L.; Kleim, M.; Weber, S.; Meyer, H.; Laghmani, K.; Kömhoff, M. MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S. Cells 2022, 11, 2546. [Google Scholar] [CrossRef] [PubMed]
- Seaayfan, E.; Nasrah, S.; Quell, L.; Radi, A.; Kleim, M.; Schermuly, R.T.; Weber, S.; Laghmani, K.; Kömhoff, M. Reciprocal Regulation of MAGED2 and HIF-1α Augments Their Expression under Hypoxia: Role of cAMP and PKA Type II. Cells 2022, 11, 3424. [Google Scholar] [CrossRef]
- Bettinelli, A.; Ciarmatori, S.; Cesareo, L.; Tedeschi, S.; Ruffa, G.; Appiani, A.C.; Rosini, A.; Grumieri, G.; Mercuri, B.; Sacco, M.; et al. Phenotypic variability in Bartter syndrome type I. Pediatr. Nephrol. 2000, 14, 940–945. [Google Scholar] [CrossRef]
- Pressler, C.A.; Heinzinger, J.; Jeck, N.; Waldegger, P.; Pechmann, U.; Reinalter, S.; Konrad, M.; Beetz, R.; Seyberth, H.W.; Waldegger, S. Late-onset manifestation of antenatal Bartter syndrome as a result of residual function of the mutated renal Na+-K+-2Cl− co-transporter. J. Am. Soc. Nephrol. 2006, 17, 2136–2142. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nozu, K.; Narita, I.; Nagata, M.; Nozu, Y.; Fu, X.J.; Matsuo, M.; Iijima, K.; Gejyo, F. Atypical phenotype of type I Bartter syndrome accompanied by focal segmental glomerulosclerosis. Pediatr. Nephrol. 2009, 24, 415–418. [Google Scholar] [CrossRef]
- Ji, W.; Foo, J.N.; O’Roak, B.J.; Zhao, H.; Larson, M.G.; Simon, D.B.; Newton-Cheh, C.; State, M.W.; Levy, D.; Lifton, R.P. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat. Genet. 2008, 40, 592–599. [Google Scholar] [CrossRef]
- Monette, M.Y.; Rinehart, J.; Lifton, R.P.; Forbush, B. Rare mutations in the human Na-K-Cl cotransporter (NKCC2) associated with lower blood pressure exhibit impaired processing and transport function. Am. J. Physiol. Renal Physiol. 2011, 300, F840–F847. [Google Scholar] [CrossRef]
- Acuna, R.; Martinez-de-la-Maza, L.; Ponce-Coria, J.; Vazquez, N.; Ortal-Vite, P.; Pacheco-Alvarez, D.; Bobadilla, N.A.; Gamba, G. Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J. Hypertens. 2011, 29, 475–483. [Google Scholar] [CrossRef]
- Seaayfan, E.; Demaretz, S.; Defontaine, N.; Laghmani, K. Rare Mutations in NKCC2 Gene Associated with Protection From Hypertension Differentially Regulate NKCC2 Isoforms A and F. J. Am. Soc. Nephrol. 2014, 25, 660A. [Google Scholar]
- Eizirik, D.c.L.; Cardozo, A.K.; Cnop, M. The Role for Endoplasmic Reticulum Stress in Diabetes Mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamazaki, O.; Ishizawa, K.; Tamura, Y.; Wang, Q.; Ueno, M.; Hayama, Y.; Fujigaki, Y.; Shibata, S. Upregulation of renal Na–K–2Cl cotransporter 2 in obese diabetes mellitus via a vasopressin receptor 2-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 524, 710–715. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laghmani, K. Protein Quality Control of NKCC2 in Bartter Syndrome and Blood Pressure Regulation. Cells 2024, 13, 818. https://doi.org/10.3390/cells13100818
Laghmani K. Protein Quality Control of NKCC2 in Bartter Syndrome and Blood Pressure Regulation. Cells. 2024; 13(10):818. https://doi.org/10.3390/cells13100818
Chicago/Turabian StyleLaghmani, Kamel. 2024. "Protein Quality Control of NKCC2 in Bartter Syndrome and Blood Pressure Regulation" Cells 13, no. 10: 818. https://doi.org/10.3390/cells13100818




