Genetic Map Construction and Primary Quantitative Trait Locus Analysis of Low-Light-Stress-Related Traits in Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic Data Collection
2.3. DNA Extraction and SLAF-seq
2.4. Map Construction and QTL Analysis
2.5. Data Analysis
3. Results
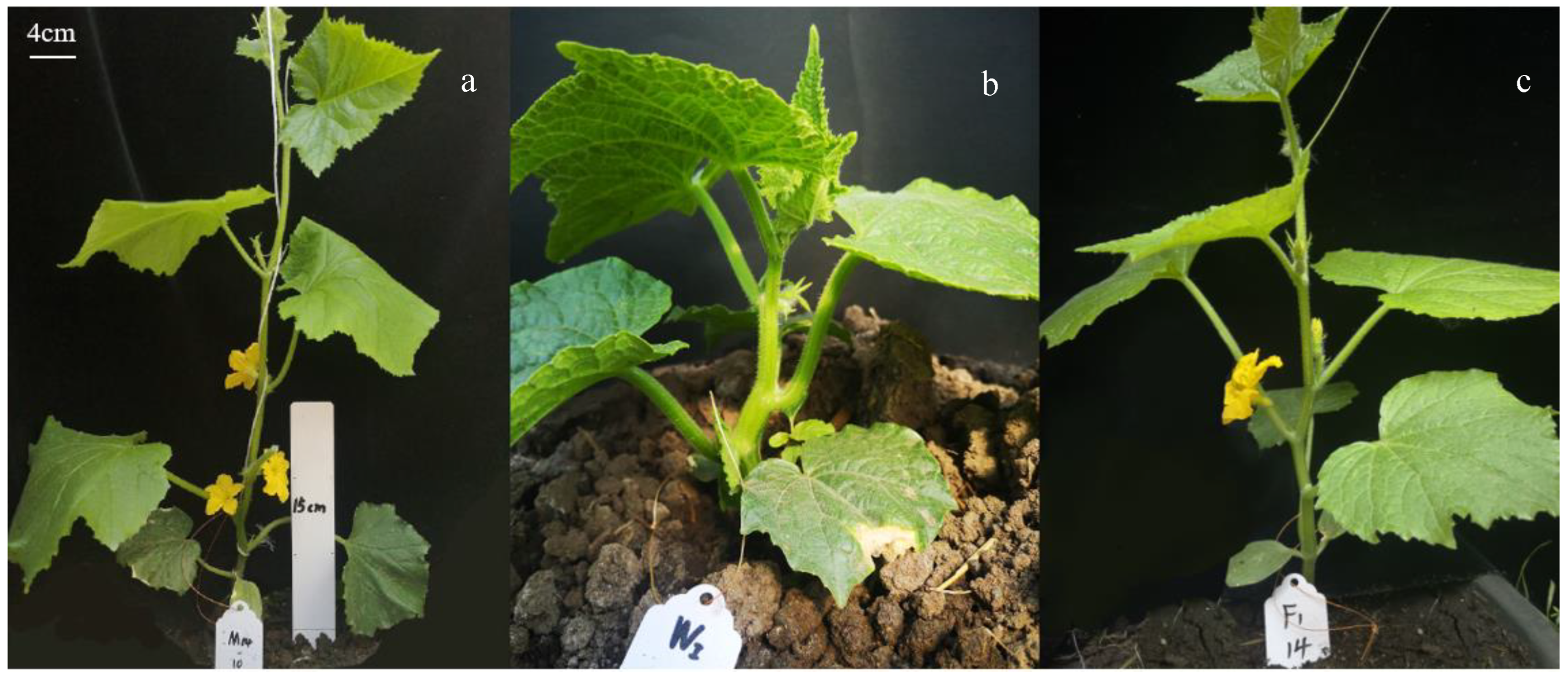
3.1. Phenotypic Characteristics of Parents of Different Tolerance and F1 under Low-Light Stress
3.2. Phenotypic Analysis of an F2 Isolated Population under Low Light
3.3. Quantity Analysis of SLAF-seq
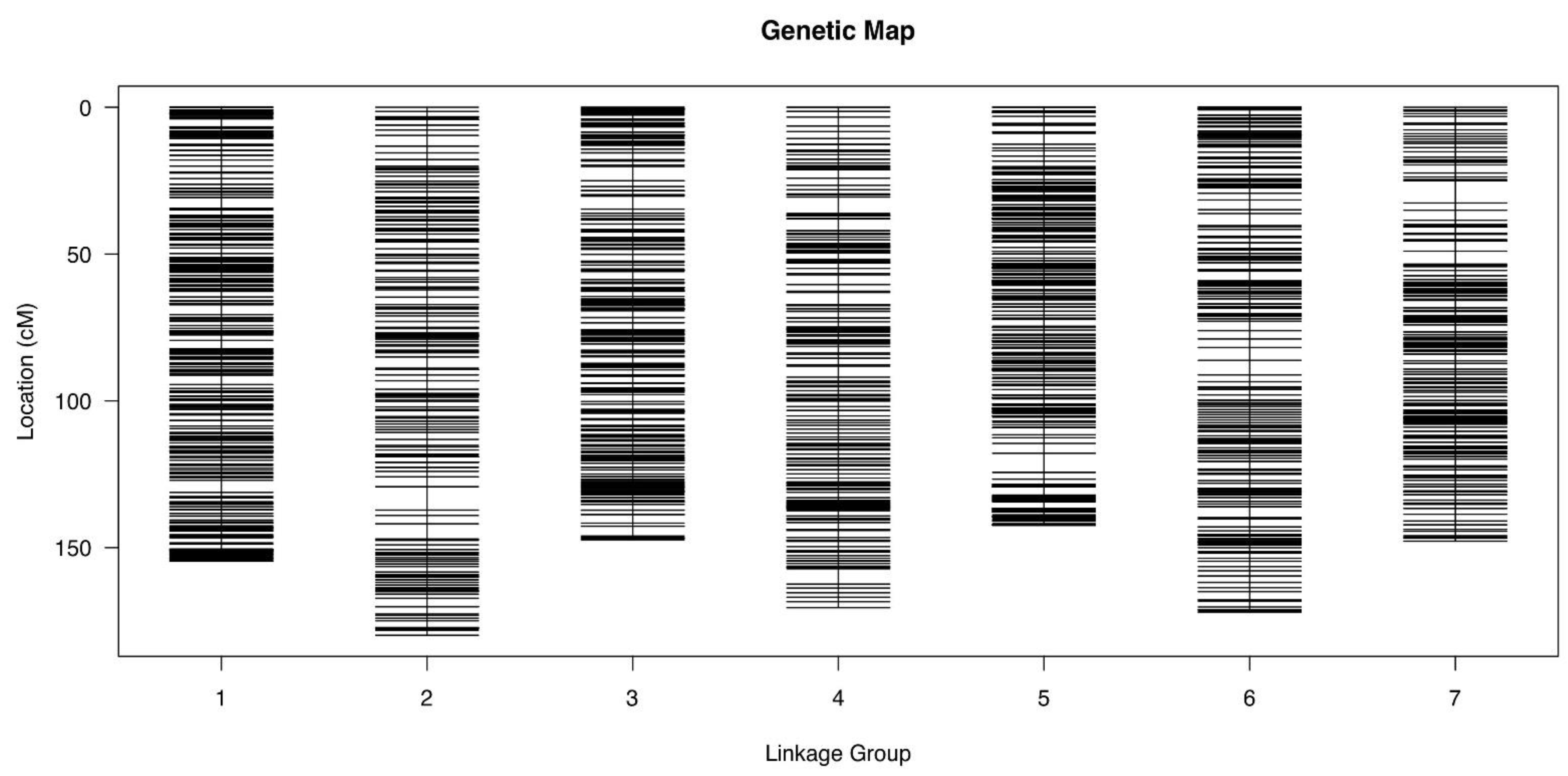
3.4. Marker Development and Construction of SLAF Genetic Map
3.5. QTL Analysis
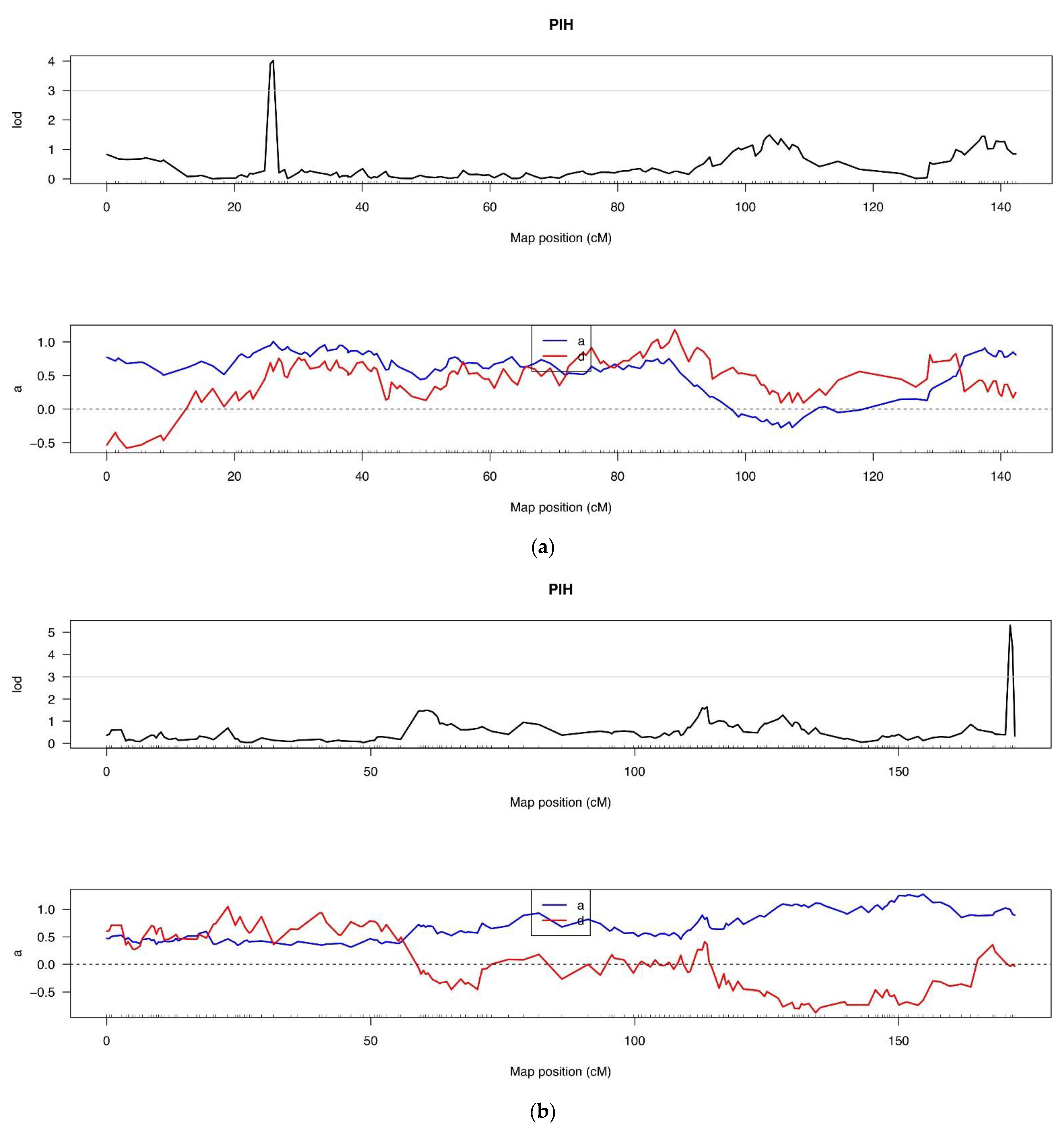
3.5.1. QTL Analysis of Plant Height under Low-Light Conditions
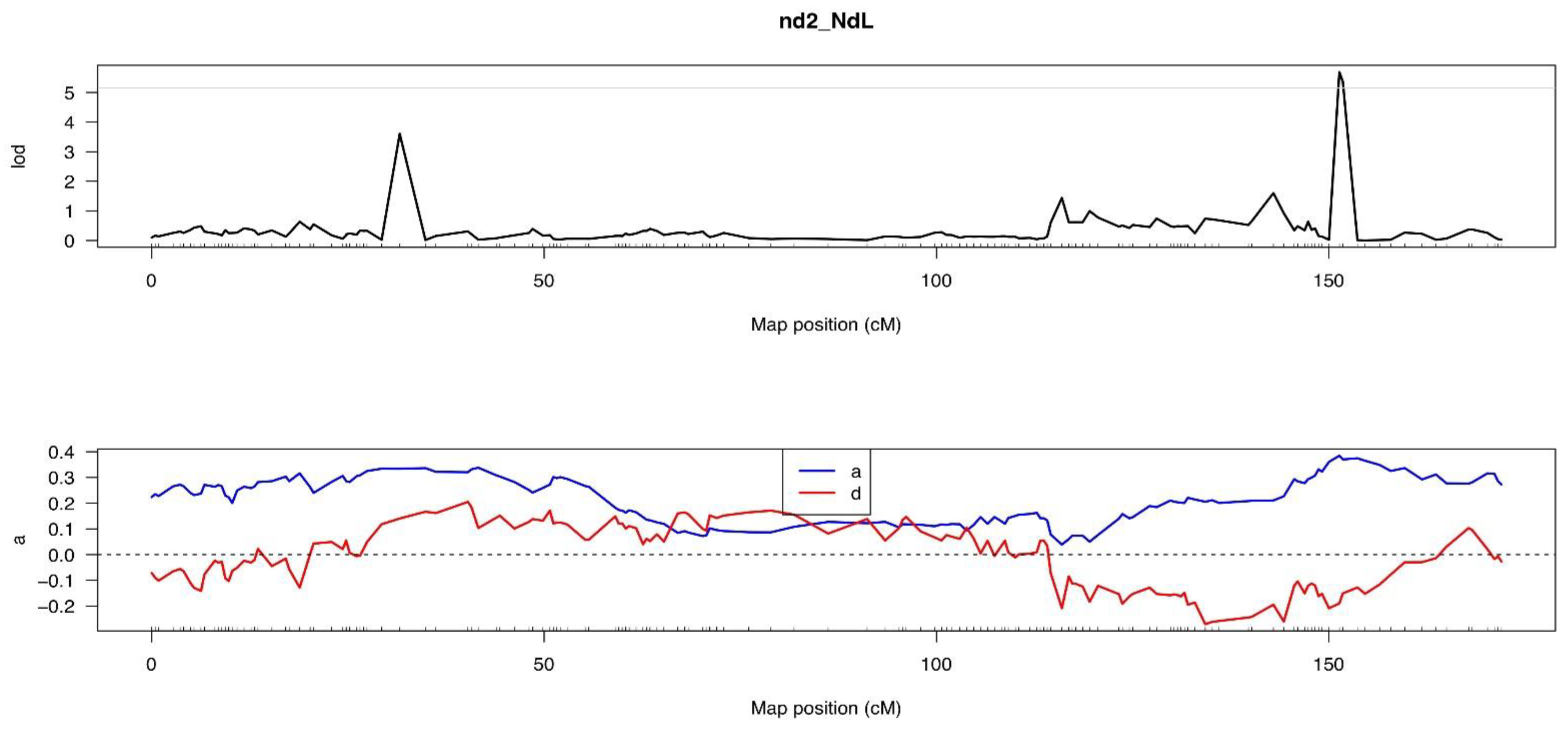
3.5.2. The Second Internode Length
3.6. Candidate Gene Screening and Function Prediction
3.6.1. QTL Localization
3.6.2. Candidate Genes of Plant Height
3.6.3. Candidate Genes of the Second Internode Length
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, D.P. Research progress on the origin and classification of cucumber plants. China Cucurbits Veg. 2017, 30, 1–3. [Google Scholar]
- FAOSTAT, The Production/Yield quantities of cucumbers and gherkins in world total between 1994 and 2022. Available online: https://www.fao.org/faostat/en/#search/Cucumbers%20and%20gherkins (accessed on 16 December 2023).
- Zhang, S.P.; Miao, H.; Bo, K.L.; Dong, S.Y.; Gu, X.F. Research progress on cucumber genetic breeding during “the Thirteenth Five-year Plan” in China. China Veg. 2021, 4, 16–26. [Google Scholar]
- Wang, X.F. Monographs on Vegetable Cultivation: Northern Edition; China Agriculture Press: Beijing, China, 2018; Volume 7, p. 10. [Google Scholar]
- Ai, X.Z.; Ma, X.Z.; Yu, L.M.; Xing, Y.X. Effect of long-term suboptimal temperature and short-term low temperature under low light density on cucumber growth and its photosynthesis. Chin. J. Appl. Ecol. 2004, 15, 2091–2094. [Google Scholar]
- Li, D.D.; Yu, F.S.; Zhang, Y.Z.; Hu, K.H.; Dai, D.Y.; Song, S.W.; Zhang, F.; Sa, R.N.; Lian, H.; Sheng, Y.Y. Integrative analysis of different low-light-tolerant cucumber lines in response to low-light stress. Front. Plant Sci. 2023, 13, 1093859. [Google Scholar] [CrossRef] [PubMed]
- Liebig, H.P.; Krug, H. Response of cucumber to climate. Acta Horti. 1991, 287, 47–50. [Google Scholar] [CrossRef]
- Zhang, X.F.; Linghu, S.F.; Sheng, Y.Y.; Wen, Y.H.; Xue, S.T.; Li, D.D. Analysis of plant characters and fruit quality in different cucumber combination under low light condition. J. Sichuan Agric. Univ. 2022, 40, 395–401, 420. [Google Scholar]
- Gao, W.R.; Sun, Y.J.; Han, B.; Li, D.C.; Fei, C.; Wang, X.S.; Xu, G. Effect of low light on plant growth and fruit development of watermelon. Chin. Agric. Sci. Bull. 2022, 38, 39–45. [Google Scholar]
- Wang, X.W.; Wang, Y.J.; Fu, Q.S.; Zhao, B.; Guo, Y.D. Effects of low light stress on morphological trait, physiological characters and leaf ultrastructure of tomato (Lycopersicon esculentum L.) seedlings. Acta Agric. Boreali-Sin. 2009, 24, 144–149. [Google Scholar]
- Yang, W.J.; Yu, X.H.; Jiang, X.M.; Gao, H.; Cheng, Y.; Zhang, F.; Li, Y.D.; Sun, D.X. Effects of low light stress on photosynthetic characteristics and root growth of tomato seedlings. Jiangsu Agric. Sci. 2017, 45, 112–114. [Google Scholar]
- Gu, X.F.; Zhang, C.Z.; Feng, L.L.; Fang, X.J.; Zhang, T.M. Genetic analysis of cucumber seedling traits in protected land. Acta Hortic. Sin. 2002, 29, 443–446. [Google Scholar]
- Cao, Q.W.; Zhang, Y.N.; Wang, Y.Q.; Yang, G.L.; Sun, X.L.; Li, L.B. Mixed genetic model analysis of main gene + polygene for cucumber internode length. J. Agric. Biotechnol. 2018, 26, 205–212. [Google Scholar]
- Serquen, F.C.; Bacher, J.; Staub, J.E. Mapping and QTL analysis of horticultural traits in a narrow cross in cucumber (Cucumis sativus L.) using random-amplified polymorphic DNA markers. Mol. Breed. 1997, 3, 257–268. [Google Scholar] [CrossRef]
- Xin, M.; Qin, Z.W.; Zhou, X.Y. Genetic analysis of cucumber plant height and its molecular markers. J. Northeast. Agric. Univ. 2008, 39, 34–38. [Google Scholar]
- Ji, Y.; Xu, Q.; Miao, M.M.; Liang, G.H.; Gao, H.J.; Luo, J.J.; Chen, X.H. Construction of cucumber genetic map and QTL mapping of plant height-related traits. Acta Hortic. Sin. 2009, 36, 1450–1456. [Google Scholar]
- Shi, J.L.; Chen, J.F.; Lou, Q.F.; Qian, C.T.; Meng, J.L. Genetic variation analysis and QTL detection of cucumber introgressive progeny. Acta Hortic. Sin. 2012, 39, 687–694. [Google Scholar]
- Miao, H.; Gu, X.F.; Zhang, S.P.; Zhang, Z.H.; Huang, S.W.; Wang, Y. QTL of cucumber plant height in different environments using permanent population. Sci. Agric. Sin. 2012, 45, 4552–4560. [Google Scholar]
- Xu, X.W.; Xu, R.X.; Zhu, B.Y.; Yu, T.; Qu, W.Q.; Lu, L.; Xu, Q.; Qi, X.H.; Chen, X.H. A high-density genetic map of cucumber derived from specific length amplified fragment sequencing (SLAF-seq). Front. Plant Sci. 2015, 5, 768. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, G.Y.; Dong, T.T.; Chen, B.; Du, H.S.; Li, C.B.; Zhang, F.L.; Zhang, H.Y.; Xu, Y.; Wang, Q.; et al. High-density genetic map construction and QTL mapping of first flower node in pepper (Capsicum annuum L.). BMC Plant Biol. 2019, 19, 167. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Wang, G.C.; Wang, D.; Sheng, Y.Y. Construction of SLAF map and QTL analysis of fruit-related traits in melon. Sci. Agric. Sin. 2021, 54, 4196–4206. [Google Scholar]
- Dai, D.Y.; Fan, L.; Zeng, S.; Wang, L.; Sheng, Y.Y.; Li, Z.Z.; Zhang, F. Construction of SLAF genetic linkage map and main QTL mapping of fruit stalk length in melon. J. Agric. Biotechnol. 2021, 29, 656–662. [Google Scholar]
- Zhang, C.H.; Chen, D.M.; Yang, F.Q.; Zhao, T.S.; Li, Y.; Zhao, G.D.; Zhao, Y.B. Construction of SLAF map and QTL analysis of fruit rust gene in apple. Acta Agric. Boreali-Sin. 2019, 34, 37–44. [Google Scholar]
- Wei, Q.Z.; Wang, W.H.; Hu, T.H.; Hu, H.J.; Wang, J.L.; Bao, C.L. Construction of a SNP-Based genetic map using SLAF-Seq and QTL analysis of morphological traits in eggplant. Front. Genet. 2020, 11, 178. [Google Scholar] [CrossRef]
- Dong, H.; Yang, L.; Li, L.; Zhang, J.J.; Fang, Q.F.; Song, S.J.; Yang, L.; Li, H.S. Construction of high-density genetic map of cultivated strawberry based on SLAF-seq technology. Acta Agric. Boreali-Sin. 2020, 35, 52–57. [Google Scholar]
- Hou, S.S.; Niu, H.H.; Tao, Q.Y.; Wang, S.H.; Gong, Z.H.; Li, S.; Weng, Y.Q.; Li, Z. A mutant in the CsDET2 gene leads to a systemic brassinosteriod deficiency and super compact phenotype in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2017, 130, 1693–1703. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.Q.; Qin, Y.G.; Pan, Y.P.; Wang, X.F.; Weng, Y.Q.; Chen, P.; Li, Y.H. The cytochrome P450 gene CsCYP85A1 is a putative candidate for super compact-1 (Scp-1) Plant Architecture Mutation in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 266. [Google Scholar] [CrossRef]
- Yang, L.M.; Liu, H.Q.; Zhao, J.Y.; Pan, Y.P.; Cheng, S.Y.; Lietzow, C.D.; Wen, C.L.; Zhang, X.L.; Weng, Y.Q. LITTLELEAF (LL) encodes a WD40 repeat domain-containing protein associated with organ size variation in cucumber. Plant J. 2018, 95, 834–847. [Google Scholar] [CrossRef]
- Gao, D.L.; Zhang, C.Z.; Zhang, S.; Hu, B.W.; Wang, S.H.; Zhang, Z.H.; Huang, S.W. Mutation in a novel gene SMALL AND CORDATE LEAF 1 affects leaf morphology in cucumber. J. Integr. Plant Biol. 2017, 59, 736–741. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, L.M.; Pathak, M.; Li, D.W.; He, X.M.; Weng, Y.Q. Fine genetic mapping of cp: A recessive gene for compact (dwarf) plant architecture in cucumber, Cucumis sativus L. Theor. Appl. Genet. 2011, 123, 973–983. [Google Scholar] [CrossRef]
- Xu, L.L.; Wang, C.; Cao, W.; Zhou, S.M.; Wu, T. CLAVATA1-type receptor-like kinase CsCLAVATA1 is a putative candidate gene for dwarf mutation in cucumber. Mol. Genet. Genom. 2018, 293, 1393–1405. [Google Scholar] [CrossRef]
- Chen, F.F.; Fu, B.B.; Pan, Y.P.; Zhang, C.W.; Wen, H.F.; Weng, Y.Q.; Chen, P.; Li, Y.H. Fine mapping identifies CsGCN5 encoding a histone acetyltransferase as putative candidate gene for tendril-less1 mutation (td-1) in cucumber. Theor. Appl. Genet. 2017, 130, 1549–1558. [Google Scholar] [CrossRef]
- Yan, S.S.; Ning, K.; Wang, Z.Y.; Liu, X.F.; Zhong, Y.T.; Ding, L.; Zi, H.L.; Cheng, Z.H.; Li, X.X.; Shan, H.Y.; et al. CsIVP functions in vasculature development and downy mildew re sistance in cucumber. PLoS Biol. 2020, 18, e3000671. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Li, R.Q.; Li, Y.R.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Cui, H.Q.; Liu, F.; Wang, R.H.; Sheng, Y.Y.; Wu, X. Evaluation and analysis of downy mildew resistance in cucumber under low light stress. J. Plant Prot. 2016, 43, 621–626. [Google Scholar]
- Zhu, W.Y.; Huang, L.; Chen, L.; Yang, J.T.; Wu, J.N.; Qu, M.L.; Yao, D.Q.; Guo, C.L.; Lian, H.L.; He, H.L.; et al. A high-density genetic linkage map for cucumber (Cucumis sativus L.): Based on specific length amplified fragment (SLAF) se quencing and QTL analysis of fruit traits in cucumber. Front. Plant Sci. 2016, 7, 437. [Google Scholar] [CrossRef]
- Xu, X.W.; Ji, J.; Xu, Q.; Qi, X.H.; Chen, X.H. Inheritance and quantitative trail loci mapping of adventitious root numbers in cucumber seedlings under waterlogging conditions. Mol. Genet. Genomics. 2017, 292, 353–364. [Google Scholar] [CrossRef]
- Li, Z.G.; Hu, Q.; Zhou, M.; Vandenbrink, J.; Li, D.Y.; Menchyk, N.; Reighard, S.; Norris, A.; Liu, H.B.; Sun, D.F.; et al. Heterologous expression of OsSIZ1, a rice SUMO E3 ligase, enhances broad abiotic stress tolerance in transgenic creeping bentgrass. Plant Biotechnol. J. 2013, 11, 432–445. [Google Scholar] [CrossRef]
- Li, Q.; Serio, R.J.; Schofield, A.; Liu, H.; Rasmussen, S.R.; Hofius, D.; Stone, S.L. Arabidopsis RING-type E3 ubiquitin ligase XBAT35.2 promotes proteasome-dependent degradation of ACD11 to attenuate abiotic stress tolerance. Plant J. 2020, 104, 1712–1723. [Google Scholar] [CrossRef]
- Tan, B.; Lian, X.D.; Cheng, J.; Zeng, W.F.; Zheng, X.B.; Wang, W.; Ye, X.; Li, J.D.; Li, Z.Q.; Zhang, L.L.; et al. Genome-wide identification, and transcriptome profiling reveal that E3 ubiquitin ligase genes relevant to ethylene, auxin and abscisic acid are differentially expressed in the fruits of melting flesh and stony hard peach varieties. BMC Genome 2019, 20, 892. [Google Scholar] [CrossRef]
- Ren, H.M.; Zhang, Y.T.; Zhong, M.Y.; Hussian, J.; Tang, Y.T.; Liu, S.K.; Qi, G.N. Calcium signaling-mediated transcriptional re programming during abiotic stress response in plants. Theor. Appl. Genet. 2023, 136, 210. [Google Scholar] [CrossRef]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B-like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Zhang, X.Y.; Chen, K.; Zhu, X.H.; Zhao, Y. Screening for Arabidopsis mutants with altered Ca2+ signal response using ae quorin-based Ca2+ reporter system. STAR Protoc. 2021, 23, 100558. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, X.; Mei, Q.; Qiu, L.; Chen, P.; Li, W.H.; Ma, F.W. MdbHLH4 negatively regulates apple cold tolerance by inhibiting MdCBF1/3 expression and promoting MdCAX3L-2 expression. Plant Physiol. 2023, 191, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Vidal, D.; Gil, M.T.; Alvarez-Flórez, F.; Moysset, L.; Simón, E. Protein kinase activity in Cucumis sativus cotyledons: Effect of calcium and light. Phytochemistry 2007, 68, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.J.; Vernonica, E.; Franklin, T. Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 2010, 151, 7–33. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, C.; Wang, M.Y.; Wan, D.; Huang, F.F.; Jiang, Z.H.; Crawford, B.M.; Vo-Dinh, T.; Yuan, F.; Wu, F.H.; et al. Flg22-induced Ca2+ increases undergo desensitization and resensitization. Plant Cell Environ. 2021, 44, 3563–3575. [Google Scholar] [CrossRef]

| Traits | Range | Mean (cm) | Standard Deviation (SD) | Kurtosis (SEK) | Skewness (SES) | Coefficient of Variation (CV) |
|---|---|---|---|---|---|---|
| Plant height (2021) | 15.90–62.50 | 37.088 ± 0.799 | 9.145 | −0.660 | −0.036 | 0.247 |
| Plant height (2022) | 4.50–18.0 | 11.635 ± 0.197 | 2.442 | −0.130 | 0.205 | 0.210 |
| Second internode length (2021) | 0.40–2.50 | 1.173 ± 0.040 | 0.462 | −0.090 | 0.347 | 0.394 |
| Second internode length (2022) | 0.50–4.30 | 1.783 ± 0.056 | 0.687 | 1.117 | 0.701 | 0.385 |
| Items | Mapped Reads (%) | Properly Mapped Reads (%) | Number of SNPs | Number of Heterozygosity SNPs | Number of Homozygosity SNPs | Heter Ratio (%) |
|---|---|---|---|---|---|---|
| WI | 98.33 | 82.98 | 528,629 | 89,444 | 439,185 | 16.92 |
| M14 | 92.53 | 75.53 | 642,036 | 41,026 | 601,010 | 6.39 |
| F2 | 90.46 | 79.46 | 100,868.51 | 27,093.28 | 73,775.23 | 26.86 |
| Linkage Group | Number of Markers | Total Distance/c M | Average Distance/c M | Max Gap/c M | Gaps ≤ 5 c M |
|---|---|---|---|---|---|
| Chr.1 | 427 | 154.52 | 0.36 | 4.11 | 100% |
| Chr.2 | 248 | 179.85 | 0.73 | 7.94 | 99.19% |
| Chr.3 | 376 | 147.35 | 0.39 | 4.88 | 100% |
| Chr.4 | 261 | 170.45 | 0.66 | 5.60 | 99.23% |
| Chr.5 | 390 | 142.34 | 0.36 | 6.51 | 99.74% |
| Chr.6 | 280 | 171.99 | 0.62 | 4.96 | 100% |
| Chr.7 | 250 | 147.79 | 0.59 | 7.52 | 99.60% |
| Total | 2233 | 1114.29 | 0.50 | 7.94 | 99.68% |
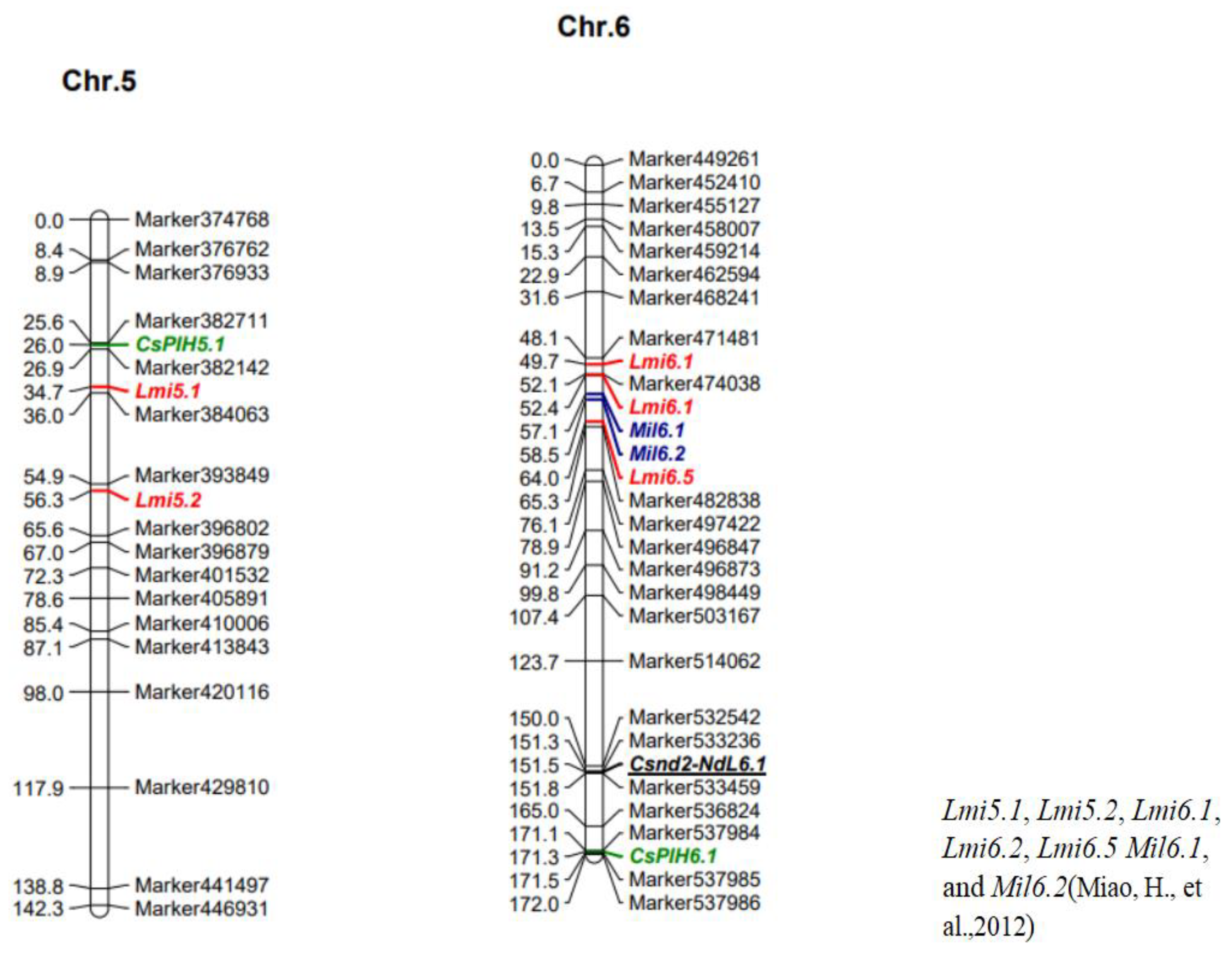
| QTL Position | LOD Value | Chr. | Start (c M) | Genome Position | End (c M) | Genome Position | Max LOD | ADD | DOM | PVE (%) | Candidate Gene Numbers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CsPlH5.1 | 3 | 5 | Marker 382711 | 4,812,907 | Marker 382610 | 5,159,042 | 4.013 | 1.005 | 0.563 | 9.446 | 41 |
| (25.61) | (26.05) | ||||||||||
| CsPlH6.1 | 3 | 6 | Marker 537985 | 31,658,938 | Marker 537984 | 31,658,958 | 5.318 | 0.997 | −0.037 | 6.887 | 1 |
| (171.10) | (171.55) | ||||||||||
| Csnd2_NdL6.1 | 5.148 | 6 | Marker 533236 | 29,572,188 | Marker 533459 | 29,604,215 | 5.689 | 0.384 | −0.19 | 14.18 | 7 |
| (151.34) | (151.78) |
| No. | Gene ID | Function Annotation |
|---|---|---|
| 1 | CsGy5G007180 | ribulose bisphosphate carboxylase/oxygenase activase 2, chloroplastic-like |
| 2 | CsGy5G007200 | AT-hook motif nuclear-localized protein |
| 3 | CsGy5G007220 | vesicle transport v-SNARE 12-like |
| 4 | CsGy5G007240 | transcription termination factor MTERF5, chloroplastic-like |
| 5 | CsGy5G007310 | ultraviolet-B receptor UVR8 isoform X2 |
| 6 | CsGy5G007340 | protein DA1-related 2 |
| 7 | CsGy5G007360 | subtilase 4.13 |
| 8 | CsGy6G036070 | pentatricopeptide repeat-containing protein At4g21065 |
| No. | Gene ID | Function Annotation |
|---|---|---|
| 1 | CsGy6G032280 | E3 ubiquitin-protein ligase RGLG2-like |
| 2 | CsGy6G032290 | uncharacterized protein At4g26485 |
| 3 | CsGy6G032300 | Calcineurin B-like protein 3 |
| 4 | CsGy6G032310 | BTB/POZ domain-containing protein At5g47800-like |
| 5 | CsGy6G032320 | Phosphoglycerate phosphatase |
| 6 | CsGy6G032330 | pentatricopeptide repeat-containing protein At2g04860 |
| 7 | CsGy6G032340 | Unknown Protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Linghu, S.; Zhang, Y.; Song, S.; Cao, J.; Hu, K.; Zhang, Y.; Yu, F.; Han, Y. Genetic Map Construction and Primary Quantitative Trait Locus Analysis of Low-Light-Stress-Related Traits in Cucumber. Agronomy 2024, 14, 1061. https://doi.org/10.3390/agronomy14051061
Li D, Linghu S, Zhang Y, Song S, Cao J, Hu K, Zhang Y, Yu F, Han Y. Genetic Map Construction and Primary Quantitative Trait Locus Analysis of Low-Light-Stress-Related Traits in Cucumber. Agronomy. 2024; 14(5):1061. https://doi.org/10.3390/agronomy14051061
Chicago/Turabian StyleLi, Dandan, Shaofeng Linghu, Yuting Zhang, Siwen Song, Jiawen Cao, Kaihong Hu, Yanzhao Zhang, Fushun Yu, and Yehui Han. 2024. "Genetic Map Construction and Primary Quantitative Trait Locus Analysis of Low-Light-Stress-Related Traits in Cucumber" Agronomy 14, no. 5: 1061. https://doi.org/10.3390/agronomy14051061
APA StyleLi, D., Linghu, S., Zhang, Y., Song, S., Cao, J., Hu, K., Zhang, Y., Yu, F., & Han, Y. (2024). Genetic Map Construction and Primary Quantitative Trait Locus Analysis of Low-Light-Stress-Related Traits in Cucumber. Agronomy, 14(5), 1061. https://doi.org/10.3390/agronomy14051061





