Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Growth and Treatment
2.2. Seedling Growth Measurements
2.3. Histochemical Staining
2.4. Fluorescent Detection In Vivo
2.5. Determination of Relative Conductivity
2.6. Assay of Enzyme Activity
2.7. Evaluation of Celllular Redox Status
2.8. Determination of Metabolites
2.9. Determination of Total Soluble Protein Content
2.10. Data Analysis
3. Results
3.1. Salinity Inhibited the Growth of Chinese Cabbage Seedlings
3.2. Thymol Promoted the Growth of Chinese Cabbage Seedlings upon Saline Stress
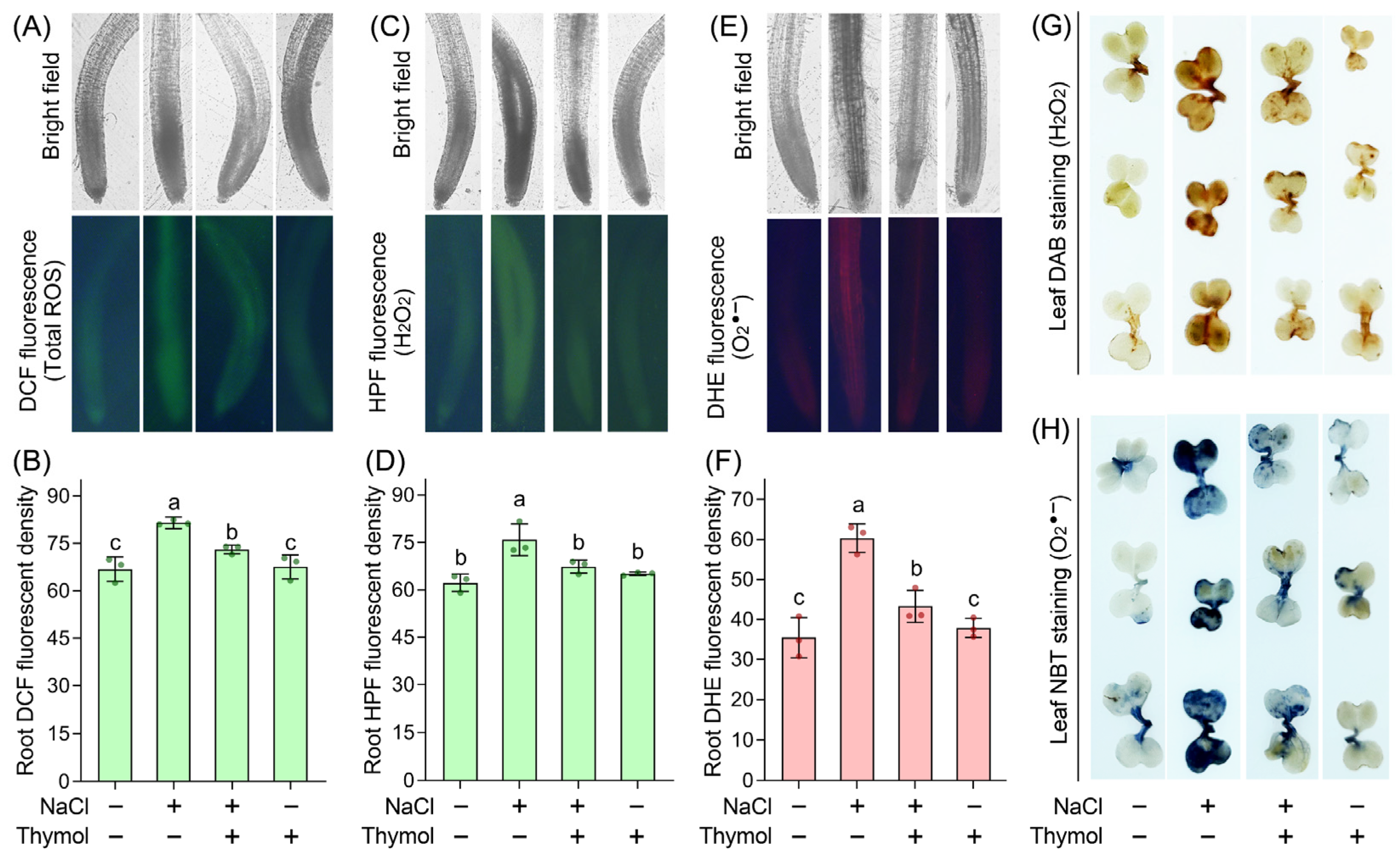
3.3. Thymol-Suppressed ROS Accumulation in Chinese Cabbage Seedlings upon Saline Stress
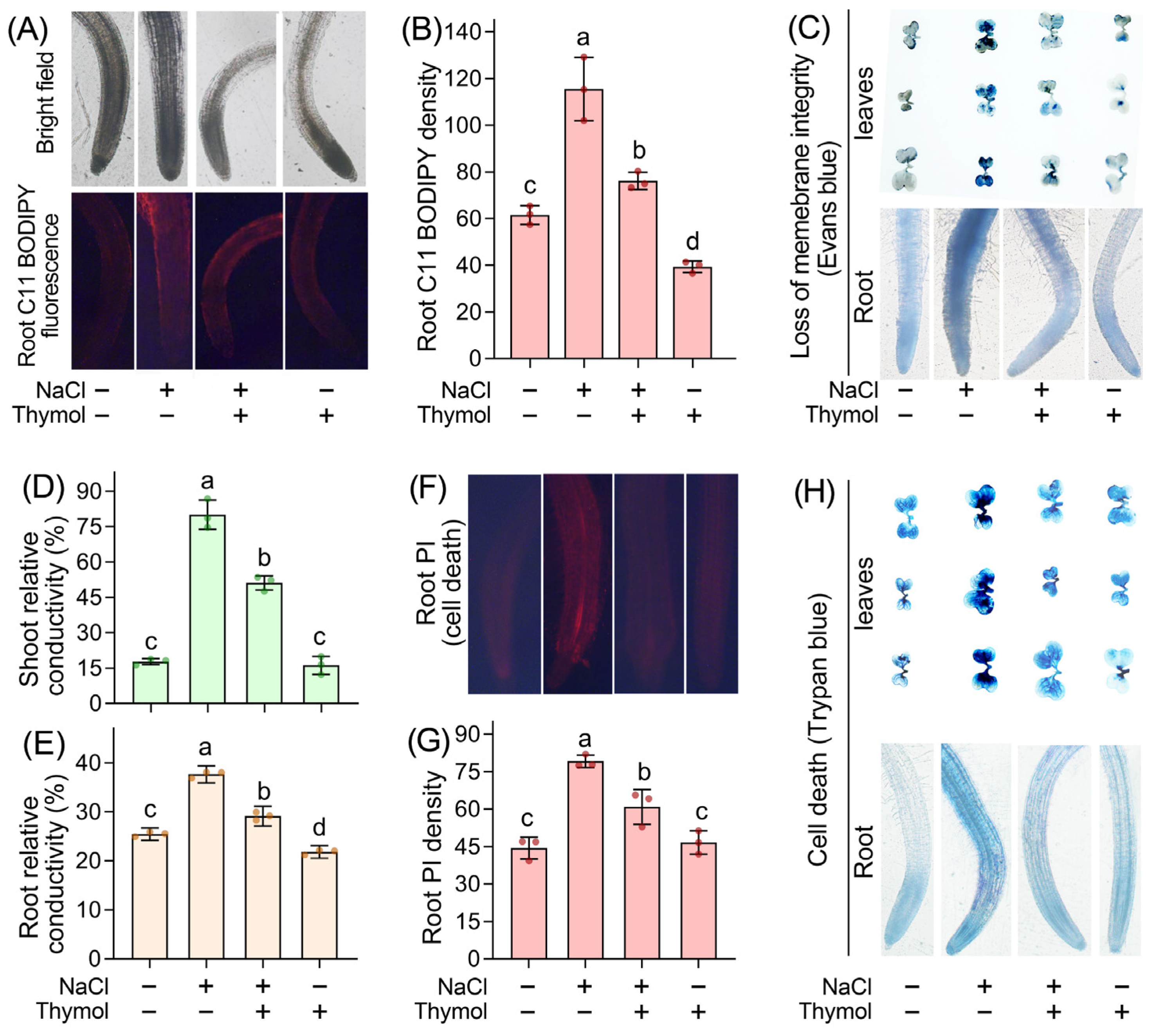
3.4. Thymol Attenuated Saline-Induced Oxidative Damage in Chinese Cabbage Seedlings
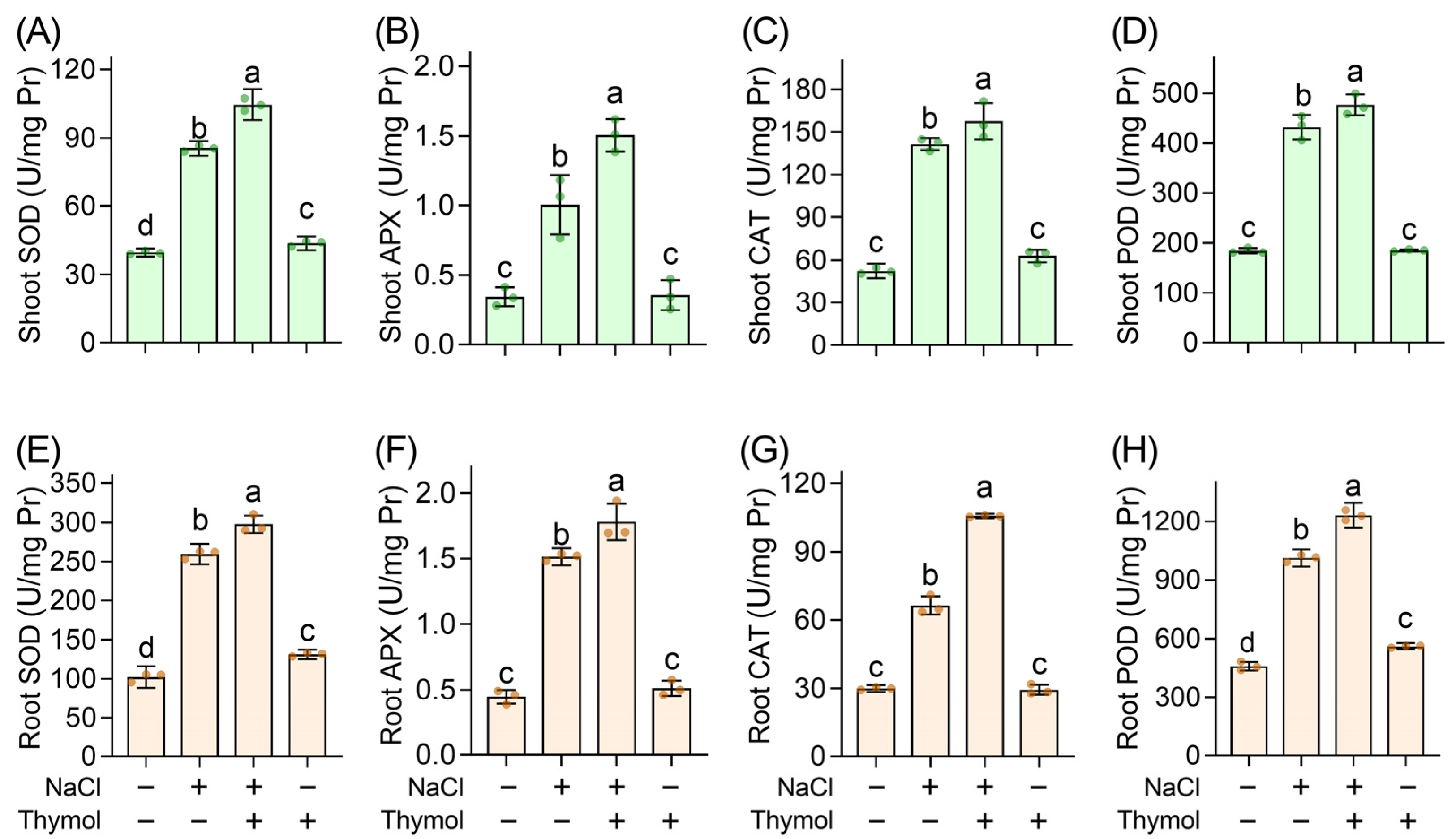
3.5. Thymol Activated Antioxidative Enzymes in Chinese Cabbage Seedlings upon Saline Stress
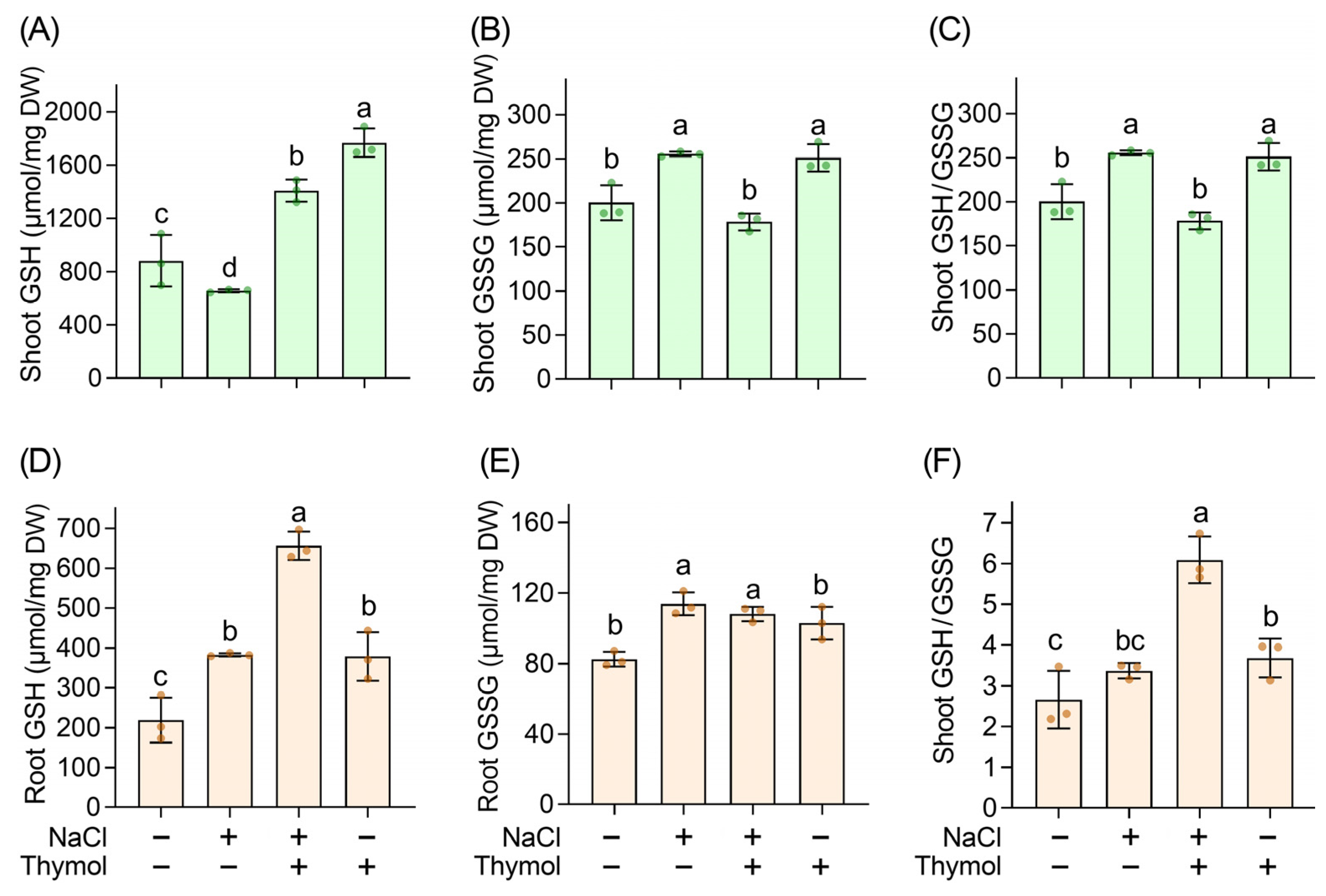
3.6. Thymol Adjusted Redox Balance in Chinese Cabbage Seedlings upon Saline Stress
3.7. Thymol Enhanced Antioxidant Content in Chinese Cabbage Seedlings upon Saline Stress
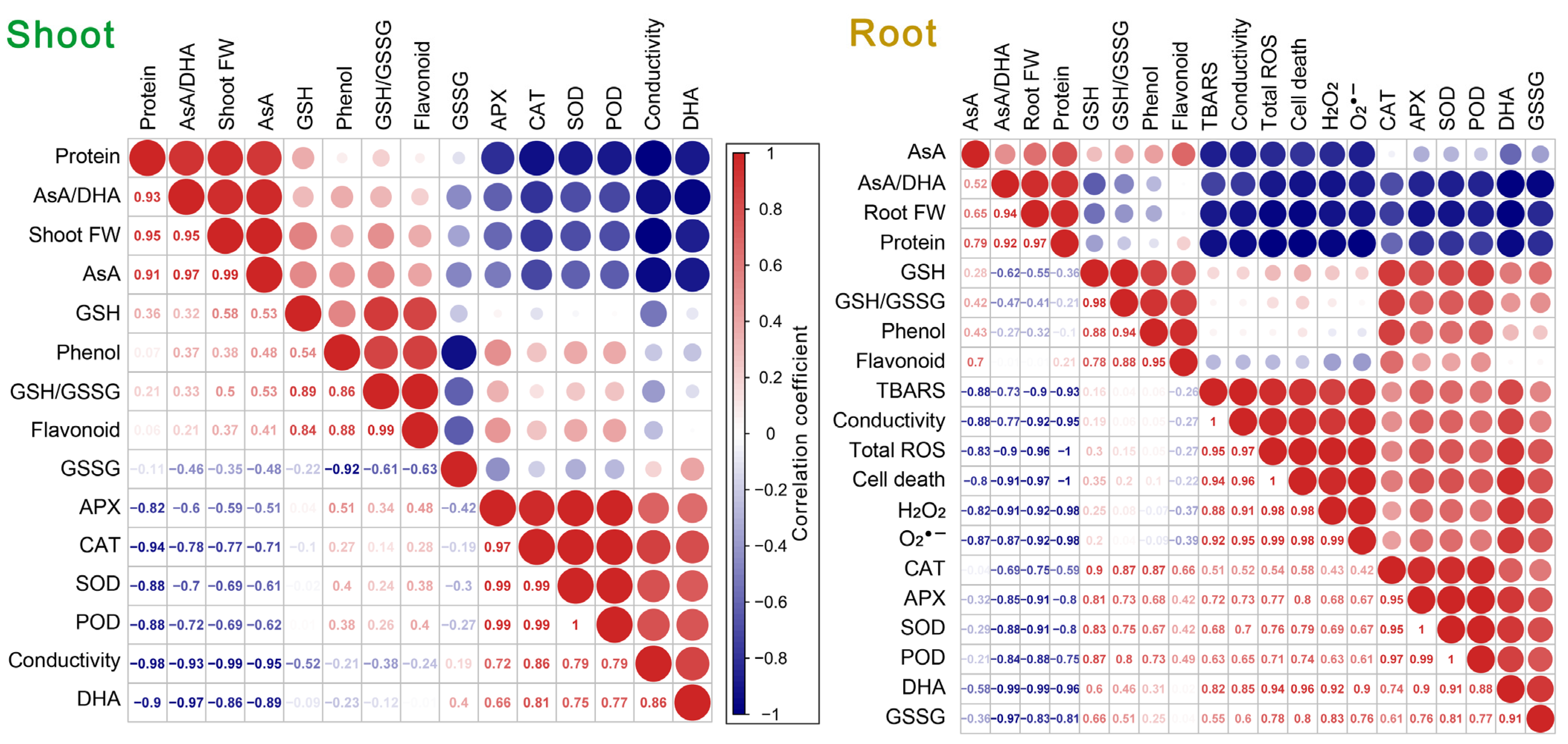
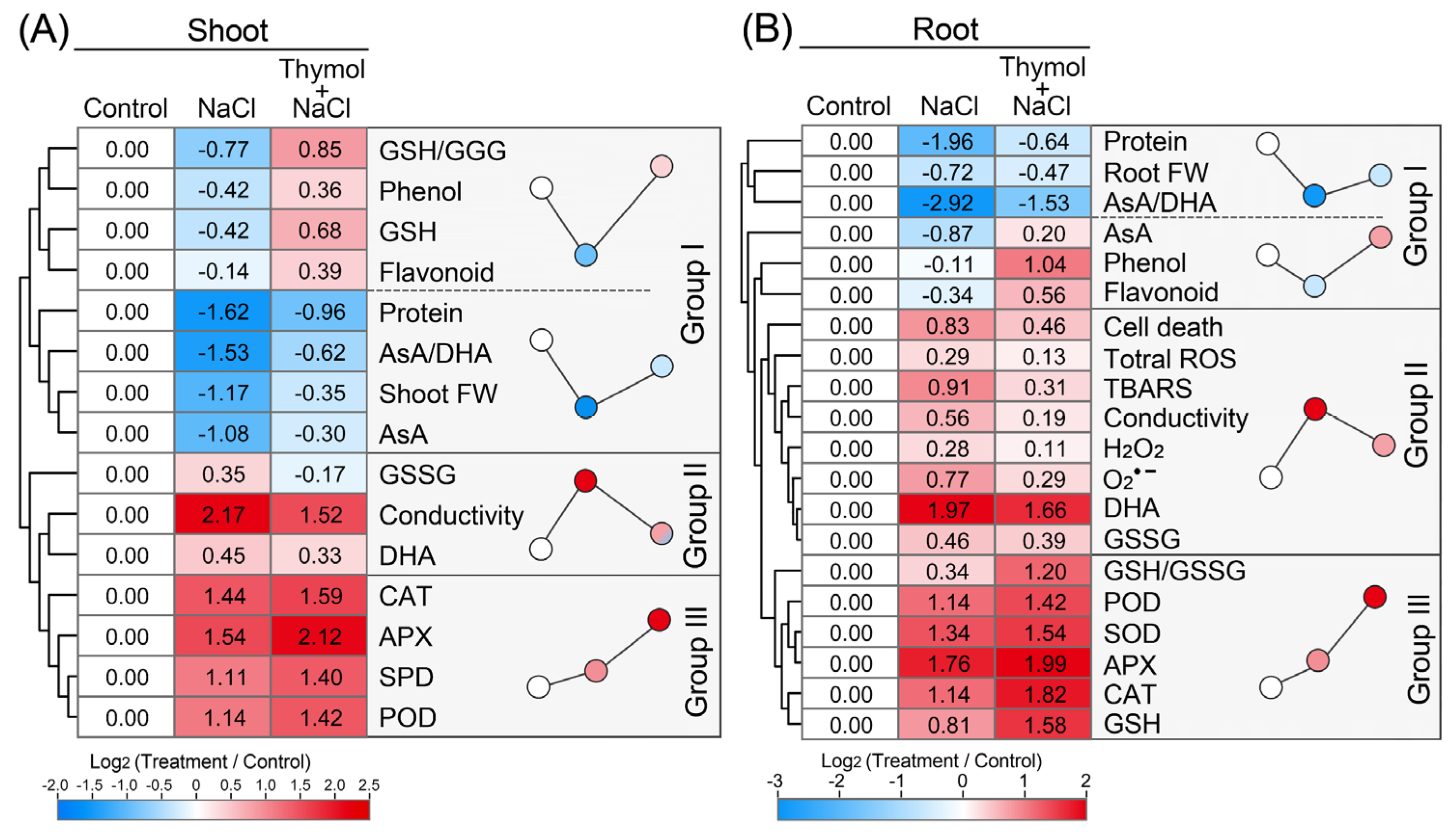
3.8. Correlation and Cluster Analysis
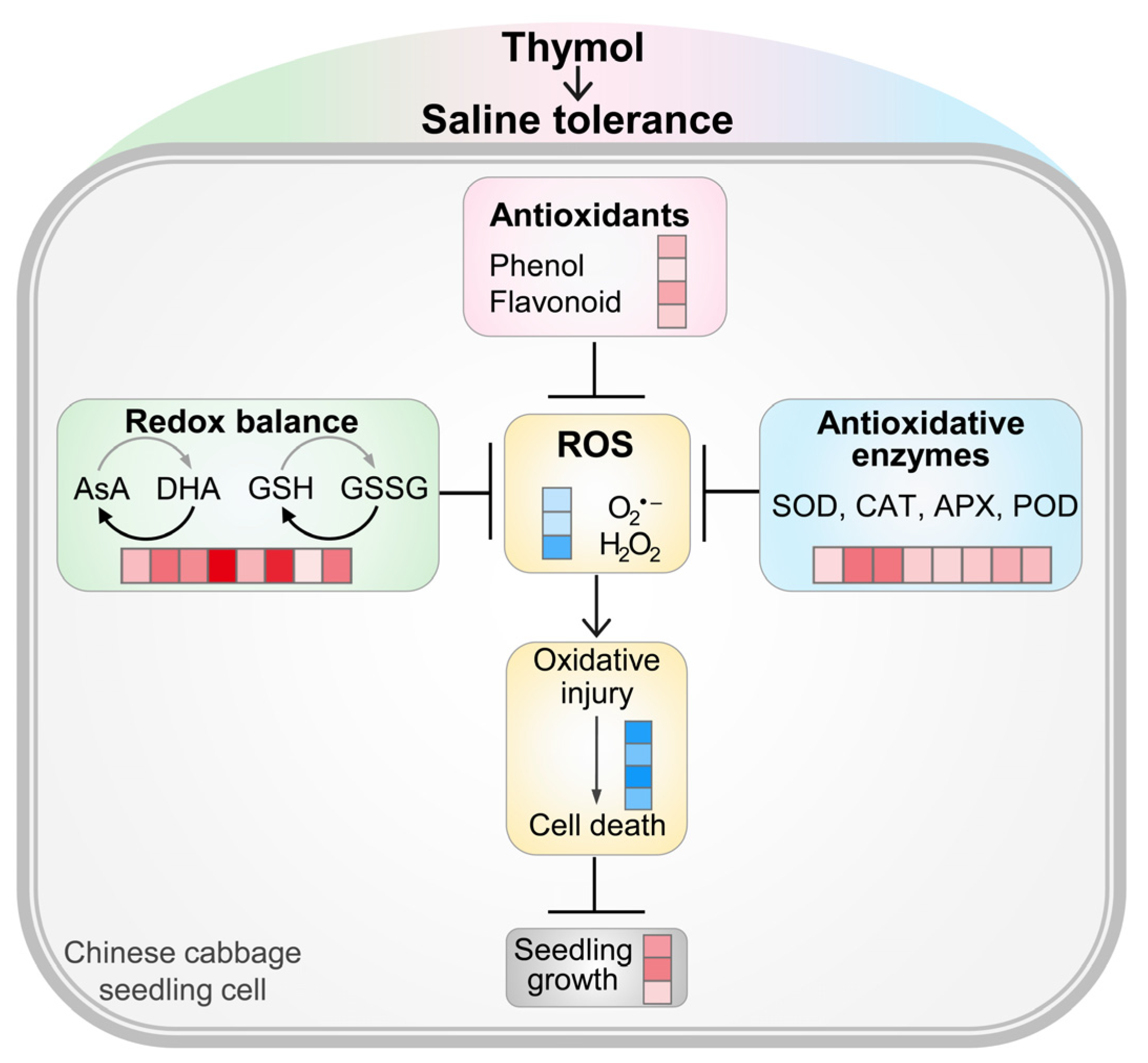
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meddich, A. Biostimulants for Resilient Agriculture—Improving Plant Tolerance to Abiotic Stress: A Concise Review. Gesunde Pflanz. 2023, 75, 709–727. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Wang, T.T.; Shi, Z.Q.; Hu, L.B.; Xu, X.F.; Han, F.X.; Zhou, L.G.; Chen, J. Thymol ameliorates cadmium-induced phytotoxicity in the root of rice (Oryza sativa) seedling by decreasing endogenous nitric oxide generation. J. Agric. Food Chem. 2017, 65, 7396–7405. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; An, G.; Wang, N.; Pang, B.; Shi, Z.; Bai, H.; Zhang, L.; Chen, J.; Xu, W. Thymol ameliorates ammonium toxicity via repressing polyamine oxidase-derived hydrogen peroxide and modulating ammonium transporters in rice root. Food Prod. Process. Nutri. 2021, 3, 7. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Kong, X.-W.; Wang, N.; Wang, T.-T.; Chen, J.; Shi, Z. Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol. Environ. Saf. 2020, 188, 109894. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef]
- Ponce, K.S.; Guo, L.; Leng, Y.; Meng, L.; Ye, G. Advances in sensing, response and regulation mechanism of salt tolerance in rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Souana, K.; Taïbi, K.; Ait Abderrahim, L.; Amirat, M.; Achir, M.; Boussaid, M.; Mulet, J.M. Salt-tolerance in Vicia faba L. is mitigated by the capacity of salicylic acid to improve photosynthesis and antioxidant response. Sci. Hortic. 2020, 273, 109641. [Google Scholar] [CrossRef]
- Challabathula, D.; Analin, B.; Mohanan, A.; Bakka, K. Differential modulation of photosynthesis, ROS and antioxidant enzyme activities in stress-sensitive and -tolerant rice cultivars during salinity and drought upon restriction of COX and AOX pathways of mitochondrial oxidative electron transport. J. Plant Physiol. 2022, 268, 153583. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Alharby, H.F.; Nahar, K.; Al-Zahrani, H.S.; Hakeem, K.R.; Hasanuzzaman, M. Enhancing salt tolerance in soybean by exogenous boron: Intrinsic study of the ascorbate-glutathione and glyoxalase pathways. Plants 2021, 10, 2085. [Google Scholar] [CrossRef]
- Yan, R.; Liu, J.; Zhang, S.; Guo, J. Exogenous melatonin and salicylic acid enhance the drought tolerance of hibiscus (Hibiscus syriacus L.) by regulating photosynthesis and antioxidant system. J. Soil Sci. Plant Nutr. 2024, 24, 497–511. [Google Scholar] [CrossRef]
- do Carmo, M.A.P.; dos Santos, H.O.; e Oliveira, J.B.R.; da Silva, I.G.; dos Santos Guaraldo, M.M.; Souza Pereira, W.V. Signaling Molecules for Increasing Urochloa ruziziensis Tolerance to Abiotic Stresses. J. Soil Sci. Plant Nutr. 2024, 24, 870–883. [Google Scholar] [CrossRef]
- Lakhdar, A.; Trigui, M.; Montemurro, F. An overview of biostimulants’ effects in saline soils. Agronomy 2023, 13, 2092. [Google Scholar] [CrossRef]
- Sadak, M.S.; Hanafy, R.S.; Elkady, F.M.A.M.; Mogazy, A.M.; Abdelhamid, M.T. Exogenous calcium reinforces photosynthetic pigment content and osmolyte, enzymatic, and non-enzymatic antioxidants abundance and alleviates salt stress in bread wheat. Plants 2023, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.A.; Mousa, N.H.S.; Hanafy, R.S.; Latef, A.A.H.A. Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative stress in wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, D.; Zhang, Z.; Li, Y.; Shi, S.; Yang, Y. Calcium signal regulated carbohydrate metabolism in wheat seedlings under salinity stress. Physiol. Mol. Biol. Plants 2024, 30, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hao, Y.; Lu, H.; Li, P.; Chen, J.; Shi, Z.; Xie, Y.; Mo, H.; Hu, L. Nano-thymol emulsion inhibits Botrytis cinerea to control postharvest gray mold on tomato fruit. Agronomy 2022, 12, 2973. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Stanely Mainzen Prince, P. Protective effects of thymol on altered plasma lipid peroxidation and nonenzymic antioxidants in isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2012, 26, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Wang, S.J.; Yang, Z.M. Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 2008, 70, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen; Han, C.; Zhang, Y.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.; van Liebergen, L.C.; Op den Kamp, J.A.; Post, J.A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ling, T.; Xue, Y.; Xu, C.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. Thymol mitigates cadmium stress by regulating glutathione levels and reactive oxygen species homeostasis in tobacco seedlings. Molecules 2016, 21, 1339. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wu, C.; Xuan, Z.; Cheng, Y.; Xiong, R.; Su, Z.; Wang, D. Lead exposure dose-dependently affects oxidative stress, AsA-GSH, photosynthesis, and mineral content in pakchoi (Brassica chinensis L.). Front. Plant Sci. 2022, 13, 1007276. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, F.; Cui, R.; Wang, S.; Cui, Y.; Lu, X.; Huang, H.; Ni, K.; Liu, X.; Jiang, T.; et al. GhIMP10D, an inositol monophosphates family gene, enhances ascorbic acid and antioxidant enzyme activities to confer alkaline tolerance in Gossypium hirsutum L. BMC Plant Biol. 2023, 23, 447. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Huang, Y.; Lian, S.; Saleem, M.; Li, B.; Wang, C. Improving the biocontrol efficacy of Meyerozyma guilliermondii Y-1 with melatonin against postharvest gray mold in apple fruit. Postharvest Biol. Technol. 2021, 171, 111351. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Zhou, Q.; Xin, Y.; Wang, G.; Xu, L.-a. De novo transcriptome analysis revealed genes involved in flavonoid biosynthesis, transport and regulation in Ginkgo biloba. Ind. Crops Prod. 2018, 124, 226–235. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.92. Available online: https://github.com/taiyun/corrplot (accessed on 13 November 2021).
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production, antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant Superoxide Dismutases: Function Under Abiotic Stress Conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Tyagi, S.; Shumayla; Singh, S.P.; Upadhyay, S.K. Role of Superoxide Dismutases (SODs) in Stress Tolerance in Plants. In Molecular Approaches in Plant Biology and Environmental Challenges; Singh, S.P., Upadhyay, S.K., Pandey, A., Kumar, S., Eds.; Springer: Singapore, 2019; pp. 51–77. [Google Scholar]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and redox markers in plant cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar] [CrossRef]
- Teixeira, F.K.; Menezes-Benavente, L.; Margis, R.; Margis-Pinheiro, M. Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: Inferences from the rice genome. J. Mol. Evol. 2004, 59, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar] [PubMed]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S.A.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Kruk, I.; Michalska, T.; Lichszteld, K.; Kładna, A.; Aboul-Enein, H.Y. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 2000, 41, 1059–1064. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Lv, W.; Yang, L.; Xu, C.; Shi, Z.; Shao, J.; Xian, M.; Chen, J. Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Front. Plant Sci. 2017, 8, 232. [Google Scholar] [CrossRef]
- Namdari, H.; Izad, M.; Rezaei, F.; Amirghofran, Z. Thymol as a reciprocal regulator of T cell differentiation: Promotion of regulatory T cells and suppression of Th1/Th17 cells. Int. Immunopharmacol. 2019, 67, 417–426. [Google Scholar] [CrossRef]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol isolated from Thymus vulgaris L. inhibits colorectal cancer cell growth and metastasis by suppressing the Wnt/β-Catenin Pathway. Drug Des. Devl. Ther. 2020, 14, 2535–2547. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Chen, J.; Wang, L.; Li, J.; Shi, Z.; Yang, L.; Yu, X. Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress. Agronomy 2024, 14, 1059. https://doi.org/10.3390/agronomy14051059
Sun C, Chen J, Wang L, Li J, Shi Z, Yang L, Yu X. Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress. Agronomy. 2024; 14(5):1059. https://doi.org/10.3390/agronomy14051059
Chicago/Turabian StyleSun, Changwei, Jian Chen, Lanlan Wang, Jiajun Li, Zhiqi Shi, Lifei Yang, and Xiangyang Yu. 2024. "Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress" Agronomy 14, no. 5: 1059. https://doi.org/10.3390/agronomy14051059
APA StyleSun, C., Chen, J., Wang, L., Li, J., Shi, Z., Yang, L., & Yu, X. (2024). Thymol Deploys Multiple Antioxidative Systems to Suppress ROS Accumulation in Chinese Cabbage Seedlings under Saline Stress. Agronomy, 14(5), 1059. https://doi.org/10.3390/agronomy14051059






