An Overview of Recycling Phenolic Resin
Abstract
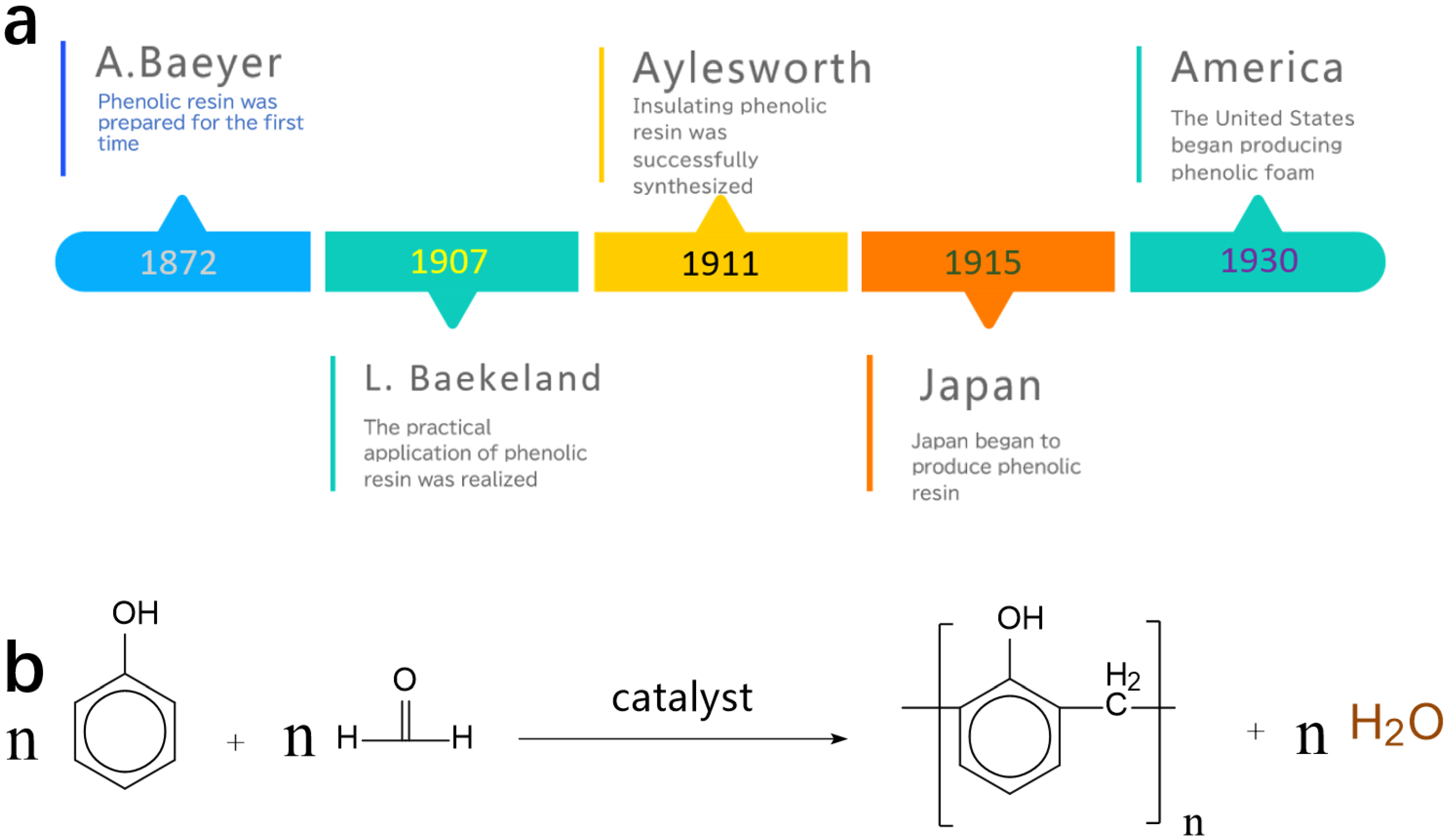
:1. Introduction
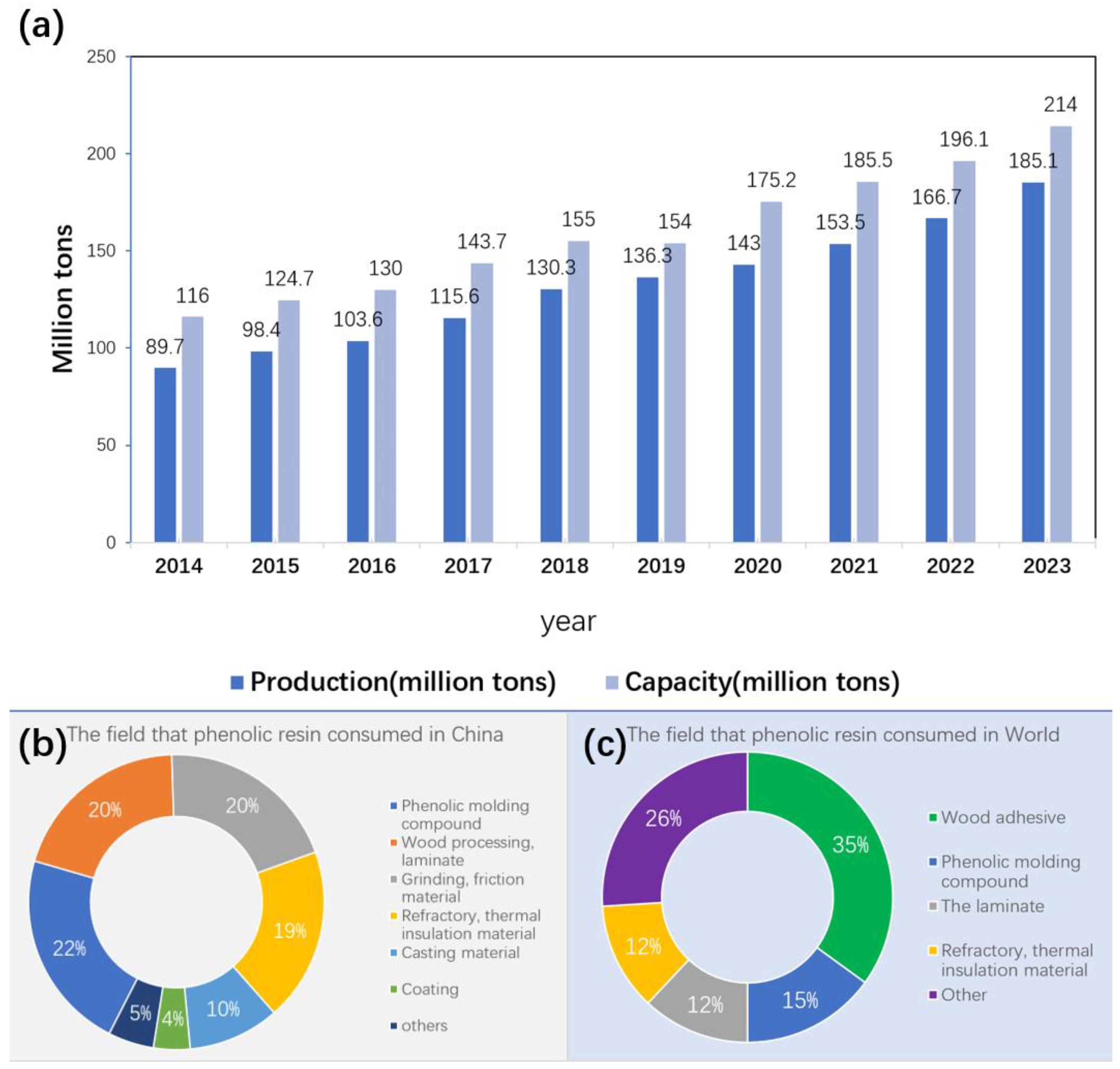
1.1. The Consumption of Phenolic Resin in the Global Market
1.2. Environmental Hazards of Phenolic Resins
- (1)
- Alkaline system: The synthesis equations of phenolic resins in alkaline systems are shown in Figure 3. Under the alkaline condition, phenol reacts with OH- in the solution to form phenoxy anion and the electrons on the oxygen ions in the phenoxy anion form an extended pi bond with the benzene ring. Due to the electron-withdrawing effect of oxygen ions, the electron density of the ortho and para positions and nucleophilicity of the whole structure increase, which leads to the addition reaction mainly concentrated on the ortho and para positions of phenol. In formaldehyde, the electronegativity of the oxygen atom is larger than that of the carbon atom, resulting in a greater attraction of the oxygen atom to the valence electrons showing negative electricity, and positive electricity in the carbon atom. Formaldehyde reacts with the active center to produce ortho- or para- hydroxymethyl-phenol (Figure 3a). The hydroxymethyl-phenol can continue to react with formaldehyde to form di-hydroxymethyl-phenol and tri-hydroxymethyl-phenol [5] (Figure 3b). The process of the formation of methylene bridges is shown in Figure 3c. Since addition reactions can occur in the ortho position or para positions, there are many types of chemical bonds between dimers and oligomers.
- (2)
- Acid system: It is easy to form linear phenolic resin under acidic conditions because the condensation rate is much higher than the addition rate under acidic conditions. The specific chemical reaction equations are shown in Figure 3d.
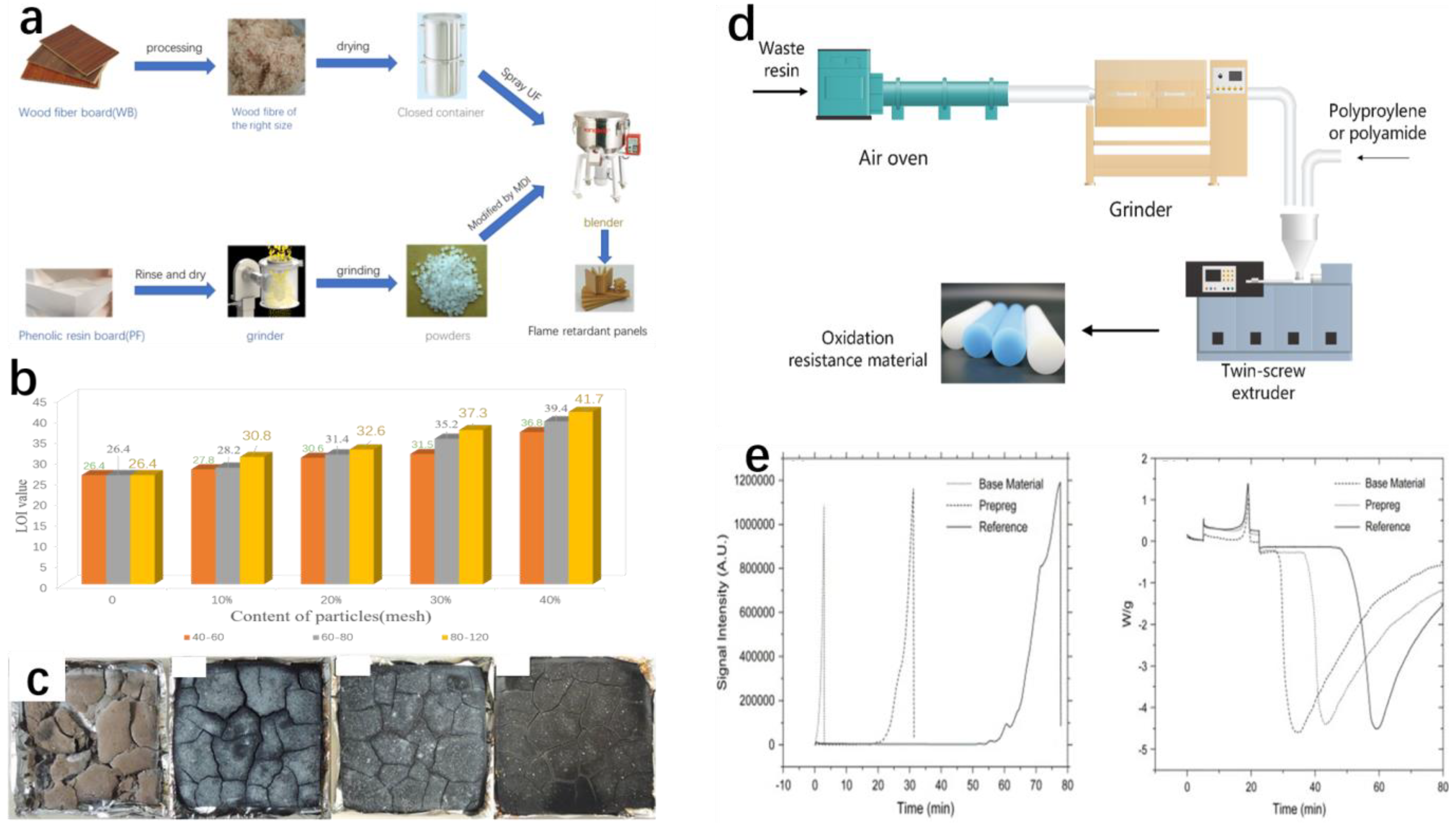
2. Mechanical Recycling
2.1. Mechanical Grinding and Reincorporation
2.2. Mechanical Recycling and Special Applications
3. Chemical Recycling
3.1. Depolymerization
3.2. Conversion into Aromatic Amines
3.3. Recycling to Carbon-Based Functional Materials
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Louis, P. Phenolic resins: 100 Years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar]
- Kazuhisa, H.; Masakatsu, A. Phenolic resins—100 years of progress and their future. React. Funct. Polym. 2013, 73, 256–269. [Google Scholar]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and respects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Li, H.Q. Principle, Technology and Application of Adhesive; South China University of Technology Press: Guangzhou, China, 2017. [Google Scholar]
- Chaussoy, N.; Brandt, D.; Gerard, J.F. Formaldehyde-Free and Phenol-Free Non-toxic Phenolic Resins with High Thermostability. ACS Appl. Polym. Mater. 2023, 5, 5630–5640. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, Z.-R.; Bai, Y.-H. Human Cancer Risk from the Inhalation of Formaldehyde in Different Indoor Environments in Guiyang City, China. Bull. Environ. Contam. Toxicol. 2008, 81, 200–204. [Google Scholar] [CrossRef]
- Songur, A.; Ozen, O.A.; Sarsilmaz, M. The Toxic Effects of Formaldehyde on the Nervous System. Rev. Environ. Contam. Toxicol. 2009, 203, 105–118. [Google Scholar]
- Liu, Y.; Yu, D.; Xiao, S. Effects of chronic exposure to formaldehyde on micronucleus rate of bone marrow cells in male mice. J. Pak. Med. Assoc. 2017, 67, 933–935. [Google Scholar]
- Kim, K.-H.; Jahan, S.A.; Lee, J.-T. Exposure to Formaldehyde and Its Potential Human Health Hazards. J. Environ. Sci. Health Part C 2011, 29, 277–299. [Google Scholar] [CrossRef]
- Maleki, A.; Mahvi, A.H.; Mesdaghinia, A.; Naddafi, K. Degradation and Toxicity Reduction of Phenol by Ultrasound Waves. Bull. Chem. Soc. Ethiop. 2007, 21, 33–38. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Magos, L. Patty’s Industrial Hygiene and Toxicology. J. Appl. Toxicol. 1996, 16, 539–540. [Google Scholar] [CrossRef]
- Craig, A.W. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. Br. J. Cancer 1973, 27, 190. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, L.; Kim, D.; Cui, R.; Lee, J.; An, Y.-J. Deriving hazardous concentrations of phenol in soil ecosystems using a species sensitivity distribution approach. J. Hazard. 2020, 399, 123036. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM fragrance ingredient safety assessment, phenol, CAS Registry Number108-95-2. Food Chem. Toxicol. 2021, 149, 111909. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Zhu, B.; Zhu, M.Y. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Chen, R.; Deng, S.; Cui, T.; Duan, S.; Jia, Q.; Zhang, L. Progress in recycling and reutilization of waste polyethylene terephthalate. Prog. Rubber Plast. Recycl. Technol. 2024, 40, 77–97. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Uzosike, C.C.; Yee, L.H.; Padilla, R.V. Small-Scale Mechanical Recycling of Solid Thermoplastic Wastes: A Review of PET, PEs, and PP. Energies 2023, 16, 1406. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Li, D.; Ma, Y.; Zhou, S.C.; Wang, Y.; Zhang, D.H. Bio-based hyperbranched epoxy resins: Synthesis and recycling. Chem. Soc. Rev. 2024, 53, 624–655. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal solid waste management and landfilling technologies: A review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Liu, H. Study on thermal degradation of phenolic resin. Appl. Mech. Mater. 2013, 422, 24–28. [Google Scholar] [CrossRef]
- Jackson, W.M.; Conley, R.T. High Temperature Oxidative Degradation of Phenol-Formaldehyde Polycondensates. J. Appl. Polym. Sci. 1964, 8, 2163–2193. [Google Scholar] [CrossRef]
- Mong, G.R.; Chong, C.T.; Chong, W.W.F.; Ng, J.-H.; Ong, H.C.; Ashokkumar, V.; Tran, M.-V.; Karmakar, S.; Goh, B.H.H.; Yasin, M.F.M. Progress and challenges in sustainable pyrolysis technology: Reactors, feedstocks and products. Fuel 2022, 324, 124777. [Google Scholar] [CrossRef]
- Cao, H.; Xin, Y.; Wang, D.; Yuan, Q. Pyrolysis characteristics of cattle manures using a discrete distributed activation energy model. Bioresour. Technol. 2014, 172, 219–225. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Goracy, K. The use of recycled fibre composites as reinforcement for thermosets. Mech. Compos. Mater. 1994, 29, 352–356. [Google Scholar] [CrossRef]
- Palmer, J.; Ghita, O.R.; Savage, L.; Evans, K.E. Successful closed-loop recycling of thermoset composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 490–498. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Bartl, A.; Mihalyi, B.; Marini, I. Applications of renewable fibrous materials. Chem. Biochem. Eng. Q. 2004, 18, 21–28. [Google Scholar]
- Bledzki, A.K.; Kurek, K.; Barth, C.H. Development of a Thermoset part with SMC reclaim. Soc. Plast. Eng. Annu. Tech. Conf. 1992, 50, 1558–1560. [Google Scholar]
- Liu, Z.; Shi, L.; Wu, Z.; Zhao, K. Experimental Study on The Recycling Technology of Waste Thermosetting Phenolic Resin Based on Mechanical and Physical Method. Adv. Mater. Res. 2012, 482–484, 2445–2449. [Google Scholar] [CrossRef]
- Hu, J.; Dong, H.; Song, S. Research on Recovery Mechanism and Process of Waste Thermosetting Phenolic Resins Based on Mechanochemical Method. Adv. Mater. Sci. Eng. 2020, 2020, 1384194. [Google Scholar] [CrossRef]
- Bernardeau, F.; Perrin, D.; Caro, A.-S.; Benezet, J.-C.; Ienny, P. Valorization of waste thermoset material as a filler in thermoplastic: Mechanical properties of phenolic molding compound waste-filled PP composites. J. Appl. Polym. Sci. 2018, 135, 45894. [Google Scholar] [CrossRef]
- Weon, J.-I.; Sue, H.-J. Mechanical properties of talc- and CaCO3-reinforced high-crystallinity polypropylene composites. J. Mater. Sci. 2006, 41, 2291–2300. [Google Scholar] [CrossRef]
- Yang, L.; Runt, J.; Kuo, M.-C.; Huang, K.-S.; Yeh, J.-T. Regeneration and utilization of waste phenolic formaldehyde resin: A performance investigation. J. Appl. Polym. Sci. 2019, 136, 47445. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, S.; Chen, Z. Influence of particle size and addition of recycling phenolic foam on mechanical and flame retardant properties of wood-phenolic composites. Constr. Build. Mater. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Wood fibres as reinforcements in natural fibre composites: Structure, properties, processing and applications. In Natural Fibre Composites; Woodhead Publishing: Sawston, UK, 2014; pp. 3–65. [Google Scholar]
- Zheng, Y.; Jiang, Z.; Sun, Z.; Ren, H. Effect of microwave-assisted curing on bamboo glue strength: Bonded by thermosetting phenolic resin. Constr. Build. Mater. 2014, 68, 320–325. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xu, Y.; Wang, C.; Chu, F. Preparation and characterization of phenolic foams with eco-friendly halogen-free flame retardant. J. Therm. Anal. Calorim. 2013, 114, 1143–1151. [Google Scholar] [CrossRef]
- Shalbafan, A.; Benthien, J.T.; Welling, J.; Barbu, M.C. Flat pressed wood plastic composites made of milled foam core particleboard residues. Eur. J. Wood Prod. 2013, 71, 805–813. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Strydom, M.J. Critical materials and processing challenges affecting the interface and functional performance of wood polymer composites (WPCs). Mater. Chem. Phys. 2016, 171, 290–302. [Google Scholar] [CrossRef]
- Hashim, R.; Sulaiman, O.; Kumar, R.N.; Tamyez, P.F.; Murphy, R.J.; Ali, Z. Physical and mechanical properties of flame-retardant urea formaldehyde medium density fiberboard. J. Mater. Process. Technol. 2009, 209, 635–640. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.S.; Kim, S.; Kim, H.J.; Lee, B.; Deng, Y.; Feng, Q.; Luo, J. Evaluating the flammability of wood-based panels and gypsum particle board using a cone calorimeter. Constr. Build. Mater. 2011, 25, 3044–3050. [Google Scholar] [CrossRef]
- Gröning, M.; Eriksson, H.; Hakkarainen, M.; Albertsson, A.-C. Phenolic prepreg waste as functional filler with antioxidant effect in polypropylene and polyamide-6. Polym. Degrad. Stab. 2006, 91, 1815–1823. [Google Scholar] [CrossRef]
- Yamada, K.; Hung, P.; Yoshimura, K.; Taniguchi, S.; Lim, B.O.; Sugano, M. Effect of unsaturated fatty acids and antioxidants on immunoglobulin production by mesenteric lymph node lymphocytes of Sprague-Dawley rats. J. Biochem. 1996, 120, 138–144. [Google Scholar] [CrossRef]
- Gröning, M.; Hakkarainen, M.; Albertsson, A.-C. Recycling of glass fibre reinforced phenolic prepreg waste Part 2. Milled prepreg as functional filler in PP and PA6. Polym. Polym. Compos. 2004, 12, 501–509. [Google Scholar] [CrossRef]
- Asokan, P.; Osmani, M.; Price, A.D.F. Assessing the recycling potential of glass fibre reinforced plastic waste in concrete and cement composites. J. Clean. Prod. 2009, 17, 821–829. [Google Scholar] [CrossRef]
- Hulme, A.J.; Goodhead, T.C. Cost effective reprocessing of polyurethane by hot compression molding. J. Mater. Process. Technol. 2003, 139, 322–326. [Google Scholar] [CrossRef]
- Dweik, H.S.; Ziara, M.M.; Hadidoun, M.S. Enhancing Concrete Strength and Thermal Insulation Using Thermoset Plastic Waste. Int. J. Polym. Mater. 2008, 57, 635–656. [Google Scholar] [CrossRef]
- Panyakapo, P.; Panyakapo, M. Reuse of thermosetting plastic waste for lightweight concrete. Waste Manag. 2008, 28, 1581–1588. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Zhao, X.; Du, R.; Xu, S.; Wang, Y.-Z. Multiple functional materials from crushing waste thermosetting resins. Mater. Horiz. 2021, 8, 234–243. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials—Current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Kouparitsas, C.E.; Kartalis, C.N.; Varelidis, P.C.; Tsenoglou, C.J.; Papaspyrides, C.D. Recycling of the fibrous fraction of reinforced thermoset composites. Polym. Compos. 2002, 23, 682–689. [Google Scholar] [CrossRef]
- Ingrassia, L.P.; Lu, X.; Ferrotti, G.; Canestrari, F. Renewable materials in bituminous binders and mixtures: Speculative pretext or reliable opportunity? Resour. Conserv. Recycl. 2019, 144, 209–222. [Google Scholar] [CrossRef]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Kazemi, M.; Kabir, S.F.; Fini, E.H. State of the art in recycling waste thermoplastics and thermosets and their applications in construction. Resour. Conserv. Recycl. 2021, 174, 105776. [Google Scholar] [CrossRef]
- Ohemeng, E.A.; Ekolu, S.O. Strength prediction model for cement mortar made with waste LDPE plastic as fine aggregate. J. Sustain. Cem.-Based Mater. 2019, 8, 228–243. [Google Scholar] [CrossRef]
- Saxena, R.; Siddique, S.; Gupta, T.; Sharma, R.K.; Chaudhary, S. Impact resistance and energy absorption capacity of concrete containing plastic waste. Construct. Build. Mater. 2018, 176, 415–421. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Z.; Tian, Q.; Xue, S.; Qiu, F.; Peng, X.; Zhang, T. Waste to treasure: A superwetting fiber membrane from waste PET plastic for water-in-oil emulsion separation. J. Clean. Prod. 2023, 396, 136502. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Xi, W.; Zhou, H.; Yang, P.; Yao, J.; Jiang, X.; Wu, D. Upcycling plastic waste to carbon materials for electrochemical energy storage and conversion. Chem. Eng. J. 2023, 461, 141962. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, D.; Yue, X.; Xu, J.; Qiu, F.; Zhang, T. A recyclable and regenerated aerogel membrane derived from waste plastic for emulsion separation. J. Environ. Chem. Eng. 2022, 10, 108221. [Google Scholar] [CrossRef]
- Adibfar, M.; Kaghazchi, T.; Asasian, N.; Soleimani, M. Conversion of Poly(Ethylene Terephthalate) Waste into Activated Carbon: Chemical Activation and Characterization. Chem Eng Technol. 2014, 37, 979–986. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Zhang, J.; Huo, P. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef]
- Zhuang, Z.; Xiong, Q.; Zhang, T.; Yang, D.; Qiu, F.; Yue, X. Upcycling of waste brick powder and PET as robust and flexible Janus membrane with asymmetric wettability for switchable emulsion separation. J. Environ. Chem. Eng. 2023, 11, 111171. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Li, J.; Zhou, W.; Zhang, J.; Liu, X.; Song, X.; Wang, H.; Huo, P. Removal of polyethylene terephthalate plastics waste via Co–CeO2 photocatalyst–activated peroxymonosulfate strategy. Chem. Eng. J. 2024, 479, 147781. [Google Scholar] [CrossRef]
- Qiao, W.M.; Yoon, S.H.; Mochida, I.; Yang, J.H. Waste polyvinylchloride derived pitch as a precursor to develop carbon fibers and activated carbon fibers. Waste Manag. 2007, 27, 1884–1890. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, X.; Meng, Y.; Shen, P. Preparation of hollow carbon spheres by carbonization of polystyrene/polyaniline core-shell polymer particles. New Carbon Mater. 2011, 26, 389–395. [Google Scholar] [CrossRef]
- Garforth, A.A.; Ali, S.; Hernández-Martínez, J.; Akah, A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. Sci. 2004, 8, 419–425. [Google Scholar] [CrossRef]
- Gusse, A.C.; Miller, P.D.; Volk, T.J. White-Rot Fungi Demonstrate First Biodegradation of Phenolic Resin. Environ. Sci. Technol. 2006, 40, 4196–4199. [Google Scholar] [CrossRef]
- Barr, D.P.; Aust, S.D. Mechanisms white rot fungi use to degrade pollutants. Environ. Sci. Technol. 1994, 28, 78A–87A. [Google Scholar] [CrossRef]
- Cameron, M.D.; Timofeevski, S.; Aust, S.D. Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl. Microbiol. Biotechnol. 2000, 54, 751–758. [Google Scholar] [CrossRef]
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Milstein, O.; Gersonde, R.; Hutterman, A.; Chen, M.-J.; Meister, J. Fungal Bioremediation of Lignin Graft Copolymers from Ethene Monomers. J. Macromol. Sci. A 1996, 33, 685–702. [Google Scholar] [CrossRef]
- Chen, M.J.; Gunnells, D.W.; Gardner, D.J.; Milstein, O.; Gersonde, R.; Feine, H.J.; Huttermann, A.; Frund, R.; Ludemann, H.D.; Meister, J.J. Graft copolymers of lignin with 1-ethenylbenzene. 2. Properties. Macromolecules 1996, 29, 1389–1398. [Google Scholar] [CrossRef]
- Williams, J.R.; Clifford, A.A.; Al-Saidi, S.H.R. Supercritical Fluids and Their Applications in Biotechnology and Related Areas. Mol. Biotechnol. 2002, 22, 263–286. [Google Scholar] [CrossRef]
- Tagaya, H.; Suzuki, Y.; Asou, T.; Kadokawa, J.; Chiba, K. Reaction of Model Compounds of Phenol Resin and Molding Materials of Phenol Resin in Supercritical Water for Chemical Recycling of Polymer Waste. Chem. Lett. 1998, 27, 937–938. [Google Scholar] [CrossRef]
- Ozaki, J.; Djaja, S.K.I.; Oya, A. Chemical Recycling of Phenol Resin by Supercritical Methanol. Ind. Eng. Chem. Res. 2002, 39, 245–249. [Google Scholar] [CrossRef]
- Yang, J.D.; Zhang, T.; Cai, J.T.; Niu, B.; Zhang, Y.Y.; Long, D.H. Investigating the pyrolysis mechanisms of three archetypal ablative resins through pyrolysis experiments and ReaxFF MD simulations. Mater. Today Commun. 2023, 36, 106683. [Google Scholar] [CrossRef]
- Bennett, A.; Payne, D.R.; Court, R.W. Pyrolytic and Elemental Analysis of Decomposition Products from a Phenolic Resin. Macromol. Symp. 2014, 339, 38–47. [Google Scholar] [CrossRef]
- Yu, C.; Luo, W.B.; Zhong, S.L. Ga/ZSM-5 Catalyzed Pyrolysis of Phenolic Resin for the Selective Preparation of Monocyclic Aromatic. Adv. N&R Energy 2023, 11, 1–7. [Google Scholar]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Chemical Recycling of Printed Circuit Board Waste by Depolymerization in Sub- and Supercritical Solvents. Waste Biomass Valorization 2015, 6, 959–965. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, N.; Wang, C.Z.; Li, H.; Jia, S.Y.; Fan, H.H.; Cui, X.J.; Hou, X.L.; Deng, T.S. Affinity of K+ to organic matter promotes reactions: Degradation of super stable phenolic epoxy vinyl ester resin to value-added chemicals. Green Chem. 2023, 25, 5213–5221. [Google Scholar] [CrossRef]
- Freeman, H.S. Aromatic amines: Use in azo dye chemistry. Front. Biosci. Landmark 2013, 18, 145–164. [Google Scholar] [CrossRef]
- Arora, P.K. Bacterial degradation of monocyclic aromatic amines. Front. Microbiol. 2015, 6, 152548. [Google Scholar] [CrossRef]
- Xu, L.; He, Z.; Zhang, H.; Wu, S.; Dong, C.; Fang, Z. Production of aromatic amines via catalytic co-pyrolysis of lignin and phenol-formaldehyde resins with ammonia over commercial HZSM-5 zeolites. Bioresour. Technol. 2020, 320, 124252. [Google Scholar] [CrossRef]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. Production of phenols from pyrolysis of sugarcane bagasse lignin: Catalyst screening using thermogravimetric analysis-thermal desorption-gas chromatography-mass spectroscopy. J. Anal. Appl. Pyrolysis 2019, 138, 120–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Hu, B.; Deng, J.; Yao, Q.; Zhang, X.; Liu, X.; Fu, Y.; Lu, Q. Direct conversion of cellulose and raw biomass to acetonitrile by catalytic fast pyrolysis in ammonia. Green Chem. 2019, 21, 812–820. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Liu, C.; Tao, L.; Huang, Y.; Zheng, Z. Integrated production of aromatic amines, aromatic hydrocarbon and N-heterocyclic bio-char from catalytic pyrolysis of biomass impregnated with ammonia sources over Zn/HZSM-5 catalyst. J. Energy Inst. 2019, 93, 210–223. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Q.; Zhang, Y.; Fu, Y. Integrated production of aromatic amines and N-doped carbon from lignin via ex situ catalytic fast pyrolysis in the presence of ammonia over zeolites. ACS Sustain. Chem. Eng. 2017, 5, 2960–2969. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, Q.; Dong, Q.; Zhang, L.Y.; Fang, Z. Co-production of phenolic oil and CaO/char deoxidation catalyst via catalytic fast pyrolysis of phenol formaldehyde resin with Ca(OH)2. J. Anal. Appl. Pyrolysis 2019, 142, 104663. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Shen, D.; Gu, S.; Luo, K.; Wang, S.; Fang, M. The pyrolytic degradation of wood derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.; Lu, C.; Li, S.; Zhu, M. Molten salt technique for synthesis of carbon-based materials for supercapacitor. Green Chem. 2023, 25, 10209–10234. [Google Scholar] [CrossRef]
- Tong, Y.; Jiang, B.; Chen, X.; Ren, X.; Lu, J.; Ding, L. Synergistic degradation of methylene blue by laser cavitation and activated carbon fiber. Opt. Laser Technol. 2022, 155, 108417. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, W.; Xie, H.; Zhang, K. Two types of nitrogen and sulfur co-doped carbons derived from soybean sprouts enabling high performance lithium-sulfur batteries. J. Energy Storage 2023, 68, 107790. [Google Scholar] [CrossRef]
- Chen, Z.; He, R. Molecular simulation of the effects of activated carbonstructure on the adsorption performance for volatile organiccompounds in fumes from gasoline. Environ. Prog. Sustain. Energy 2023, 42, e14156. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Y.; Ma, Y. Salt-assisted synthesis of advanced carbon-based materials for energy-related applications. Green Chem. 2023, 25, 10263–10303. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, G.; Chen, L.; Zhong, J.; Chen, Y.; Zhu, G.; Chuan, X.; Zhang, J.; Shen, X. Defect engineering of hollow porous N, S co-doped carbon spheres-derived materials for high-performance hybrid supercapacitors. Chem. Eng. J. 2024, 480, 148213. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, J.; Li, W.; Wang, A.; Tang, Y.; Zhang, M.; Xie, J.; Wang, Z.; Hu, Z. Stabilizing sulfur doped manganese oxide active sites with phosphorus doped hierarchical nested square carbon for efficient asymmetric supercapacitor. Chem. Eng. J. 2023, 468, 143574. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, X.; Zhou, H.; Cao, J.; Pu, J.; Ji, Z.; Kong, L.; Yuan, A. Vanadium hexacyanoferrate nanoparticles connected by cross-linked carbon nanotubes conductive networks for aqueous zinc-ion batteries. Chem. Eng. J. 2022, 448, 137657. [Google Scholar] [CrossRef]
- Zhang, W.J.; Sun, M.X.; Yan, X.H.; You, M.Y.; Li, Y.L.; Zhu, Y.H.; Zhang, M.Y.; Zhu, W.; Javed, M.S.; Pan, J.M.; et al. Pseudocapacitive brookite phase vanadium dioxide assembled on carbon fiber felts for flexible supercapacitor with outstanding electrochemical performance. J. Energy Storage 2022, 47, 103593. [Google Scholar] [CrossRef]
- Yuan, C.; Xu, H.; El-khodary, S.A.; Ni, G.; Esakkimuthu, S.; Zhong, S.; Wang, S. Recent advances and challenges in biomass-derived carbon materials for supercapacitors: A review. Fuel 2024, 362, 130795. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Dou, Z.; Liu, Y. Removal of gaseous Hg0 using biomass porous carbons modified by an environmental-friendly photochemical technique. Chem. Eng. J. 2023, 457, 141152. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, C.; He, S.; Jiang, D.; Cao, B.; Wang, S. Machine learning prediction of specific capacitance in biomass derived carbon materials: Effects of activation and biochar characteristics. Fuel 2023, 331, 125718. [Google Scholar] [CrossRef]
- Ren, M.; Ji, X.; Kong, F.; Zhou, C.; Yagoub, A.E.A.; Liang, J.; Ye, X.P.; Gu, Z.; Ma, Q.; Fan, X.; et al. Preparation and characterization of bio-inspired full-biomass-derived aerogel with vertically aligned structure. Chem. Eng. J. 2023, 475, 146425. [Google Scholar] [CrossRef]
- Yoo, J.; Yang, I.; Kwon, D.; Jung, M.; Kim, M.-S.; Jung, J. Low-Cost Carbon Xerogels Derived from Phenol–Formaldehyde Resin for Organic Electric Double-Layer Capacitors. Energy Technol. 2021, 9, 2000918. [Google Scholar] [CrossRef]
- Veselov, G.B.; Vedyagin, A.A. Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects. Materials 2023, 16, 6566. [Google Scholar] [CrossRef]
- Liu, L.L.; Tian, S.; Zhu, Y.S.; Tang, W.; Li, L.L.; Wu, Y.P. Nanoporous Carbon as Anode Material of High Rate Capability for Lithium Ion Batteries. J. Chin. Chem. Soc. 2012, 59, 1216–1219. [Google Scholar] [CrossRef]
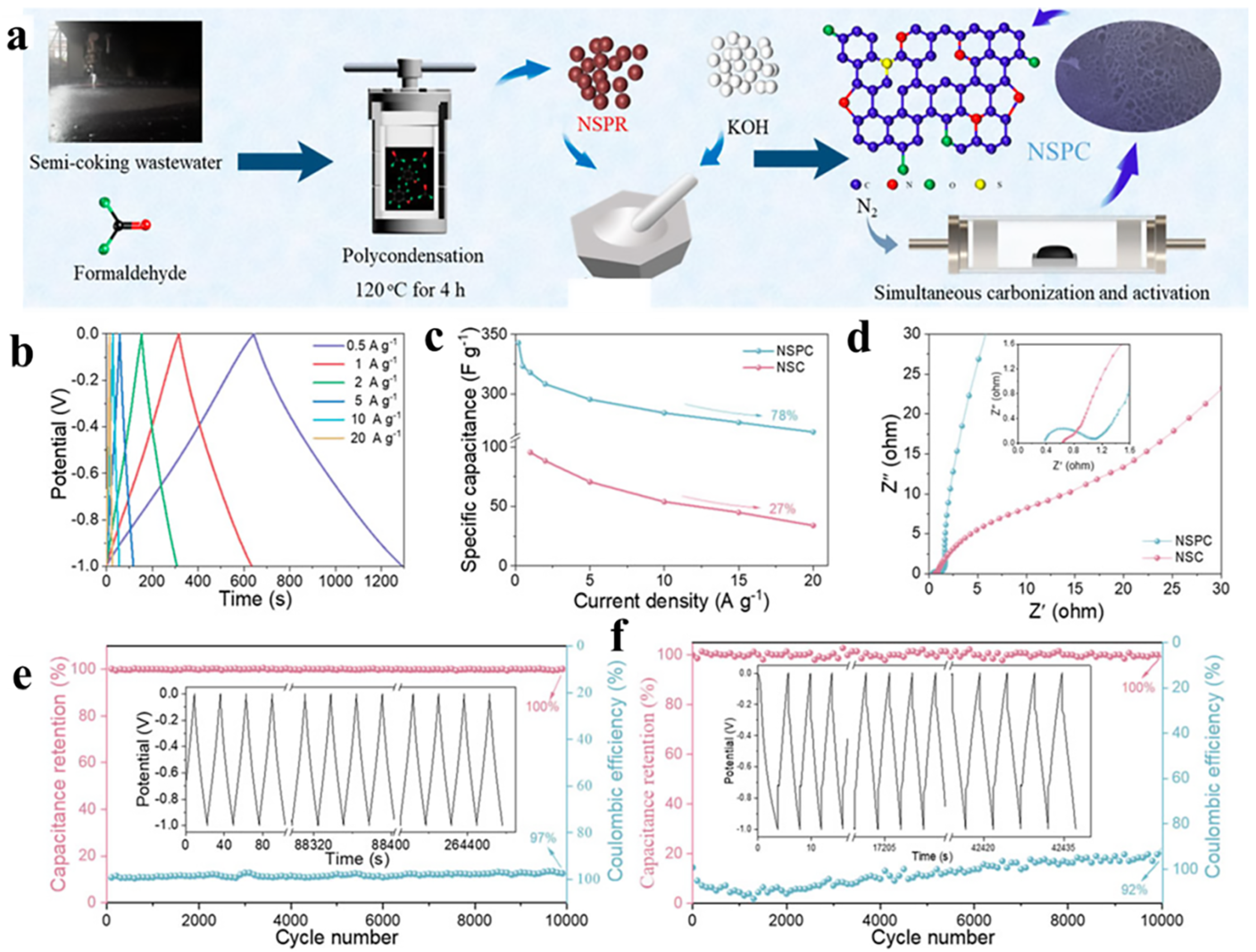
- Yan, L.; Wang, X.; Wang, Y.; Li, J.; Liu, Q.; Zhong, X.; Chang, Y.; Li, Q.; Verm, S.K. Self-doped N, S porous carbon from semi-coking wastewater-based phenolic resin for supercapacitor electrodes. Front. Chem. 2022, 10, 21394. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Shao, S.; Wang, Y.; Ma, L.; Huang, Z.; Li, X. Sustainable preparation of hierarchical porous carbon from discarded shells of crustaceans for efficient CO2 capture. Fuel 2024, 355, 129287. [Google Scholar] [CrossRef]
- Hao, C.; Ni, C.; Wang, X.; Pan, Y.; Wu, Q.; Wu, J.; Wang, X. Fabrication of three-dimensional CuS2@CoNi2S4 core–shell rod-like structures as cathode and thistle-derived carbon as anode for hybrid supercapacitors. Chem. Eng. J. 2023, 465, 143024. [Google Scholar] [CrossRef]
- Zhang, Y.; Ran, H.; Liu, X.; Zhang, X.; Zhu, T.; Yin, J.; Zhang, J.; Zhu, L.; He, J.; Li, H.; et al. One-step solvent-free approach to constructing microporous BCNO to enhance adsorptive desulfurization. Chem. Eng. J. 2023, 477, 147019. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of activated carbons prepared from wheat straw via ZnCl2 and KOH activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Rajak, V.; Kumar, S.; Thombre, N.; Mandal, A. Synthesis of activated charcoal from saw-dust and characterization for adsorptive separation of oil from oil-in-water emulsion. Chem. Eng. Commun. 2018, 205, 897–913. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.Y.; Gao, B.Y.; Li, A.M. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhang, X.; Wang, R.; Kou, L.; Li, R.; Fan, C. Preoxidation-assisted nitrogen enrichment strategy to decorate porous carbon spheres for catalytic adsorption/oxidation of methyl mercaptan. RSC Adv. 2020, 10, 37644–37656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, R.; Wang, Y.; Ren, G.; Zhang, X.; Li, R.; Fan, C. Synthesis of millimeter-sized porous carbon spheres derived from different precursors for CO2 capture. J. Porous Mater. 2021, 28, 81–91. [Google Scholar] [CrossRef]
- Liu, S.; Rao, L.; Yang, P.; Wang, X.; Wang, L.; Ma, R.; Yue, L.; Hu, X. Superior CO2 uptake on nitrogen doped carbonaceous adsorbents from commercial phenolic resin. J. Environ. Sci. 2020, 93, 109–116. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Chuah, C.Y.; Karahan, H.E.; Birer, Ö.; Bae, T.-H. Sub-Ångström-level engineering of ultramicroporous carbons for enhanced sulfur hexafluoride capture. Carbon 2019, 155, 56–64. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, Y.-S.; Chiu, C.-Y.; Hung, K.-Y.; Chou, S.-S. Microwave-Catalyzed Conversion of Phenolic Resin Waste to Activated Carbon and Its Applications for Removing Ammonium from Water. Catalysts 2021, 11, 783. [Google Scholar] [CrossRef]
- Grant, E.; Halstead, B.J. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar]
- Jones, D.A.; Lelyveld, T.; Mavrofidis, S.; Kingman, S.; Miles, N. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Appleton, T.; Colder, R.; Kingman, S.; Lowndes, I.; Read, A.G. Microwave technology for energy-efficient processing of waste. Appl. Energy 2005, 81, 85–113. [Google Scholar] [CrossRef]
- Luque, R.; Menendez, J.A.; Arenillas, A.; Cot, J. Microwave-assisted pyrolysis of biomass feedstocks: The way forward? Energy Environ. Sci. 2012, 5, 5481–5488. [Google Scholar] [CrossRef]
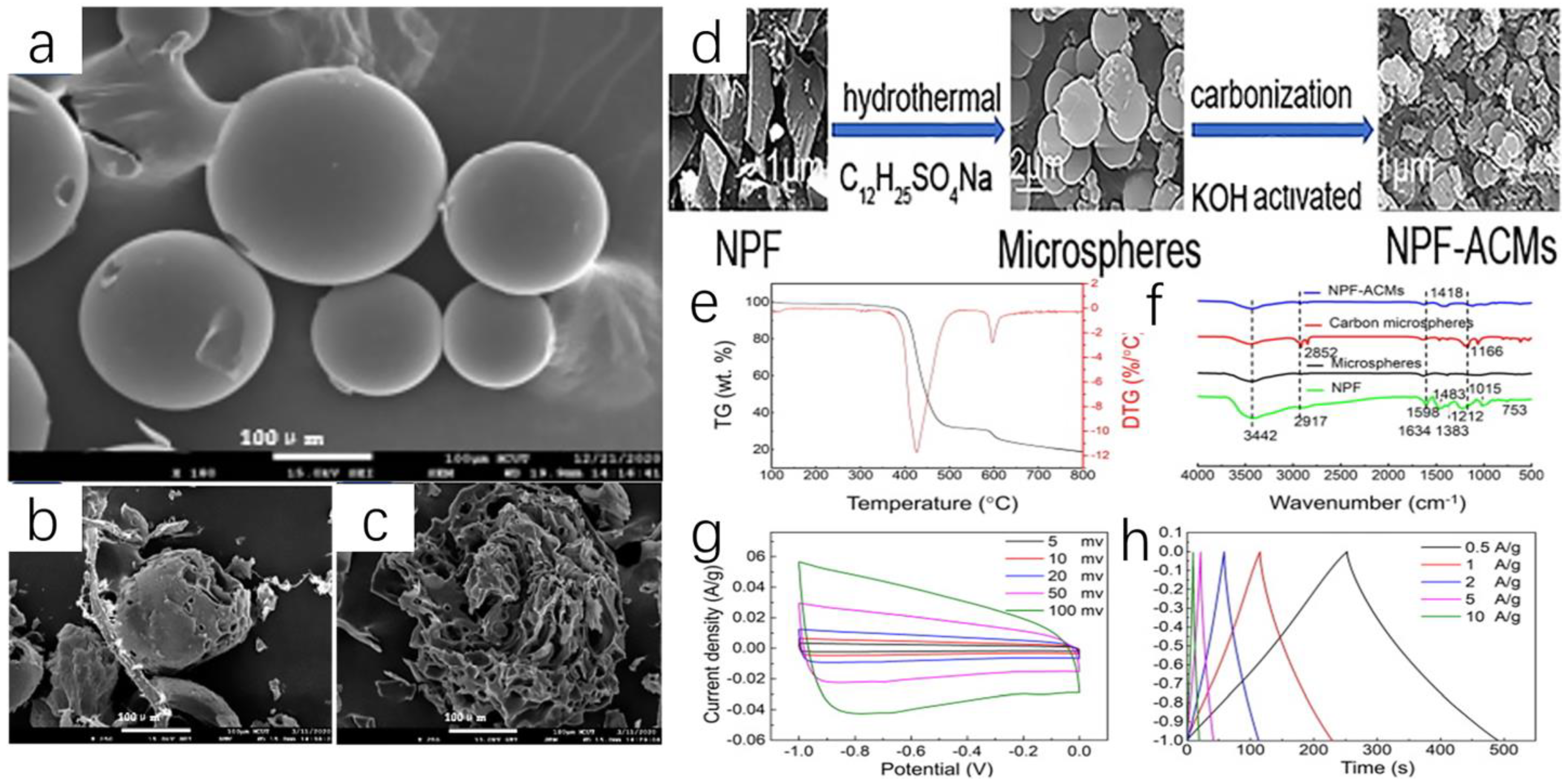
- Guan, M.; Li, H.; Chen, X.; Zhang, S. Preparation and electrochemical performance of activated carbon microspheres from recycled novolak phenol formaldehyde. Waste Manag. 2021, 120, 635–641. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, Y.; Guo, Q.X. Separation and recycle of phenol from wastewater by liquid-liquid extraction. Sep. Sci. Technol. 2003, 38, 2579–2596. [Google Scholar] [CrossRef]
- Qu, J.Y.; Han, Q.; Gao, F.; Qiu, J.S. Carbon foams produced from lignin-phenol-formaldehyde resin for oil/water separation. New Carbon Mater. 2017, 32, 86–91. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, H.; Yan, Y.; Tang, C. Preparation of core-shell silicon/porous carbon powders. Carbon 2010, 48, 316. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wu, L.; Tong, K.; Zhang, Q.; Liu, Y.; Zhang, J.F.; Liu, J.M.; Shu, S.S.; Xu, J.H.; Hu, Y. Study on the preparation of carbon nanotubes by iron modified phenolic resin pyrolysis. Conf. Ser. Mater. Sci. Eng. 2018, 423, 012088. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Sun, G.; Wang, J. Synthesis and characterization of activated carbon with a high mesoporosity. Carbon 2011, 49, 3707. [Google Scholar] [CrossRef]







| Raw Waste Material | Formulation | Condition | Application | Ref. |
|---|---|---|---|---|
| Phenolic resin (PF) | Feed particle diameter of 0.43 mm, feed volume of 60 g | Rotation speed of 2820 r/min, time of 80 min | Sheet material | [35] |
| Fiber-reinforced polymer (FRP) | 5% glass fiber reinforced plastic waste fiber | In water at 20 °C and in oven at 50 °C | Concrete material | [51] |
| Polyurethane (PU) | 70 wt% recyclate granules, 30 wt% resin binder | - | Sandwich materials | [52] |
| Melamine–formaldehyde (MF) | 0–60 wt% MF waste | - | Concrete material | [53] |
| Thermosetting plastics | The ratio of cement, sand, fly ash and plastic: 1.0:0.8:0.3:0.9 | - | Lightweight concrete | [54] |
| Epoxy resin (EP) | the particle size of 0.45 mm, the column thickness of 2 cm | - | Separating oil–water mixtures | [55] |
| Bulk molding compound (BMC) | - | - | Fillers or partial Reinforcement | [56] |
| Glass–polyester composites | 40 wt% recovered glass fibers | Mixing temperature: 200 °C for 8 min; torque level: 800 gm | New thermoplastic composites | [57] |
| Glass fiber-reinforced SMC | 10 wt% direct weight substitution | Cured at 145 °C and 4.1 MPa for 3 min | As reinforcement in new DMCs | [29] |
| Sample | Temperature (°C) | Nominal Time (min) | Composition (%) | ||
|---|---|---|---|---|---|
| C | H | O | |||
| Phenol resin | 74.9 | 5.5 | 19.6 | ||
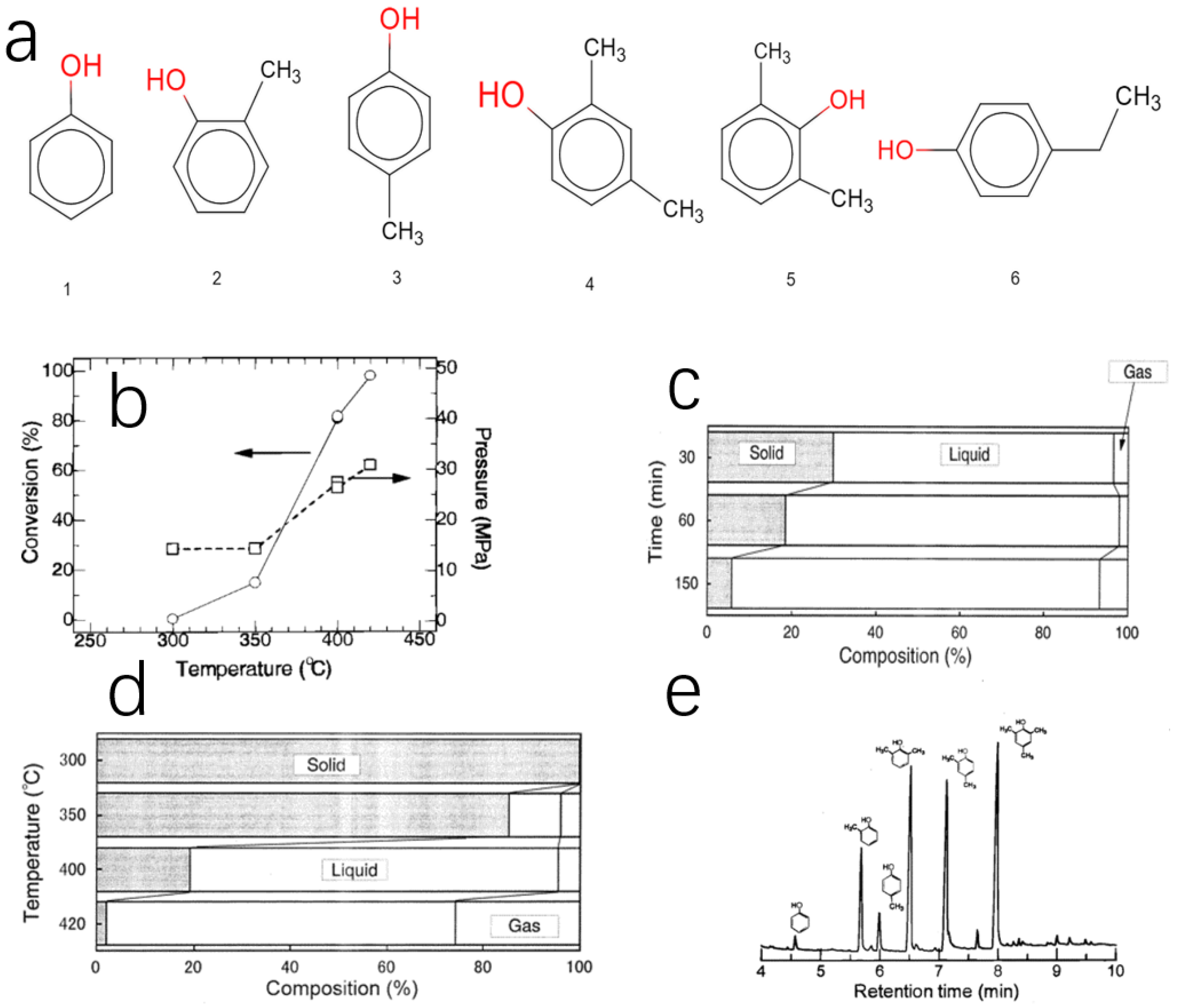
| Solid residue ① | 400 | 30 | 84.2 | 6.0 | 9.8 |
| Solid residue ② | 400 | 60 | 84.9 | 6.0 | 9.6 |
| Solid residue ③ | 400 | 150 | 89.9 | 3.9 | 6.2 |
| Sample | Reaction Condition | Reaction Product | Ref. |
|---|---|---|---|
| boron phenolic resin | at 1000 °C for 10 min | H2, CO, CH4, C2∼C4 hydrocarbons | [83] |
| high-carbon phenolic resin | at 1000 °C for 10 min | H2, CO, CO2, CH4, C2∼C4 hydrocarbons | [83] |
| silica-containing phenolic resin | at 1000 °C for 10 min | H2, CO, CH4, C2∼C4 hydrocarbons | [83] |
| resole phenolic resin | - | char, volatile organic compounds (VOCs) and permanent gases | [84] |
| phenolic resin | Ga-modified ZSM-5 catalysts; at 800 °C | monocyclic aromatic hydrocarbons | [85] |
| printed circuit boards | at 400 °C; NaOH; H2O | phenol and some phenolic compounds | [86] |
| phenolic epoxy vinyl ester resin | at 160 °C; KOH/C2H5OH-H2O | styrene and methacrylic acid, novolac glycidyl ether | [87] |
| Sample | Specific Surface Area (m2/g) * | Total Pore Volume (cm3/g) | Long-Term Adsorption/Desorption Cycling Stability | Ref. |
|---|---|---|---|---|
| ACSON | 1710 | 0.83 | 97% after ten cycles | [126] |
| Ph6M4Cs11 | 2020 | 0.968 | Almost unchanged after ten cycles | [128] |
| ACK-MW | 924 | 0.46 | - | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Jiang, X.; Li, S.; Zhu, M. An Overview of Recycling Phenolic Resin. Polymers 2024, 16, 1255. https://doi.org/10.3390/polym16091255
Zhu B, Jiang X, Li S, Zhu M. An Overview of Recycling Phenolic Resin. Polymers. 2024; 16(9):1255. https://doi.org/10.3390/polym16091255
Chicago/Turabian StyleZhu, Bing, Xinyao Jiang, Songjun Li, and Maiyong Zhu. 2024. "An Overview of Recycling Phenolic Resin" Polymers 16, no. 9: 1255. https://doi.org/10.3390/polym16091255





