Metal-Doped NASICON/Polymer Composite Solid Electrolyte for Lithium Titania Anode in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Sol–Gel Synthesis of Metal-Doped LATP Powders
2.2. Fabrication of CSEs on LTO Anode Sheets
2.3. Material and Electrochemical Characterizations
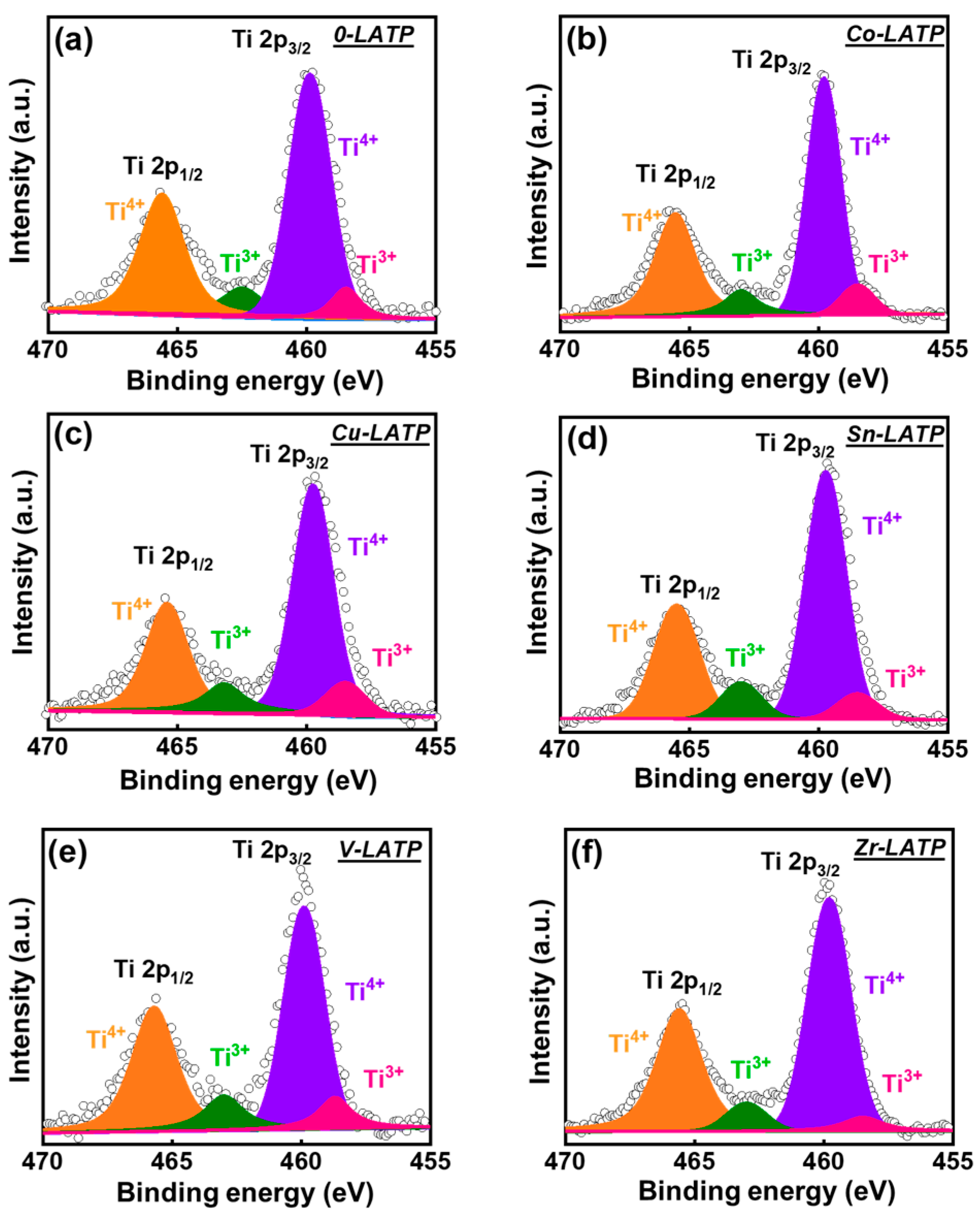
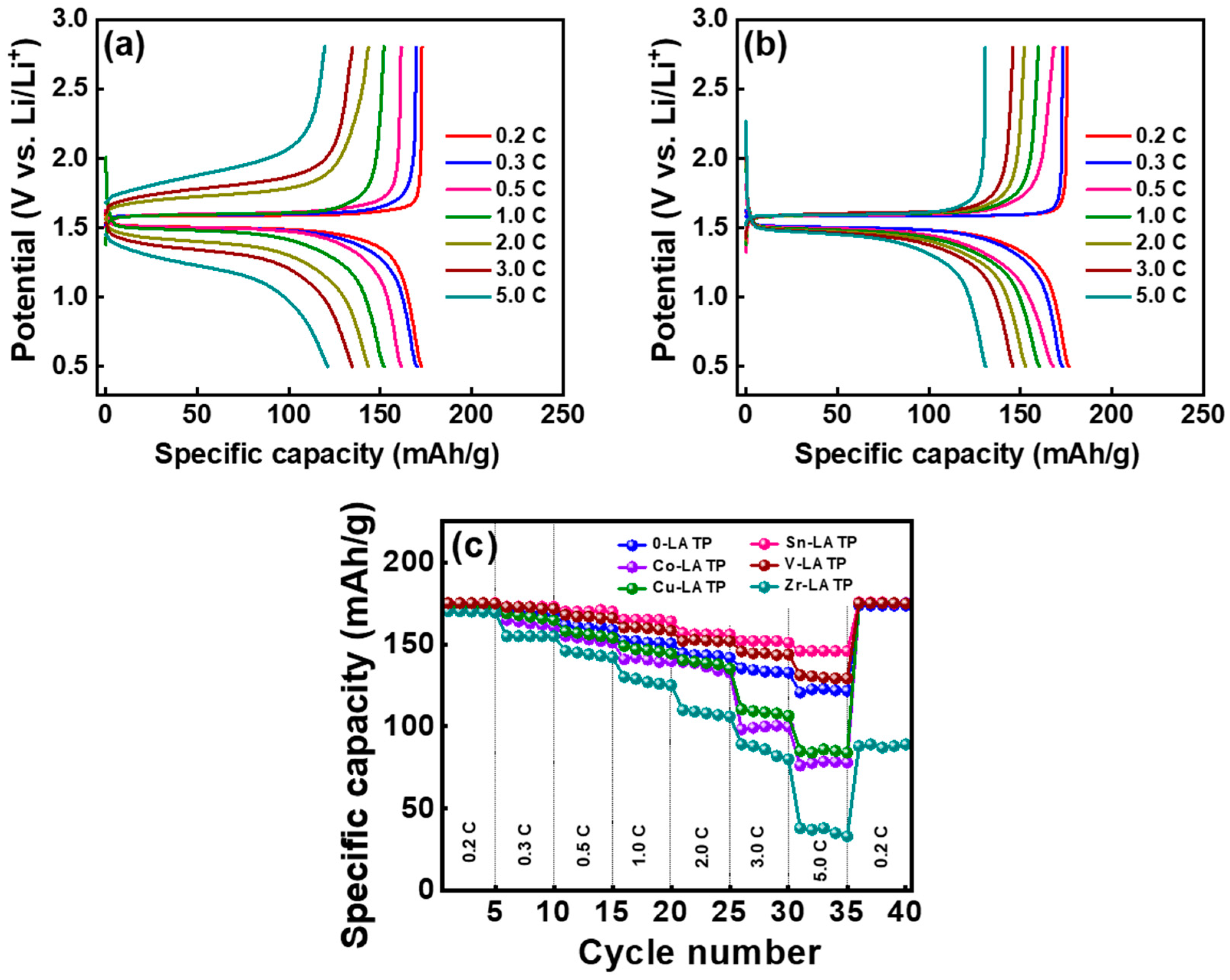
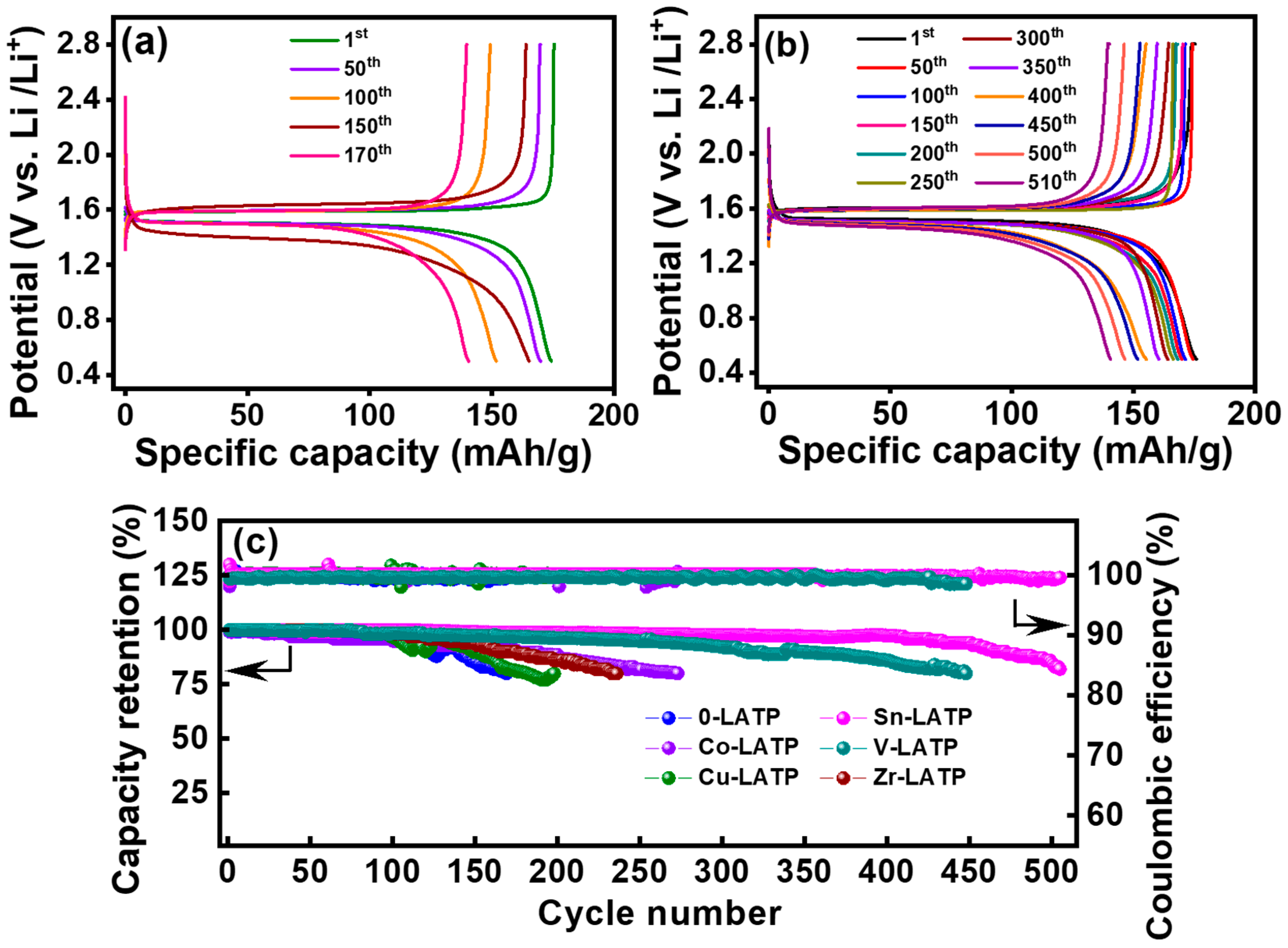
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Patra, J.; Nguyen, T.X.; Tsai, C.C.; Clemens, O.; Li, J.; Pal, P.; Chan, W.K.; Lee, C.H.; Chen, H.Y.T.; Ting, J.M.; et al. Effect of elemental modulation on phase purity and electrochemical properties of Co-free high entropy spinel oxide anodes for lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2110992. [Google Scholar] [CrossRef]
- Panda, A.; Patra, J.; Hsieh, C.T.; Huang, Y.C.; Gandomi, Y.A.; Fu, C.C.; Lin, M.H.; Juang, R.S.; Chang, J.K. Improving high-temperature performance of lithium-rich cathode by roll-to-roll atomic layer deposition of titania nanocoating for lithium-ion batteries. J. Energy Storage 2021, 44, 103348. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghamouss, F. Role of electrolytes in the stability and safety of lithium titanate-based batteries. Front. Mater. 2020, 7, 186. [Google Scholar] [CrossRef]
- Sawai, K.; Iwakoshi, Y.; Ohzuku, T. Carbon materials for lithium-ion (shuttlecock) cells. Solid State Ion. 1994, 69, 273–283. [Google Scholar] [CrossRef]
- Xu, G.; Han, P.; Dong, S.; Liu, H.; Cui, G.; Chen, L. Li4Ti5O12-based energy conversion and storage systems: Status and prospects. Coord. Chem. Rev. 2017, 343, 139–184. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamental and advances in rechargeable batteries. Infomat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolyte. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Mishra, M.; Hsu, C.W.; Rath, P.C.; Patra, J.; Lai, H.Z.; Chang, T.L.; Wang, C.Y.; Wu, T.Y.; Lee, T.C.; Chang, J.K. Ga-doped lithium lanthanum zirconium oxide electrolyte for solid-state Li batteries. Electrochim. Acta 2020, 353, 136536. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Ku, M.C.; Hsieh, C.T.; Sung, P.Y.; Chen, P.S.; Mohanty, D.; Gandomi, Y.A.; Hung, I.M.; Patra, J.; Chang, J.K. Ceramicized NASICON-based solid-state electrolytes for lithium metal batteries. J. Solid State Electrochem. 2023. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Hong, X.; He, X. Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanism and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.C.; Hsu, W.L.; Chen, C.C.; Huang, C.Y.; Wu, W.W.; Okada, S.; Dong, Q.F.; Yang, C.C.; Lee, T.C.; Chang, J.K. Dual interface design of Ga-doped Li7La3Zr2O12/polymer composite electrolytes for solid-state lithium batteries. Int. J. Energy Res. 2022, 46, 17693–17705. [Google Scholar] [CrossRef]
- Yang, C.; Liu, B.; Jiang, F.; Zhang, Y.; Xie, H.; Hitz, E.; Hu, L. Garnet/polymer hybrid ion-conducting protective layer for stable lithium metal anode. Nano Res. 2017, 10, 4256–4265. [Google Scholar] [CrossRef]
- Ding, W.Q.; Lv, F.; Xu, N.; Wu, M.T.; Liu, J.; Gao, X.P. Polyethylene oxide-based solid-state composite polymer electrolytes for rechargeable lithium batteries. ACS Appl. Energy Mater. 2021, 4, 4581–4601. [Google Scholar] [CrossRef]
- Samson, A.J.; Hofstetter, K.; Bag, S.; Thangadurai, V. A bird’s-eye view of Li-stuffed garnet-type Li7La3Zr2O12 ceramic electrolytes for advanced all-solid-state Li batteries. Energy Environ. Sci. 2019, 12, 2957–2975. [Google Scholar] [CrossRef]
- Zhu, P.; Yan, C.; Dirican, M.; Zhu, J.; Zang, J.; Selvan, R.K.; Chung, C.C.; Jia, H.; Li, Y.; Kiyak, Y.; et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries. J. Mater. Chem. A 2018, 6, 4279–4285. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.; Fan, L.; Zhang, J.; Li, X. Recent advances in Li1+xAlxTi2−x(PO4)3 solid-state electrolytes for safe lithium batteries. Energy Storage Mater. 2019, 19, 379–400. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Deng, W.; Shuai, H.; Li, S.; Xu, Z.; Li, J.; Hou, H.; Peng, H.; Zou, G.; et al. Garnet solid electrolytes for advanced all-solid-state Li batteries. Adv. Energy Mat. 2020, 11, 2000648. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; Neill, J.O.; Dura, J.A.; Wachsman, E.D. High sulfur loading and capacity retention in bilayer garnet sulfurized-polyacrylonitrile/lithium-metal batteries with gel polymer electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Yang, K.; Chen, L.; Ma, J.; He, Y.B.; Kang, F. Progress and perspective of Li1+xAlxTi2−x(PO4)3 ceramic electrolyte in lithium batteries. Infomat. 2021, 3, 1195–1217. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, X.; Xiong, X.; Zhang, Q.; Peng, B.; Chen, Y.; Wang, Z.; Fu, L.; Wu, Y. A solid electrolyte based on electrochemical active Li4Ti5O12 with PVDF for solid-state lithium metal battery. Adv. Energy Mater. 2022, 12, 2201991. [Google Scholar] [CrossRef]
- Cao, C.; Zhong, Y.; Chen, B.; Cai, R.; Shao, Z. A Li- Li4Ti5O12 composite anode for reducing interfacial resistance of solid-state batteries. Small Struct. 2023, 4, 2200374. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Lou, S.; Ma, Y.; Du, C.; Gao, Y.; Yin, G. Enhanced electrochemical performance of Li4Ti5O12 through in-situ coating 70Li2S-30P2S5 solid electrolyte for all solid-state lithium batteries. J. Alloys Compd. 2018, 752, 8–13. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K. Effect of doping of trivalent cations Ga3+, Sc3+, Y3+ in Li1.3Al0.3Ti1.7 (PO4)3 (LATP) system on Li+ ion conductivity. Phys. Rev. B Condens. 2016, 501, 90–94. [Google Scholar] [CrossRef]
- Wang, R.; Chen, B.; Liu, C.; Yin, W.; Chen, H.; Zhang, J.; Zhang, T.; Sun, L.; Liu, X. Improving the ionic conductivity of Li1+XAlXTi2-X (PO4)3 in a solid-state synthesis by regulating Li-O bond with B3+ and Y3+. J. Electrochem. Soc. 2022, 169, 120535. [Google Scholar] [CrossRef]
- Mashekova, A.; Baltash, Y.; Yegamkulov, M.; Trussov, I.; Bakenov, Z.; Mukanova, A. Polycation doping of the LATP ceramic electrolyte for Li-ion batteries. RSC Adv. 2022, 12, 29595–29601. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, Z.; Liu, S.; Huang, K.; Hai, J. Influence of rare-earth elements on the ionic conductivity of LATP electrolyte and its applications in assembled cells. J. Am. Ceram. Soc. 2024, 107, 2407–2420. [Google Scholar] [CrossRef]
- Pradeepa, P.; Raj, S.E.; Sowmya, G.; Kalaiselvimary, J.; Prabhu, M.R. Optimization of hybrid polymer electrolytes with the effect of lithium salt concentration in PEO/PVDF-HFP blends. Mater. Sci. Eng. B. 2016, 205, 6–17. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, K.; Liu, Y.; Gao, H.; Bai, Y.; Wang, X.; Wu, C. Polymer electrolytes and interfaces towards solid-state batteries: Recent advances and prospects. Energy Storage Mater. 2020, 33, 26–54. [Google Scholar] [CrossRef]
- Luo, T.; Liu, B.; Han, W.; Zhu, G.; Liang, J.; Wang, L.; Yang, J.; Wang, L.; Liu, S. Enhanced ion-electron mixing interface for high energy solid-state lithium metal batteries. J. Colloid Interface Sci 2023, 652, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolyte for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Shen, S.P.; Tang, G.; Li, H.J.; Zhang, L.; Zheng, J.C.; Luo, Y.; Yue, J.P.; Shi, Y.; Chen, Z. Low-temperature fabrication of NASICON-type LATP with superior ionic conductivity. Ceram. Int. 2022, 48, 36961. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Shi, Q.; Zhong, C.; Gong, D.; Wang, X.; Zhan, C.; Liu, G. In-situ constructed SnO2 gradient buffer layer as a tight and robust interphase toward Li metal anodes in LATP solid-state batteries. J. Energy Chem. 2023, 80, 89–98. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Zhou, G.; Yu, G.; Manthiram, A. Durability of the Li1+XTi2-XAlX(PO4)3 solid electrolyte in Lithium-sulfur batteries. ACS Energy Lett. 2016, 1, 1080–1085. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Zhao, B.; Zhang, L.; Xiao, X.; Yan, H.; Xu, G.; Ma, L.; Liu, Y. Transport and interface characteristics of Te-doped NASICON solid electrolyte Li1.3Al0.3Ti1.7(PO4)3. Electrochim. Acta 2021, 399, 139367. [Google Scholar] [CrossRef]
- He, C.; Tao, J. 2D Co6Mo6C nanosheets as robust hydrogen evolution reaction electrocatalyst. Adv. Sustain. Syst. 2018, 2, 1700136. [Google Scholar] [CrossRef]
- Chen, F.; Chen, C.; Hu, Q.; Xiang, B.; Song, T.; Zou, X.; Li, W.; Xiong, B.; Deng, M. Synthesis of CuO@CoNiLDH on Cu foam for high-performance supercapacitors. Chem. Eng. J. 2020, 401, 126145. [Google Scholar] [CrossRef]
- Liu, X.; Najam, T.; Yasin, G.; Kumar, M.; Wang, M. One-pot synthesis of high-performance tin chalcogenides/C anodes for Li-ion batteries. ACS Omega 2021, 6, 17391–17399. [Google Scholar] [CrossRef] [PubMed]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; Gryse, R.D. Determination of the V 2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectros. Relat. Phenomena 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Xing, L.; Cheng, X.; Zhang, X.; Li, H.; Liu, M. Effect of Zr modification on NH3-SCR reaction performance of Cu-Ce/SAPO-34 catalysts. Appl. Sci. 2023, 13, 4763. [Google Scholar] [CrossRef]
- Mohammad, I.M.A.; Kanagaraj, P.; Yasin, A.S.; Iqbal, W.; Liu, C. Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells. J. Alloys Compd. 2020, 816, 152513. [Google Scholar] [CrossRef]
- Rath, P.C.; Jheng, Y.S.; Chen, C.C.; Tsai, C.L.; Su, Y.S.; Yang, C.C.; Eichel, R.A.; Hsieh, C.T.; Lee, T.C.; Chang, J.K. Tape-cast- Ce-substituted Li7La3Zr2O12 electrolyte for improving electrochemical performance of solid-state lithium batteries. J. Mater. Chem. A 2022, 10, 22512–22522. [Google Scholar] [CrossRef]
- Chao, C.H.; Hsieh, C.T.; Ke, W.J.; Lee, W.L.; Lin, Y.F.; Liu, H.W.; Gu, S.; Fu, C.C.; Juang, R.S.; Mallick, B.C.; et al. Roll-to-roll atomic layer deposition of titania coating on polymeric separator for lithium ion batteries. J. Power Sources 2021, 482, 228896. [Google Scholar] [CrossRef]
- Stenina, I.; Pyrkova, A.; Yaroslavtsev, A. NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes: Effect of Al, Zr, Co-doping and synthesis method. Batteries 2023, 9, 59. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Ma, J.; Huang, J.H.; Mao, D.Y.; Wang, K.X. Realizing long-cycling solid-state Li-CO2 batteries using Zn-doped LATP ceramic electrolytes. Chem. Eng. J. 2024, 482, 148977. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar]
- Gebert, F.; Knott, J.; Gorkin, R.; Chou, S.L.; Dou, S.X. Polymer electrolytes for sodium-ion batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Ratner, R.A.; Johansson, P.; Shriver, D.F. Polymer electrolytes: Ionic transport mechanisms and relaxation coupling. MRS Bull. 2000, 25, 31–37. [Google Scholar] [CrossRef]
- Available online: https://next-gen.materialsproject.org/ (accessed on 28 April 2024).
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Munro, J.M.; Latimer, K.; Horton, M.K.; Dwaraknath, S.; Persson, K.A. An improved symmetry-based approach to reciprocal space path selection in band structure calculations. NPJ Comput. Mater 2020, 6, 112. [Google Scholar] [CrossRef]
- Xu, A.; Wang, R.; Yao, M.; Cao, J.; Li, M.; Yang, C.; Liu, F.; Ma, J. Electrochemical properties of an Sn-doped LATP ceramic electrolyte and its derived sandwich-structured composite solid electrolyte. Nanomaterials 2022, 12, 2082. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-T.; Cho, T.-S.; Chang, J.-K.; Patra, J. Metal-Doped NASICON/Polymer Composite Solid Electrolyte for Lithium Titania Anode in Lithium-Ion Batteries. Polymers 2024, 16, 1251. https://doi.org/10.3390/polym16091251
Hsieh C-T, Cho T-S, Chang J-K, Patra J. Metal-Doped NASICON/Polymer Composite Solid Electrolyte for Lithium Titania Anode in Lithium-Ion Batteries. Polymers. 2024; 16(9):1251. https://doi.org/10.3390/polym16091251
Chicago/Turabian StyleHsieh, Chien-Te, Tzu-Shaing Cho, Jeng-Kuei Chang, and Jagabandhu Patra. 2024. "Metal-Doped NASICON/Polymer Composite Solid Electrolyte for Lithium Titania Anode in Lithium-Ion Batteries" Polymers 16, no. 9: 1251. https://doi.org/10.3390/polym16091251




