Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
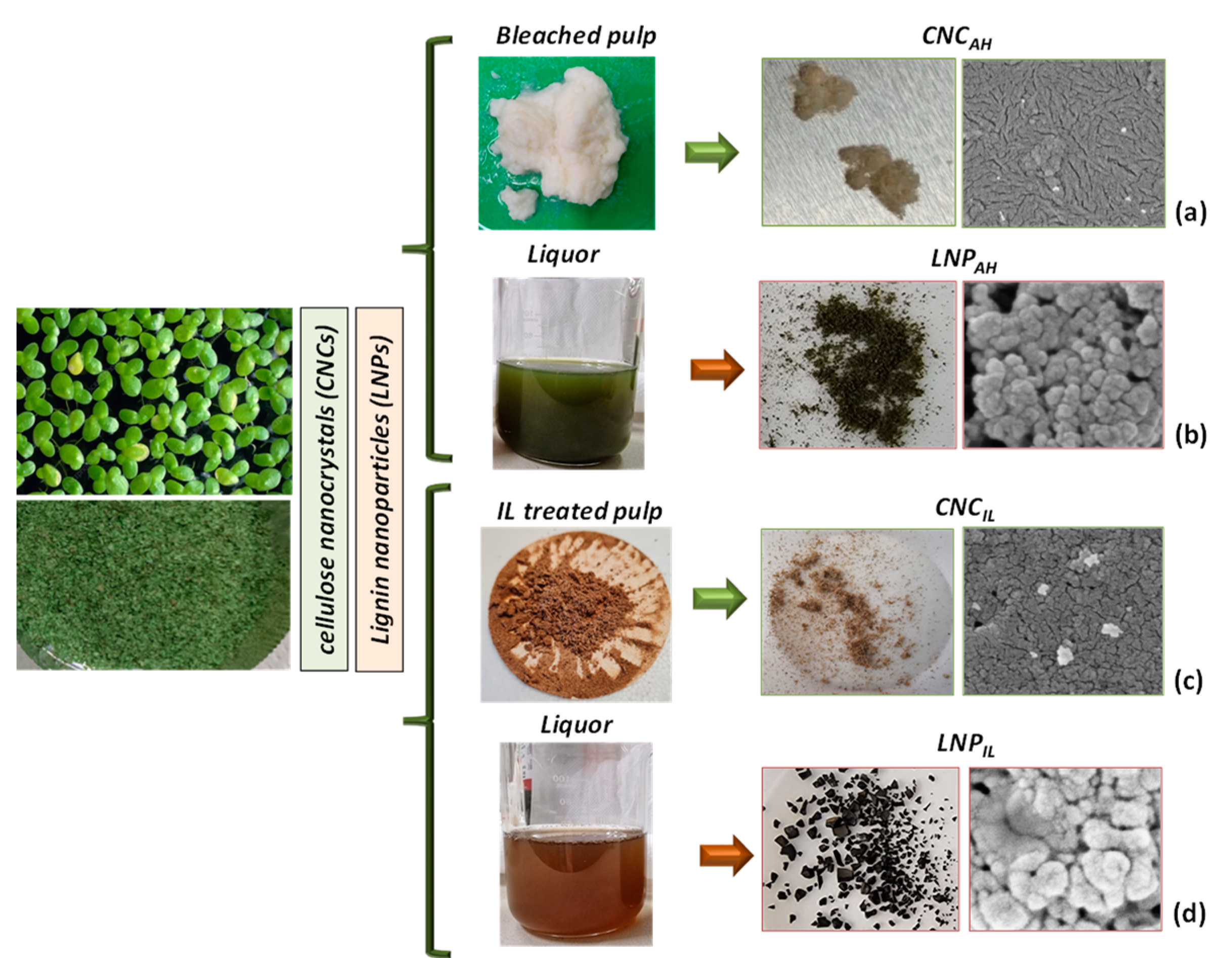
2.2. Extraction of Cellulose Nanocrystals (CNC)
2.2.1. Bleaching Treatment
2.2.2. Acid Hydrolysis after Bleaching Treatment
2.2.3. Acid Hydrolysis after Ionic Liquid Treatment
2.3. Lignin Nanoparticles Extraction (LNP)
2.3.1. Lignin Precipitation after Bleaching Treatment
2.3.2. Lignin Precipitation after IL Treatment
2.4. Characterization of Pristine Duckweed Plant, Cellulose Nanocrystals and Lignin Nanoparticles
3. Results
3.1. Characterization of Pristine Duckweed
3.2. Thermal, Morphological, and Chemical Analysis of Cellulose Nanocrystals
3.2.1. Acid Hydrolysis (CNCAH)
3.2.2. Ionic Liquid (CNCIL)
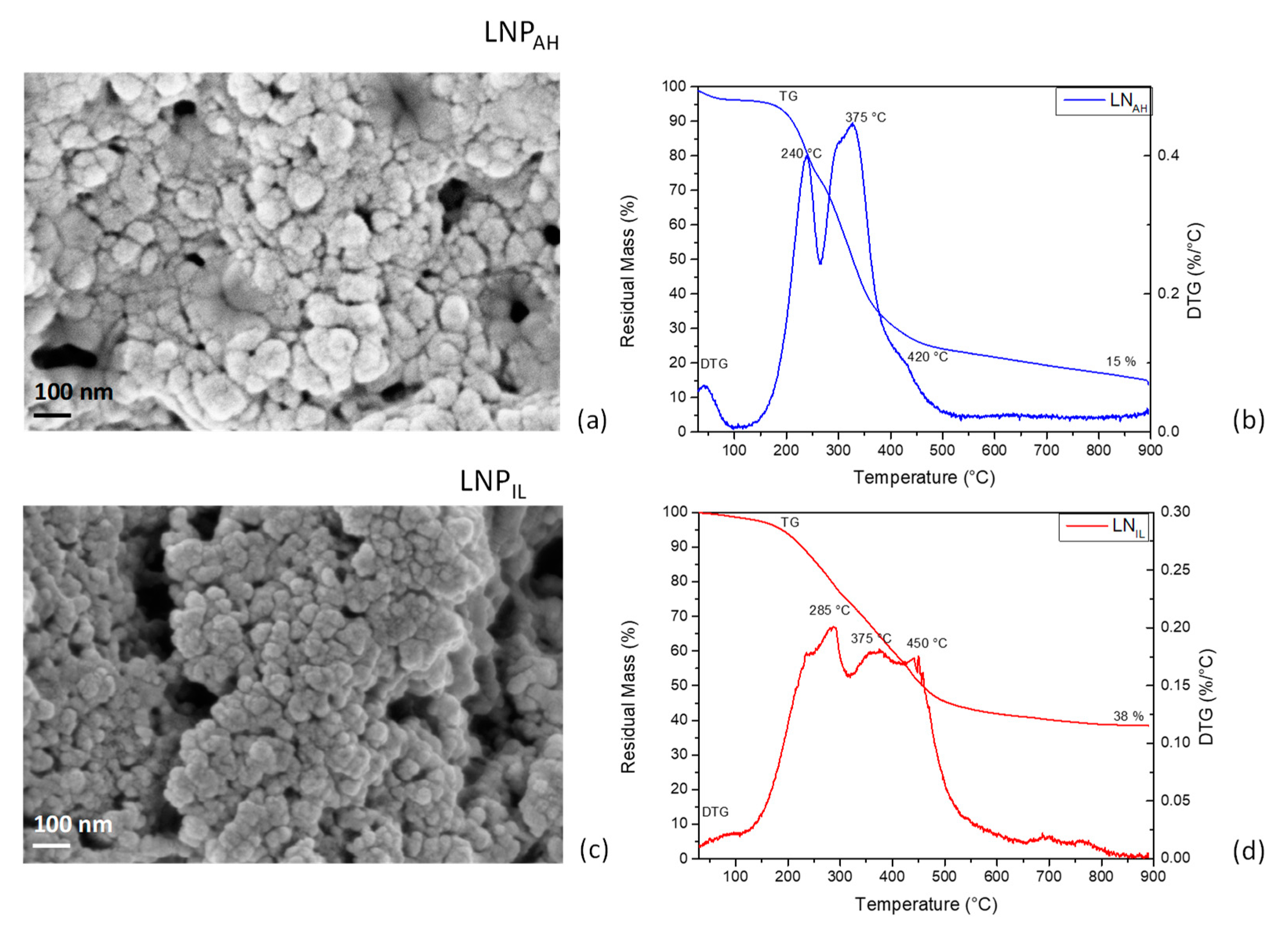
3.3. Thermal, Morphological, and Chemical Analysis of Lignin Nanoparticles
3.3.1. FTIR Analysis of Lignin Nanoparticles from Acid Hydrolysis (LNPAH) and Ionic Liquid (LNPIL) Treatments
3.3.2. Morphological and Thermal Characterization of Lignin Nanoparticles from Acid Hydrolysis (LNPAH) and Ionic Liquid (LNPIL) Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Moates, G.K.; Wellner, N.; Collins, S.R.A.; Coleman, M.J.; Waldron, K.W. Chemical Characterisation and Analysis of the Cell Wall Polysaccharides of Duckweed (Lemna minor). Carbohydr. Polym. 2014, 111, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Zhang, W.; Li, Y. Duckweed (Lemnaceae) for Potentially Nutritious Human Food: A Review. Food Rev. Int. 2023, 39, 3620–3634. [Google Scholar] [CrossRef]
- Pagliuso, D.; Grandis, A.; Fortirer, J.S.; Camargo, P.; Floh, E.I.; Buckeridge, M.S. Duckweeds as Promising Food Feedstocks Globally. Agronomy 2022, 12, 796. [Google Scholar] [CrossRef]
- Yadav, D.; Barbora, L.; Bora, D.; Mitra, S.; Rangan, L.; Mahanta, P. An Assessment of Duckweed as a Potential Lignocellulosic Feedstock for Biogas Production. Int. Biodeterior Biodegrad. 2017, 119, 253–259. [Google Scholar] [CrossRef]
- Blazey, E.B.; McClure, J.W. The Distribution and Taxonomic Significance of Lignin in the Lemnaceae. Am. J. Bot. 1968, 55, 1240–1245. [Google Scholar] [CrossRef]
- Del Buono, D.; Bartucca, M.L.; Ballerini, E.; Senizza, B.; Lucini, L.; Trevisan, M. Physiological and Biochemical Effects of an Aqueous Extract of Lemna minor L. as a Potential Biostimulant for Maize. J. Plant Growth Regul. 2021, 41, 3009–3018. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Senizza, B.; Regni, L.; Tolisano, C.; Proietti, P.; Trevisan, M.; Lucini, L.; Rouphael, Y.; Del Buono, D. Biochemical Insights into the Ability of Lemna minor L. Extract to Counteract Copper Toxicity in Maize. Plants 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarit, T.; Chanabodeechalermrung, B.; Kantrong, N.; Chittasupho, C.; Jantrawut, P. Fabrication and Evaluation of Water Hyacinth Cellulose-Composited Hydrogel Containing Quercetin for Topical Antibacterial Applications. Gels 2022, 8, 767. [Google Scholar] [CrossRef] [PubMed]
- Regni, L.; Tolisano, C.; Del Buono, D.; Priolo, D.; Proietti, P. Role of an Aqueous Extract of Duckweed (Lemna minor L.) in Increasing Salt Tolerance in Olea europaea L. Agriculture 2024, 14, 375. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, N.; Phillips, G.C.; Xu, J. Growing Lemna minor in Agricultural Wastewater and Converting the Duckweed Biomass to Ethanol. Bioresour. Technol. 2012, 124, 485–488. [Google Scholar] [CrossRef]
- Pagliuso, D.; Grandis, A.; Lam, E.; Buckeridge, M.S. High Saccharification, Low Lignin, and High Sustainability Potential Make Duckweeds Adequate as Bioenergy Feedstocks. BioEnergy Res. 2021, 14, 1082–1092. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Nawaj Alam, S.; Singh, B.; Guldhe, A. Aquatic Weed as a Biorefinery Resource for Biofuels and Value-Added Products: Challenges and Recent Advancements. Clean. Eng. Technol. 2021, 4, 100235. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Mimmo, T.; Cesco, S.; Del Buono, D. Nitrate Removal from Polluted Water by Using a Vegetated Floating System. Sci. Total Environ. 2016, 542, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheswari, B.; Sivakumar, V.M.; Thirumarimurugan, M. Lead Adsorption from Aqueous Solution Using Novel Nanoparticles Synthesized from Waste Aquatic Weeds. Nanotechnol. Environ. Eng. 2020, 5, 10. [Google Scholar] [CrossRef]
- Smriti, S.A.; Haque, A.N.M.A.; Khadem, A.H.; Siddiqa, F.; Rahman, A.N.M.M.; Himu, H.A.; Farzana, N.; Islam, M.d.A.; Naebe, M. Recent Developments of the Nanocellulose Extraction from Water Hyacinth: A Review. Cellulose 2023, 30, 8617–8641. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Prakash, P.; Babu, V.; Paul, E.J.; Bharani, R.S.A.; Kumar, J.A.; Kavisri, M.; Moovendhan, M. Aquatic Biomass Cellulose Fabrication into Cellulose Nanocomposite and Its Application in Water Purification. J. Clean. Prod. 2023, 396, 136386. [Google Scholar] [CrossRef]
- Sajjadi, M.; Ahmadpoor, F.; Nasrollahzadeh, M.; Ghafuri, H. Lignin-Derived (Nano)Materials for Environmental Pollution Remediation: Current Challenges and Future Perspectives. Int. J. Biol. Macromol. 2021, 178, 394–423. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef] [PubMed]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A.; Mahardika, M.; Park, J.-W.; Kim, H.-J. Isolation of Nanocellulose from Water Hyacinth Fiber (WHF) Produced via Digester-Sonication and Its Characterization. Fibers Polym. 2018, 19, 1618–1625. [Google Scholar] [CrossRef]
- Packiam, K.K.; Murugesan, B.; Kaliyannan Sundaramoorthy, P.M.; Srinivasan, H.; Dhanasekaran, K. Extraction, Purification and Characterization of Nanocrystalline Cellulose from Eichhornia crassipes (Mart.) Solms: A Common Aquatic Weed Water Hyacinth. J. Nat. Fibers 2022, 19, 7424–7435. [Google Scholar] [CrossRef]
- Pantamanatsopa, P.; Ariyawiriyanan, W.; Ekgasit, S. Production of Cellulose Nanocrystals Suspension with High Yields from Water Hyacinth. J. Nat. Fibers 2023, 20, 2134266. [Google Scholar] [CrossRef]
- Pintor-Ibarra, L.F.; Rivera-Prado, J.J.; Ramos-Vargas, S.; Escoto-García, T.; Rodríguez-Olalde, N.E.; Rutiaga-Quiñones, J.G. Kraft Pulping and Bleaching of Eichhornia crassipes (Mart.) Solms (Water Hyacinth). BioResources 2020, 15, 9243–9264. [Google Scholar] [CrossRef]
- Cequier, E.; Aguilera, J.; Balcells, M.; Canela-Garayoa, R. Extraction and Characterization of Lignin from Olive Pomace: A Comparison Study among Ionic Liquid, Sulfuric Acid, and Alkaline Treatments. Biomass Convers. Biorefin. 2019, 9, 241–252. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Md. Jamil, S.N.A.; Abdan, K. Nanocellulose Extraction Using Ionic Liquids: Syntheses, Processes, and Properties. Front. Mater. 2022, 9, 919918. [Google Scholar] [CrossRef]
- Tolisano, C.; Luzi, F.; Regni, L.; Proietti, P.; Puglia, D.; Gigliotti, G.; Di Michele, A.; Priolo, D.; Del Buono, D. A Way to Valorize Pomace from Olive Oil Production: Lignin Nanoparticles to Biostimulate Maize Plants. Environ. Technol. Innov. 2023, 31, 103216. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The Treatment of Duckweed with a Plant Biostimulant or a Safener Improves the Plant Capacity to Clean Water Polluted by Terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef]
- Chen, T.; Hojka, M.; Davey, P.; Sun, Y.; Dykes, G.F.; Zhou, F.; Lawson, T.; Nixon, P.J.; Lin, Y.; Liu, L.-N. Engineering α-Carboxysomes into Plant Chloroplasts to Support Autotrophic Photosynthesis. Nat. Commun. 2023, 14, 2118. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional Bionanocomposite Films of Poly(Lactic Acid), Cellulose Nanocrystals and Silver Nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Del Buono, D.; Puglia, D.; Torre, L. Revalorization of Barley Straw and Husk as Precursors for Cellulose Nanocrystals Extraction and Their Effect on PVA_CH Nanocomposites. Ind. Crops Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Sultana, S.; Sonia, Z.A.; Mahmud, M.; Mottakin, M.; Bin Haider, J.; Ahmed, S.; Hossen, M.M. An Investigation of Cellulose, Hemicellulose, and Lignin Co-Extraction from Water Hyacinth. Adv. J. Chem. Sect. A 2024, 7, 75–88. [Google Scholar] [CrossRef]
- Romero-Guzmán, E.T.; Reyes-Gutiérrez, L.R.; Marín-Allende, M.J.; González-Acevedo, Z.I.; Olguín-Gutiérrez, M.T. Physicochemical Properties of Non-Living Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor) and Their Influence on the As(V) Adsorption Processes. Chem. Ecol. 2013, 29, 459–475. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Wang, Y.; Tang, X.; He, G.; Wang, S.; Ma, Y.; Kong, Y.; Yu, C.; Zhou, G. A Submerged Duckweed Mutant with Abundant Starch Accumulation for Bioethanol Production. GCB Bioenergy 2020, 12, 1078–1091. [Google Scholar] [CrossRef]
- Tran, I.T.; Heiman, J.A.; Lydy, V.R.; Kissoon, L.T. Silver Inhibits Lemna minor Growth at High Initial Frond Densities. Plants 2023, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhao, J.; Yu, X.; Lv, K.; Wang, Z.; Xing, B. Interaction of CuO Nanoparticles with Duckweed (Lemna minor L.): Uptake, Distribution and ROS Production Sites. Environ. Pollut. 2018, 243, 543–552. [Google Scholar] [CrossRef]
- Sowinski, E.E.; Gilbert, S.; Lam, E.; Carpita, N.C. Linkage Structure of Cell-Wall Polysaccharides from Three Duckweed Species. Carbohydr. Polym. 2019, 223, 115119. [Google Scholar] [CrossRef]
- Reale, L.; Ferranti, F.; Mantilacci, S.; Corboli, M.; Aversa, S.; Landucci, F.; Baldisserotto, C.; Ferroni, L.; Pancaldi, S.; Venanzoni, R. Cyto-Histological and Morpho-Physiological Responses of Common Duckweed (Lemna minor L.) to Chromium. Chemosphere 2016, 145, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Nassar, H.F.; Ibrahim, M. Duckweed-Lemna minor as Green Route for Removal of Chromium (VI) from Aqueous Solution. Int. J. Environ. Res. 2021, 15, 275–284. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman Characterization of Non-Cellulosic Polysaccharides Fractions Isolated from Plant Cell Wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef]
- Balasubramanian, U.M.; Vaiyazhipalayam Murugaiyan, S.; Marimuthu, T. Enhanced Adsorption of Cr(VI), Ni(II) Ions from Aqueous Solution Using Modified Eichhornia crassipes and Lemna minor. Environ. Sci. Pollut. Res. 2020, 27, 20648–20662. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, C.; Ma, Y.; Xu, H.; Wang, S.; Wang, Y.; Liu, X.; Zhou, G. Insights into the Structural and Physicochemical Properties of Small Granular Starches from Two Hydrophyte Duckweeds, Spirodela oligorrhiza and Lemna minor. Carbohydr. Res. 2016, 435, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Tao, L. Variation in Crystalline Type with Amylose Content in Maize Starch Granules: An X-ray Powder Diffraction Study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Ferrer, A.; Alciaturi, C.; Faneite, A.; Ríos, J. Analyses of Biomass Fibers by XRD, FT-IR, and NIR. In Analytical Techniques and Methods for Biomass; Vaz, S., Jr., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–83. ISBN 978-3-319-41414-0. [Google Scholar]
- Mazen, A.M.A.; Zhang, D.; Franceschi, V.R. Calcium Oxalate Formation in Lemna minor: Physiological and Ultrastructural Aspects of High Capacity Calcium Sequestration. New Phytol. 2004, 161, 435–448. [Google Scholar] [CrossRef] [PubMed]
- De Morais Teixeira, E.; Corrêa, A.C.; Manzoli, A.; de Lima Leite, F.; de Oliveira, C.R.; Mattoso, L.H.C. Cellulose Nanofibers from White and Naturally Colored Cotton Fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Lee, B.-M.; Koo, D.H.; Kang, P.-H.; Jeun, J.-P. Preparation of Nanocellulose from a Kenaf Core Using E-Beam Irradiation and Acid Hydrolysis. Cellulose 2016, 23, 3039–3049. [Google Scholar] [CrossRef]
- Hourlier, D. Thermal Decomposition of Calcium Oxalate: Beyond Appearances. J. Therm. Anal. Calorim. 2019, 136, 2221–2229. [Google Scholar] [CrossRef]
- Garcia-Guinea, J.; Correcher, V.; Lozano-Diz, E.; Bañares, M.A.; Lopez-Arce, P.; García, A.M.; Moreno, D.A. Effect of Thermal Annealing on Synthetic Sodium Oxalate Crystals. J. Anal. Appl. Pyrolysis 2011, 91, 332–337. [Google Scholar] [CrossRef]
- Juárez-Luna, G.N.; Favela-Torres, E.; Quevedo, I.R.; Batina, N. Enzymatically Assisted Isolation of High-Quality Cellulose Nanoparticles from Water Hyacinth Stems. Carbohydr. Polym. 2019, 220, 110–117. [Google Scholar] [CrossRef]
- Roy, M.; Meena, S.K.; Kusurkar, T.S.; Singh, S.K.; Sethy, N.K.; Bhargava, K.; Sarkar, S.; Das, M. Carbondioxide Gating in Silk Cocoon. Biointerphases 2012, 7, 45. [Google Scholar] [CrossRef]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II Nanocrystals Produced by Sulfuric Acid Hydrolysis of Tetra Pak Cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Verch, A.; Levy, D.; Fitch, A.N.; Pokroy, B. Amorphous Biogenic Calcium Oxalate. ChemistrySelect 2016, 1, 132–135. [Google Scholar] [CrossRef]
- Trouvé, G.; Michelin, L.; Kehrli, D.; Josien, L.; Rigolet, S.; Lebeau, B.; Gieré, R. The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals 2023, 13, 967. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An Economically Viable Ionic Liquid for the Fractionation of Lignocellulosic Biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Nurdin, M.; Abimanyu, H.; Putriani, H.; Setiawan, L.O.M.I.; Maulidiyah, M.; Wibowo, D.; Ansharullah, A.; Natsir, M.; Salim, L.O.A.; Arham, Z.; et al. Optimization of OPEFB Lignocellulose Transformation Process through Ionic Liquid [TEA][HSO4] Based Pretreatment. Sci. Rep. 2021, 11, 11338. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.F.O.; Pereira, A.L.S.; Rosa, M.F.; de Santiago-Aguiar, R.S. Lignin-Rich Cellulose Nanocrystals from Coir Fiber Treated with Ionic Liquids: Preparation and Evaluation as Pickering Emulsifier. Ind. Crops Prod. 2022, 186, 115119. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose Hydrolysis Using Ionic Liquids and Inorganic Acids under Dilute Conditions: Morphological Comparison of Nanocellulose. RSC Adv. 2020, 10, 39413–39424. [Google Scholar] [CrossRef]
- Al Hakkak, J.; Grigsby, W.J.; Kathirgamanathan, K.; Edmonds, N.R. Generation of Spherical Cellulose Nanoparticles from Ionic Liquid Processing via Novel Nonsolvent Addition and Drying. Adv. Mater. Sci. Eng. 2019, 2019, 2081027. [Google Scholar] [CrossRef]
- Li, Q.; Ji, G.; Chen, Y.; Xu, Y.; Shen, J.; Wu, Y. Efficient Enzymatic Hydrolysis of Cellulose Treated by Mixed Ionic Liquids. Chem. Pap. 2020, 74, 3481–3490. [Google Scholar] [CrossRef]
- Campuzano, F.; Escobar, D.M.; Torres, L.A.M. Simple Method for Obtaining Regenerated Cellulose Nanoparticles from Delignified Coffee Parchment, and Their Use in Fabricating Blended Films. Cellulose 2023, 30, 7681–7694. [Google Scholar] [CrossRef]
- Pereira, B.; Matos, F.S.; Valente, B.F.A.; Von Weymarn, N.; Kamppuri, T.; Freire, C.S.R.; Silvestre, A.J.D.; Vilela, C. From Regenerated Wood Pulp Fibers to Cationic Cellulose: Preparation, Characterization and Dyeing Properties. Polysaccharides 2022, 3, 609–624. [Google Scholar] [CrossRef]
- Im, J.; Lee, S.; Jo, I.; Kang, J.W.; Kim, K.-S. Structural Characteristics and Thermal Properties of Regenerated Cellulose, Hemicellulose and Lignin after Being Dissolved in Ionic Liquids. J. Ind. Eng. Chem. 2022, 107, 365–375. [Google Scholar] [CrossRef]
- Chaiyo, N.; Muanghlua, R.; Niemcharoen, S.; Boonchom, B.; Seeharaj, P.; Vittayakorn, N. Non-Isothermal Kinetics of the Thermal Decomposition of Sodium Oxalate Na2C2O4. J. Therm. Anal. Calorim. 2012, 107, 1023–1029. [Google Scholar] [CrossRef]
- Rana, M.d.M.; De la Hoz Siegler, H. Influence of Ionic Liquid (IL) Treatment Conditions in the Regeneration of Cellulose with Different Crystallinity. J. Mater. Res. 2023, 38, 328–336. [Google Scholar] [CrossRef]
- Maulina, W.; Kusumaningtyas, R.; Rachmawati, Z.; Arkundato, A.; Rohman, L.; Purwandari, E. Carbonization Process of Water Hyacinth as an Alternative Renewable Energy Material for Biomass Cook Stoves Applications. In Proceedings of the 12th International Interdisciplinary Studies Seminar—Environmental Conservation and Education for Sustainable Development, Ijen Suites Hotel, Malang, Indonesia, 14–15 November 2018; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 239. [Google Scholar]
- Wilson, R.H.; Smith, A.C.; Kacurakova, M.; Saunders, P.K.; Wellner, N.; Waldron, K.W. The Mechanical Properties and Molecular Dynamics of Plant Cell Wall Polysaccharides Studied by Fourier-Transform Infrared Spectroscopy. Plant Physiol. 2000, 124, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Grząbka-Zasadzińska, A.; Skrzypczak, A.; Borysiak, S. The Influence of the Cation Type of Ionic Liquid on the Production of Nanocrystalline Cellulose and Mechanical Properties of Chitosan-Based Biocomposites. Cellulose 2019, 26, 4827–4840. [Google Scholar] [CrossRef]
- Kazachenko, A.; Akman, F.; Medimagh, M.; Issaoui, N.; Vasilieva, N.; Malyar, Y.N.; Sudakova, I.G.; Karacharov, A.; Miroshnikova, A.; Al-Dossary, O.M. Sulfation of Diethylaminoethyl-Cellulose: QTAIM Topological Analysis and Experimental and DFT Studies of the Properties. ACS Omega 2021, 6, 22603–22615. [Google Scholar] [CrossRef] [PubMed]
- Sasani, N.; Bock, P.; Felhofer, M.; Gierlinger, N. Raman Imaging Reveals In-Situ Microchemistry of Cuticle and Epidermis of Spruce Needles. Plant Methods 2021, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Agustin, M.B.; Penttilä, P.A.; Lahtinen, M.; Mikkonen, K.S. Rapid and Direct Preparation of Lignin Nanoparticles from Alkaline Pulping Liquor by Mild Ultrasonication. ACS Sustain. Chem. Eng. 2019, 7, 19925–19934. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent Developments in Modification of Lignin Using Ionic Liquids for the Fabrication of Advanced Materials—A Review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Nisha, S.S.; Nikzad, M.; Al Kobaisi, M.; Truong, V.K.; Sbarski, I. The Role of Ionic-Liquid Extracted Lignin Micro/Nanoparticles for Functionalisation of an Epoxy-Based Composite Matrix. Compos. Sci. Technol. 2019, 174, 11–19. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. A Simple Environment-Friendly Process for Preparing High-Concentration Alkali Lignin Nanospheres. Eur. Polym. J. 2019, 112, 15–23. [Google Scholar] [CrossRef]
- Amini, E.; Valls, C.; Roncero, M.B. Ionic Liquid, Ultrasound-Assisted Synthesis of Lignin Nanoparticles for Barrier-Enhanced All-Cellulose Nanocomposite Films. Wood Sci. Technol. 2023, 57, 1319–1344. [Google Scholar] [CrossRef]
- Yang, X.; Han, F.; Xu, C.; Jiang, S.; Huang, L.; Liu, L.; Xia, Z. Effects of Preparation Methods on the Morphology and Properties of Nanocellulose (NC) Extracted from Corn Husk. Ind. Crops Prod. 2017, 109, 241–247. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Preparation and Characterization of Size-Controlled Lignin Nanoparticles with Deep Eutectic Solvents by Nanoprecipitation. Molecules 2021, 26, 218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Kent, M.S.; He, L.; Varanasi, P.; Dibble, D.; Arora, R.; Deng, K.; Hong, K.; Melnichenko, Y.B.; Simmons, B.A.; et al. Effect of Ionic Liquid Treatment on the Structures of Lignins in Solutions: Molecular Subunits Released from Lignin. Langmuir 2012, 28, 11850–11857. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, M.; Klapiszewski, Ł.; Dobrowolska, A.; Piasecki, A.; Czaczyk, K.; Jesionowski, T. The Practical Utility of Imidazolium Hydrogen Sulfate Ionic Liquid in Fabrication of Lignin-Based Spheres: Structure Characteristic and Antibacterial Activity. Front. Chem. 2022, 10, 946665. [Google Scholar] [CrossRef]
- Perera, U.P.; Foo, M.L.; Chew, I.M.L. Synthesis and Characterization of Lignin Nanoparticles Isolated from Oil Palm Empty Fruit Bunch and Application in Biocomposites. Sustain. Chem. Clim. Act. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, Y.; Sharma, A.; Bai, X. Lignin Valorization through Thermochemical Conversion: Comparison of Hardwood, Softwood and Herbaceous Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6608–6617. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglia, D.; Luzi, F.; Tolisano, C.; Rallini, M.; Priolo, D.; Brienza, M.; Costantino, F.; Torre, L.; Del Buono, D. Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass. Polymers 2024, 16, 1395. https://doi.org/10.3390/polym16101395
Puglia D, Luzi F, Tolisano C, Rallini M, Priolo D, Brienza M, Costantino F, Torre L, Del Buono D. Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass. Polymers. 2024; 16(10):1395. https://doi.org/10.3390/polym16101395
Chicago/Turabian StylePuglia, Debora, Francesca Luzi, Ciro Tolisano, Marco Rallini, Dario Priolo, Monica Brienza, Ferdinando Costantino, Luigi Torre, and Daniele Del Buono. 2024. "Cellulose Nanocrystals and Lignin Nanoparticles Extraction from Lemna minor L.: Acid Hydrolysis of Bleached and Ionic Liquid-Treated Biomass" Polymers 16, no. 10: 1395. https://doi.org/10.3390/polym16101395





