Simple Electrospinning Method for Biocompatible Polycaprolactone β-Carotene Scaffolds: Advantages and Limitations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning Process
2.2. Characterization Techniques
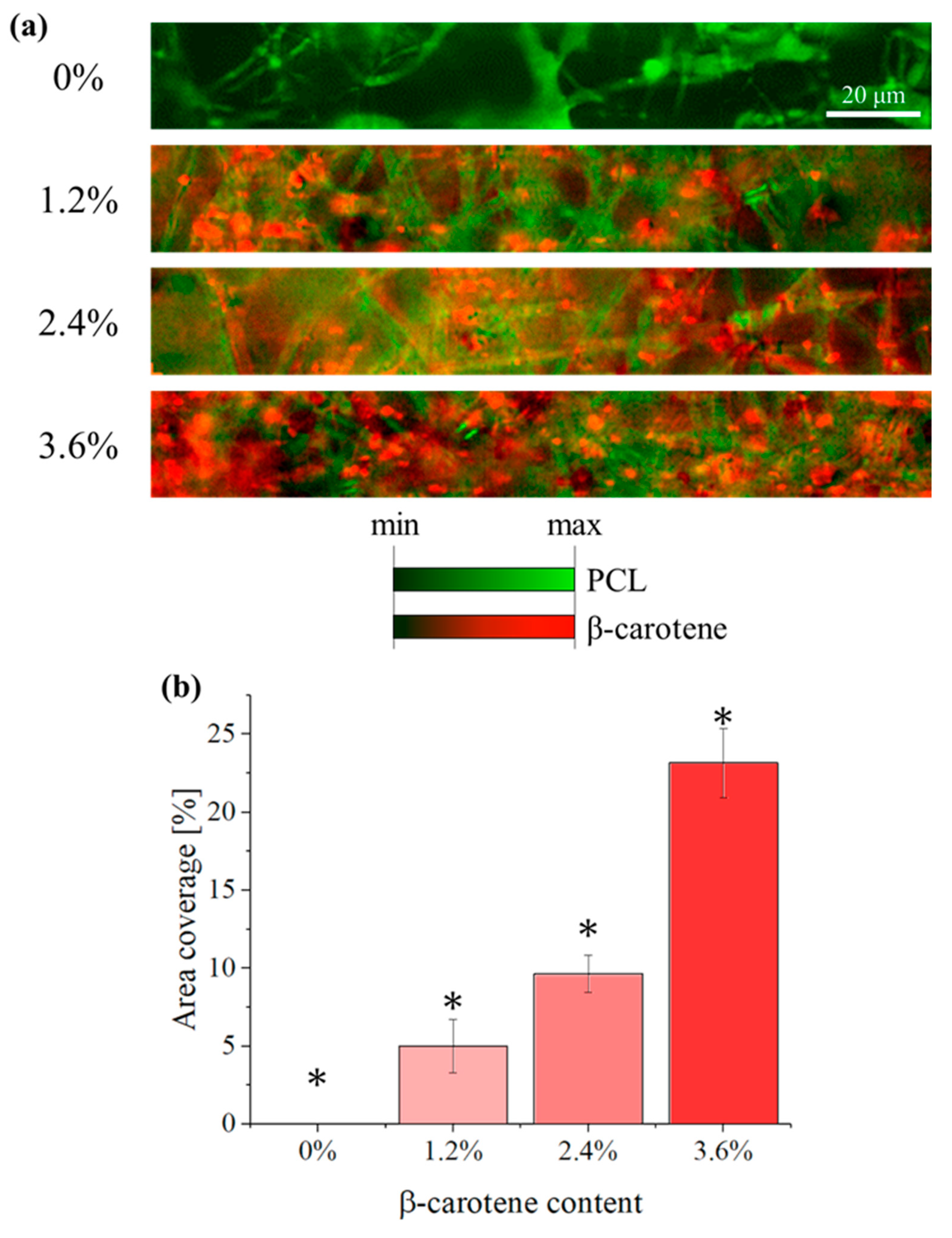
2.2.1. Confocal Imaging
2.2.2. Scanning Electron Microscope
2.2.3. Spinnability Test
2.2.4. Raman Spectroscopy
2.2.5. X-ray Diffraction
2.2.6. Differential Scanning Calorimetry
2.2.7. Mechanical Testing
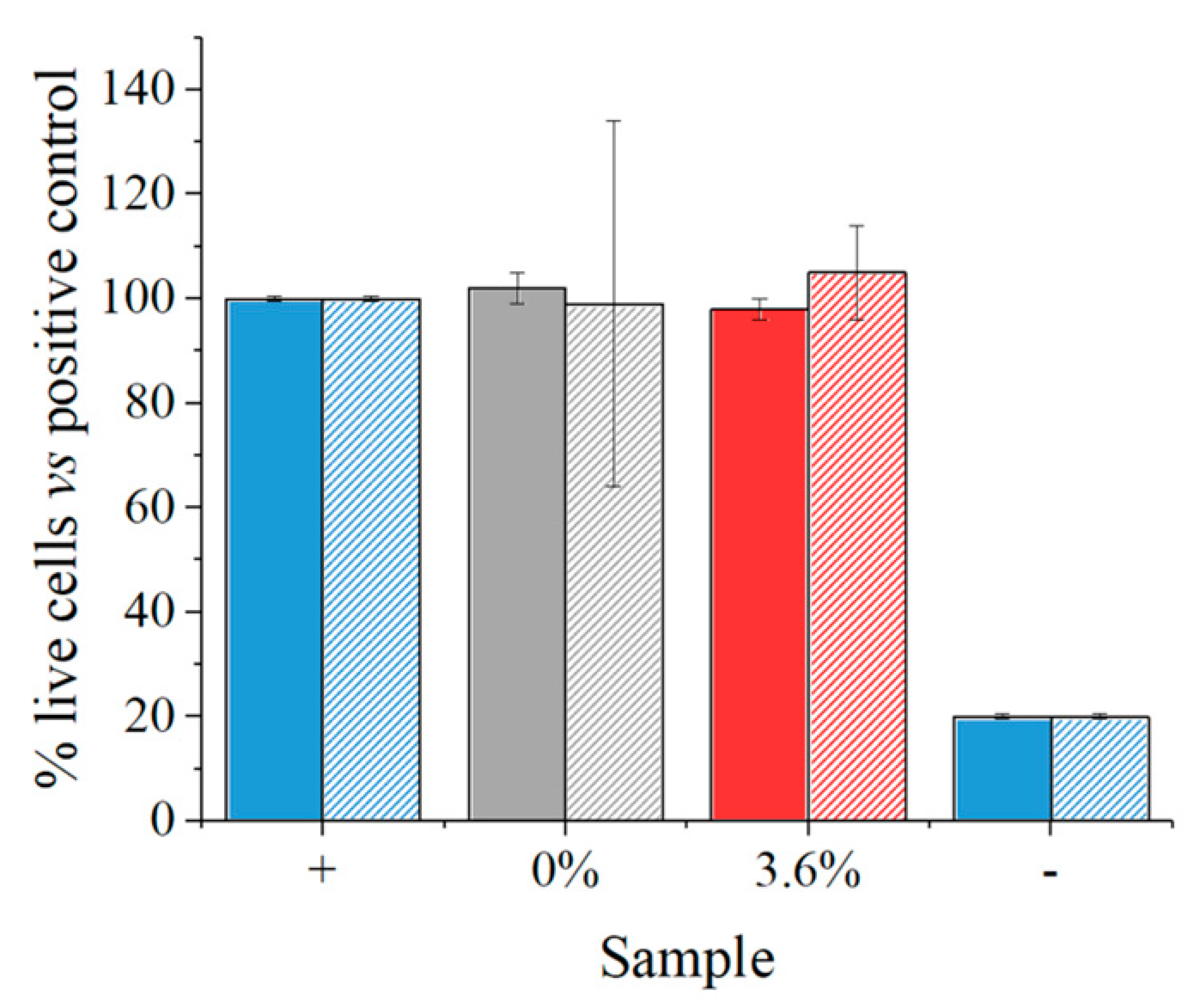
2.3. Biological Testing
2.3.1. Cell Culture
2.3.2. Cell Testing
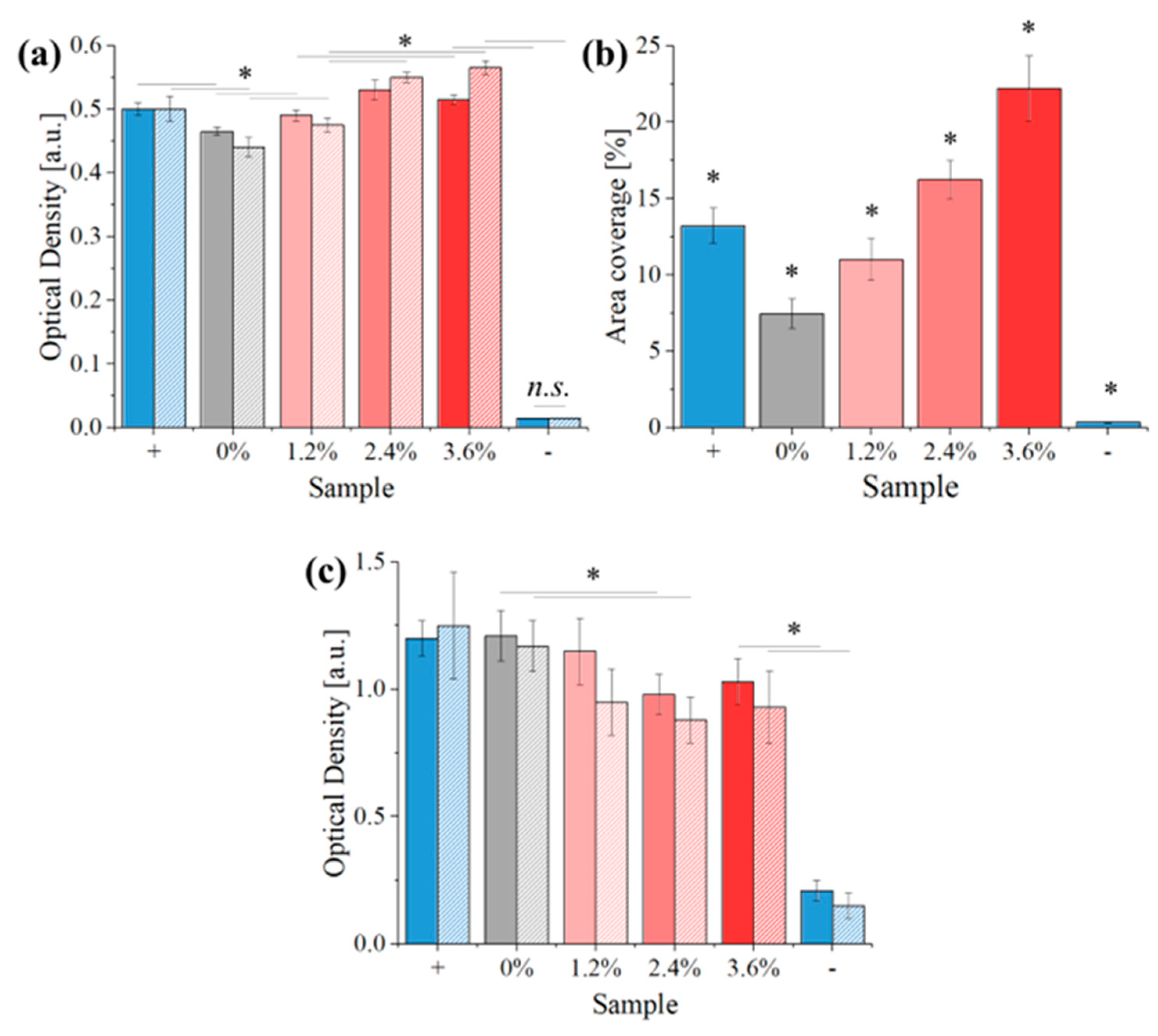
2.3.3. Bacteria Culture
2.3.4. Bacteria Testing
2.4. Statistical Analysis
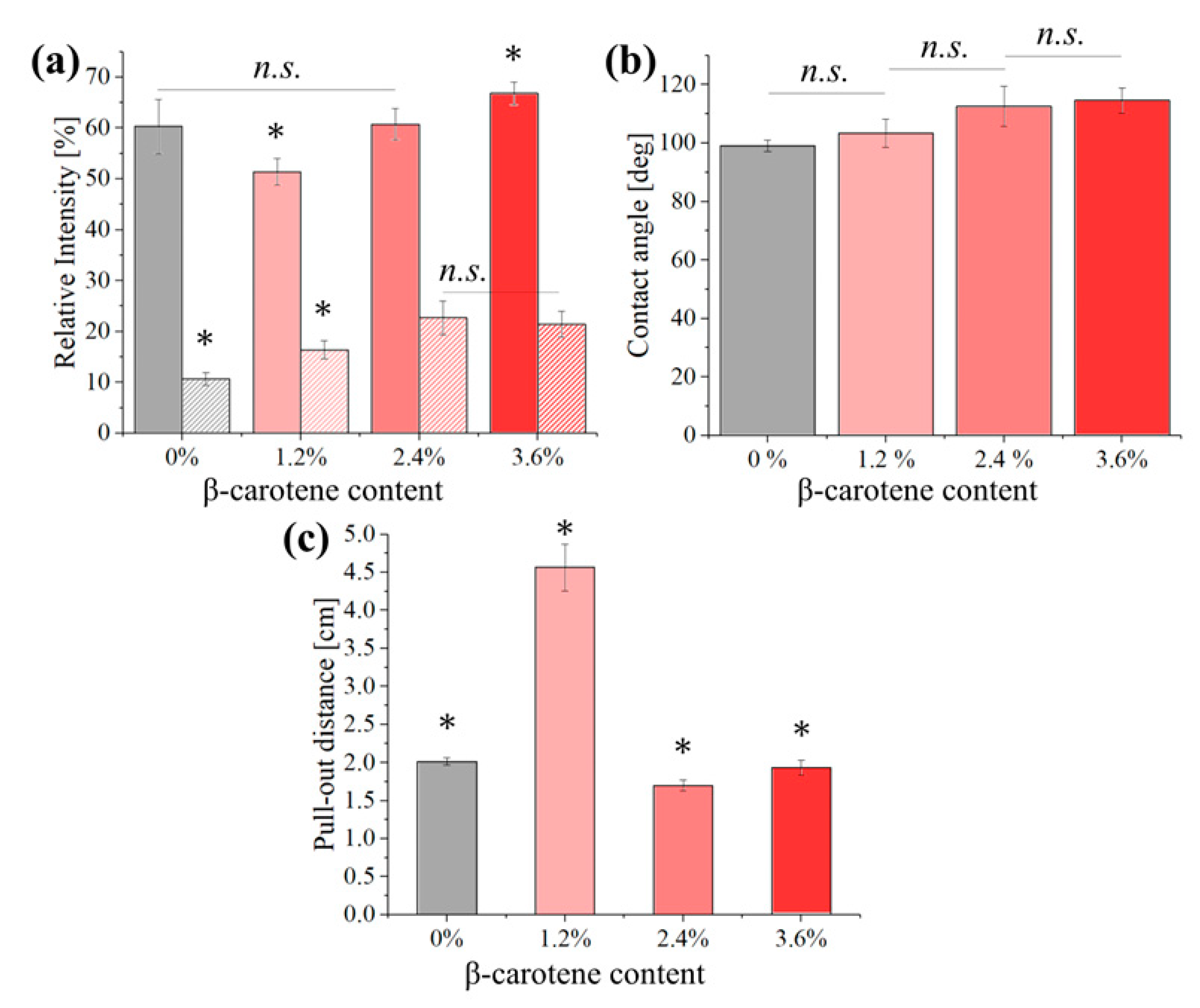
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, e290602. [Google Scholar] [CrossRef]
- Jauregui, H.O. Cell adhesion to biomaterials. The role of several extracellular matrix components in the attachment of non-transformed fibroblasts and parenchymal cells. ASAIO Trans. 1987, 33, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Leong, D.T.W.; Hutmacher, D.W. The challenge to measure cell proliferation in two and three dimensions. Tissue Eng. 2005, 11, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Fundamentals of electrospinning and processing technologies. Part. Sci. Technol. 2016, 34, 72–82. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning: Materials, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Recek, N.; Resnik, M.; Motaln, H.; Lah-Turnšek, T.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Mozetič, M. Cell Adhesion on Polycaprolactone Modified by Plasma Treatment. Int. J. Polym. Sci. 2016, 2016, e7354396. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, L.Y.; Yang, X.B.; Weng, J. Preparation of bioactive porous HA/PCL composite scaffolds. Appl. Surf. Sci. 2008, 255, 2942–2946. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, G.H. PCL/Alginate Composite Scaffolds for Hard Tissue Engineering: Fabrication, Characterization, and Cellular Activities. ACS Comb. Sci. 2015, 17, 87–99. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. Fabrication of porous PCL/elastin composite scaffolds for tissue engineering applications. J. Supercrit. Fluids 2011, 59, 157–167. [Google Scholar] [CrossRef]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Shibata, A.; Paganini-Hill, A.; Ross, R.K.; Henderson, B.E. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: A prospective study. Br. J. Cancer 1992, 66, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Harrison, M.T.; D’andrea, A.; Endsley, A.N.; Yin, F.; Kodukula, K.; Watson, D.S. β-Carotene Biosynthesis in Probiotic Bacteria. Probiotics Antimicrob. Proteins 2013, 5, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G. Carotenoids and cardiovascular disease. Curr. Atheroscler Rep. 2009, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Park, H.A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Sowers, M.; Lachance, L. VITAMINS AND ARTHRITIS: The Roles of Vitamins A, C, D, and E. Rheum. Dis. Clin. N. Am. 1999, 25, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Blake, R.J.; Garcia-Caraballo, S.; Gibson, P.G. Airway and Circulating Levels of Carotenoids in Asthma and Healthy Controls. J. Am. Coll. Nutr. 2005, 24, 448–455. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, M.; Paliyath, G. Effects of Carotenoids and Retinoids on Immune-Mediated Chronic Inflammation in Inflammatory Bowel Disease. In Functional Foods, Nutraceuticals, and Degenerative Disease Prevention; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 213–233. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470960844.ch8 (accessed on 16 February 2024).
- Paiva, S.A.R.; Russell, R.M. β-Carotene and Other Carotenoids as Antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary Intake of Carotenoids and Their Antioxidant and Anti-Inflammatory Effects in Cardiovascular Care. Mediat. Inflamm. 2013, 2013, e782137. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Okishima, N.; Sumida, S.; Okamura, K.; Doi, T.; Kishino, Y. β-carotene supplementation enhances lymphocyte proliferation with mitogens in human peripheral blood lymphocytes. Nutr. Res. 1996, 16, 211–218. [Google Scholar] [CrossRef]
- Abril, E.R.; Rybski, J.A.; Scuderi, P.; Watson, R.R. Beta-Carotene Stimulates Human Leukocytes to Secrete a Novel Cytokine. J. Leukoc. Biol. 1989, 45, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Semnani, D.; Nasari, M.; Fakhrali, A. PCL nanofibers loaded with beta-carotene: A novel treatment for eczema. Polym. Bull. 2018, 75, 2015–2026. [Google Scholar] [CrossRef]
- Lino, R.C.; de Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; da Rosa, C.G.; Nunes, M.R.; Barreto, P.L.M. Development and Characterization of Poly-ε-caprolactone Nanocapsules Containing β-carotene Using the Nanoprecipitation Method and Optimized by Response Surface Methodology. Braz Arch Biol Technol. 2020, 63, e20190184. [Google Scholar] [CrossRef]
- de Paz, E.; Martín, Á.; Duarte, C.M.M.; Cocero, M.J. Formulation of β-carotene with poly-(ε-caprolactones) by PGSS process. Powder Technol. 2012, 217, 77–83. [Google Scholar] [CrossRef]
- Dabouian, A.; Bakhshi, H.; Irani, S.; Pezeshki-Modaress, M. β-Carotene: A natural osteogen to fabricate osteoinductive electrospun scaffolds. RSC Adv. 2018, 8, 9941–9945. [Google Scholar] [CrossRef]
- Marin, E.; Yoshikawa, O.; Boschetto, F.; Honma, T.; Adachi, T.; Zhu, W.; Xu, H.; Kanamura, N.; Yamamoto, T.; Pezzotti, G. Innovative electrospun PCL/fibroin/l-dopa scaffolds scaffolds supporting bone tissue regeneration. Biomed. Mater. 2022, 17, 045010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Turng, L.S.; Li, Q. Crystalline Morphology of Electrospun Poly(ε-caprolactone) (PCL) Nanofibers. Ind. Eng. Chem. Res. 2013, 52, 4939–4949. [Google Scholar] [CrossRef]
- Lavanya, M.N.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Development of β-carotene aerosol formulations using a modified spray dryer. J. Food Process Eng. 2020, 43, e13233. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, J.; Lee, D.; Pang, Y. Metal-enhanced fluorescence and excited state dynamics of carotenoids in thin polymer films. Sci. Rep. 2019, 9, 3551. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.; Tasumi, M.; Miyazawa, T. Excitation profile of the resonance Raman effect of β-carotene. J. Mol. Spectrosc. 1974, 50, 286–303. [Google Scholar] [CrossRef]
- Moyers-Montoya, E.; García-Casillas, P.; Vargas-Requena, C.; Escobedo-González, R.; Martel-Estrada, S.A.; Martínez-Pérez, C.A. Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique. Polymers 2016, 8, 395. [Google Scholar] [CrossRef]
- Kotula, A.P.; Snyder, C.R.; Migler, K.B. Determining conformational order and crystallinity in polycaprolactone via Raman spectroscopy. Polymer 2017, 117, 1–10. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Ribeiro, D.; Sousa, A.; Nicola, P.; de Oliveira, J.M.P.F.; Rufino, A.T.; Silva, M.; Freitas, M.; Carvalho, F.; Fernandes, E. β-Carotene and its physiological metabolites: Effects on oxidative status regulation and genotoxicity in in vitro models. Food Chem. Toxicol. 2020, 141, 111392. [Google Scholar] [CrossRef] [PubMed]
- Rotondo Dottore, G.; Ionni, I.; Menconi, F.; Casini, G.; Sellari-Franceschini, S.; Nardi, M.; Vitti, P.; Marcocci, C.; Marinò, M. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves’ orbitopathy (GO). J. Endocrinol. Investig. 2018, 41, 815–820. [Google Scholar] [CrossRef]
- Venna, N.K.; Lalhruaitluanga, H.; Challa, S. Cowpea isoflavones enhance the osteoblast differentiation and antioxidant capacity in synergy with vitamin D and β-carotene: A mechanistic in vitro study. Nutr. Health 2023. [Google Scholar] [CrossRef]
- Izadi, N.; Irani, S.; Bonakdar, S.; Ghalandari, B. Bone Tissue Engineering by Cell-Imprinted Polydimethyl Silicone Surface and β-Carotene: An In Vitro Study. Iran J. Sci. Technol. Trans. A Sci. 2022, 46, 1115–1123. [Google Scholar] [CrossRef]
- Nishide, Y.; Tousen, Y.; Tadaishi, M.; Inada, M.; Miyaura, C.; Kruger, M.C.; Ishimi, Y. Combined Effects of Soy Isoflavones and β-Carotene on Osteoblast Differentiation. Int. J. Environ. Res. Public Health 2015, 12, 13750–13761. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High Serum Carotenoids Associated with Lower Risk for Bone Loss and Osteoporosis in Post-Menopausal Japanese Female Subjects: Prospective Cohort Study. PLoS ONE 2012, 7, e52643. [Google Scholar] [CrossRef]
- Schwartz, J.; Shklar, G. The selective cytotoxic effect of carotenoids and α-Tocopherol on human cancer cell lines in vitro. J. Oral Maxillofac. Surg. 1992, 50, 367–373. [Google Scholar] [CrossRef]
- Iftikhar, S.; Lietz, H.; Mobarhan, S.; Frommel, T.O. In vitro β-carotene toxicity for human colon cancer cells. Nutr. Cancer 1996, 25, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.S.; Saini, M.K.; Jin, G.F.; Awasthi, Y.C.; van Kuijk, F.J.G.M. Toxicity of oxidized β-carotene to cultured human cells. Exp. Eye Res. 2005, 81, 239–243. [Google Scholar] [CrossRef]
- Hashimoto, A.; Yamaguchi, Y.; Chiu, L.-D.; Morimoto, C.; Fujita, K.; Takedachi, M.; Kawata, S.; Murakami, S.; Tamiya, E. Time-lapse Raman imaging of osteoblast differentiation. Sci. Rep. 2015, 5, 12529. [Google Scholar] [CrossRef] [PubMed]
- Manimala, M.R.A.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In vitro anticancer activity of natural β-carotene from Dunaliella salina EU5891199 in PC-3 cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar] [CrossRef]
- Metwally, R.A.; El-Sersy, N.A.; El Sikaily, A.; Sabry, S.A.; Ghozlan, H.A. Optimization and multiple in vitro activity potentials of carotenoids from marine Kocuria sp. RAM1. Sci Rep. 2022, 12, 18203. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.H. Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. Egypt. J. Aquat. Res. 2012, 38, 185–194. [Google Scholar] [CrossRef]







| Position [cm−1] | Vibrations | Origin | References | Position [cm−1] |
|---|---|---|---|---|
| 3045 | β-carotene | [38] | 3045 | |
| 2930 | PCL | [39] | 2930 | |
| 2850 | PCL | [39] | 2850 | |
| 2675 | β-carotene | [38] | 2675 | |
| 2525 | β-carotene | [38] | 2525 | |
| 2310 | β-carotene | [38] | 2310 | |
| 1525 | β-carotene | [38] | 1525 | |
| 1470 | PCL | [40] | 1470 | |
| 1440 | PCL | [40] | 1440 | |
| 1415 | PCL | [40] | 1415 | |
| 1305 | PCL | [40] | 1305 | |
| 1285 | PCL | [40] | 1285 | |
| 1155 | β-carotene | [38] | 1155 | |
| 1110 | PCL | [40] | 1110 | |
| 1065 | PCL | [40] | 1065 | |
| 1035 | PCL | [40] | 1035 | |
| 1005 | β-carotene | [38] | 1005 | |
| 960 | PCL | [40] | 960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, O.; Basoli, V.; Boschetto, F.; Rondinella, A.; Lanzutti, A.; Zhu, W.; Greco, E.; Thieringer, F.M.; Xu, H.; Marin, E. Simple Electrospinning Method for Biocompatible Polycaprolactone β-Carotene Scaffolds: Advantages and Limitations. Polymers 2024, 16, 1371. https://doi.org/10.3390/polym16101371
Yoshikawa O, Basoli V, Boschetto F, Rondinella A, Lanzutti A, Zhu W, Greco E, Thieringer FM, Xu H, Marin E. Simple Electrospinning Method for Biocompatible Polycaprolactone β-Carotene Scaffolds: Advantages and Limitations. Polymers. 2024; 16(10):1371. https://doi.org/10.3390/polym16101371
Chicago/Turabian StyleYoshikawa, Orion, Valentina Basoli, Francesco Boschetto, Alfredo Rondinella, Alex Lanzutti, Wenliang Zhu, Enrico Greco, Florian Markus Thieringer, Huaizhong Xu, and Elia Marin. 2024. "Simple Electrospinning Method for Biocompatible Polycaprolactone β-Carotene Scaffolds: Advantages and Limitations" Polymers 16, no. 10: 1371. https://doi.org/10.3390/polym16101371






