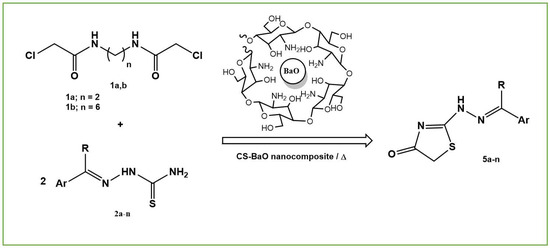
Green Synthetic Approaches of 2-Hydrazonothiazol-4(5H)-ones Using Sustainable Barium Oxide-Chitosan Nanocomposite Catalyst
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals and Instruments
2.2. Preparation of Compounds
2.2.1. Synthesis of Barium Oxide-Chitosan (BaO-CS) Nanocomposite Film
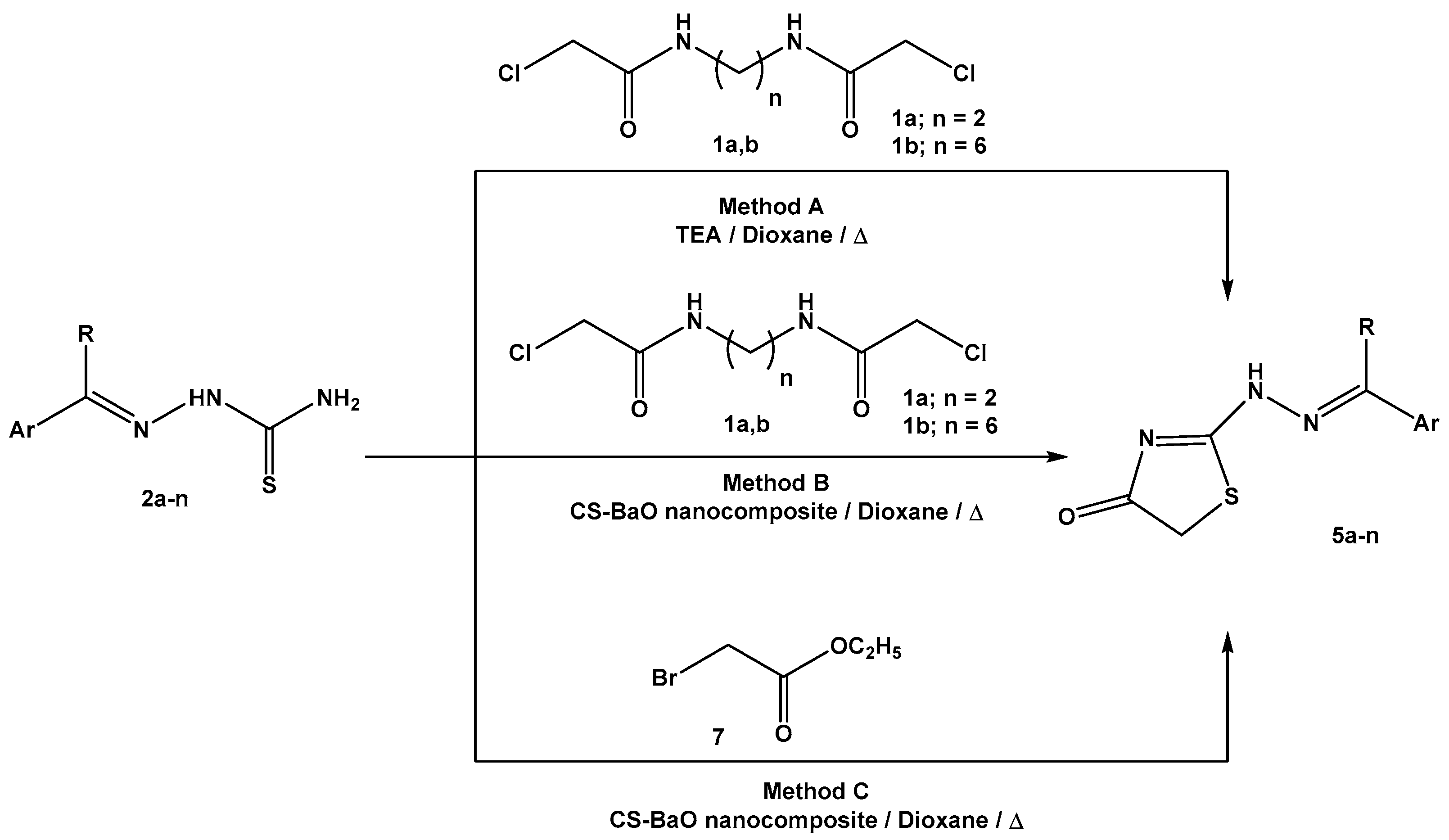
2.2.2. General Procedure for the Preparation of 2-Hydrazonothiazol-4(5H)-ones
Method A
Method B
Method C
2-(2-Benzylidenehydrazinyl)thiazol-4(5H)-one (5a)
2-[2-(4-Methoxybenzylidene)hydrazinyl]thiazol-4(5H)-one (5b)
2-[2-(4-Chlorobenzylidene)hydrazinyl]thiazol-4(5H)-one (5c)
2-[2-(4-Bromobenzylidene)hydrazinyl]thiazol-4(5H)-one (5d)
2-[2-(4-Nitrobenzylidene)hydrazinyl]thiazol-4(5H)-one (5e)
2-[2-(4-Hydroxybenzylidene)hydrazinyl]thiazol-4(5H)-one (5f)
2-[2-(3-Hydroxybenzylidene)hydrazinyl]thiazol-4(5H)-one (5g)
2-[2-(Furan-2-ylmethylene)hydrazinyl]thiazol-4(5H)-one (5h)
2-[2-(1-phenylethylidene)hydrazinyl]thiazol-4(5H)-one (5i)
2-{2-[1-(4-bromophenyl)ethylidene]hydrazinyl}thiazol-4(5H)-one (5j)
2-{2-[1-(thiophen-2-yl)ethylidene]hydrazinyl}thiazol-4(5H)-one (5k)
2-{2-[1-(1-H-indol-3-yl)ethylidene]hydrazinyl}thiazol-4(5H)-one (5m)
2-{2-[1-(2-oxo-2H-chromen-3-yl)ethylidene]hydrazinyl}thiazol-4(5H)-one (5n)
3. Results and Discussion
3.1. Structural Characterizations of Nanocatalyst: (BaO-CS) Nanocomposite Film
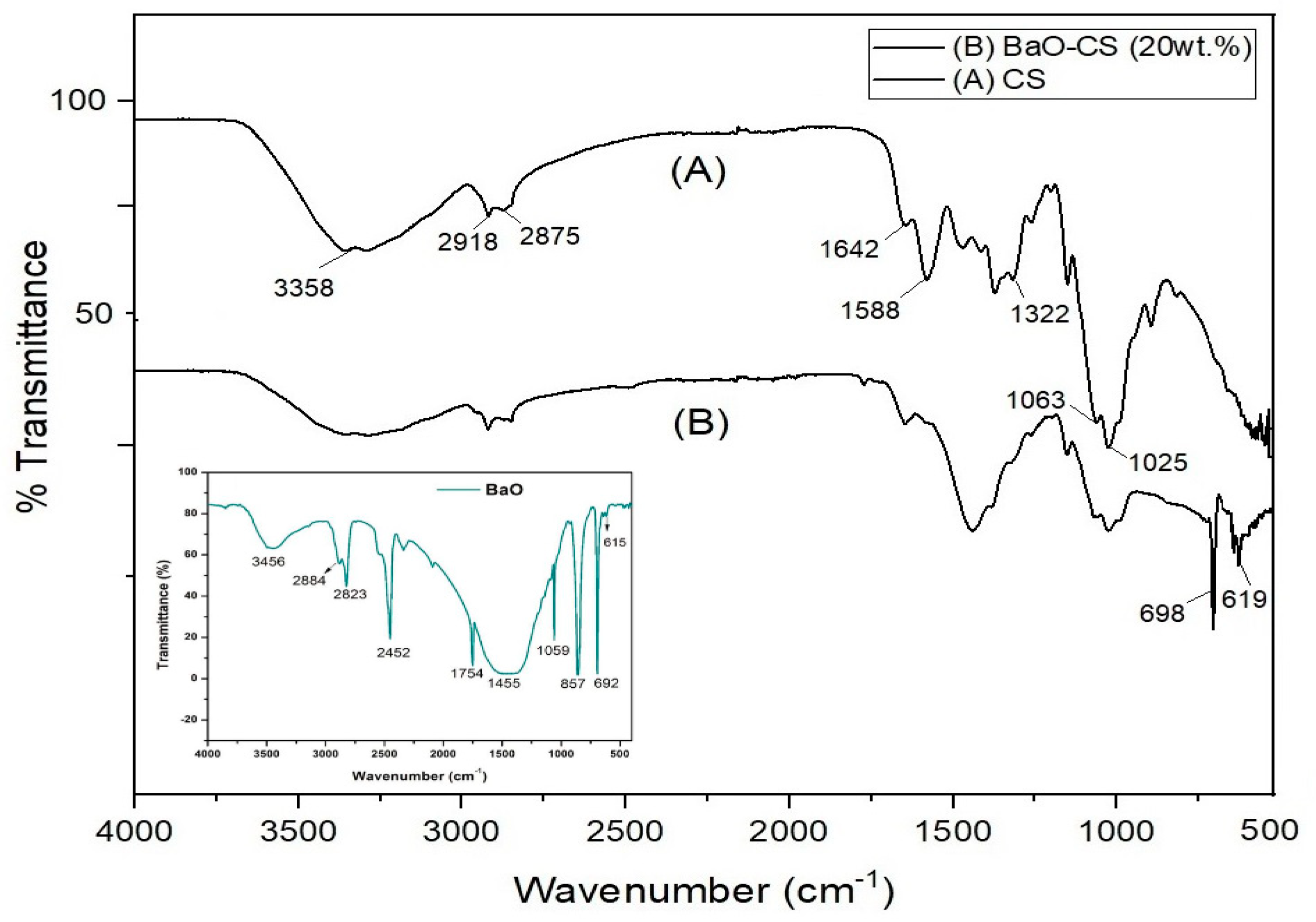
3.1.1. FTIR-Based Studies
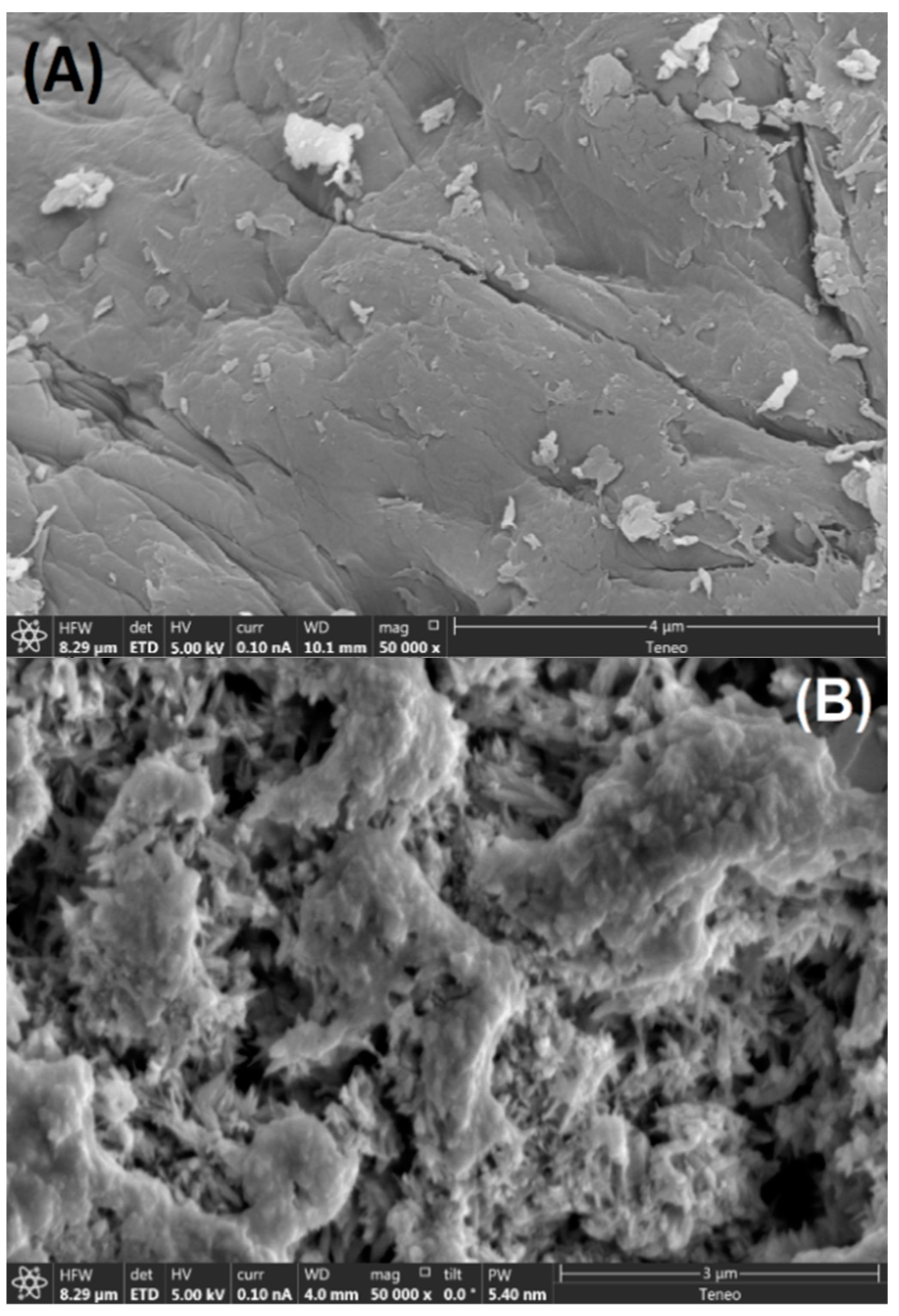
3.1.2. Characterization via FESEM (Studies on Morphological Features)
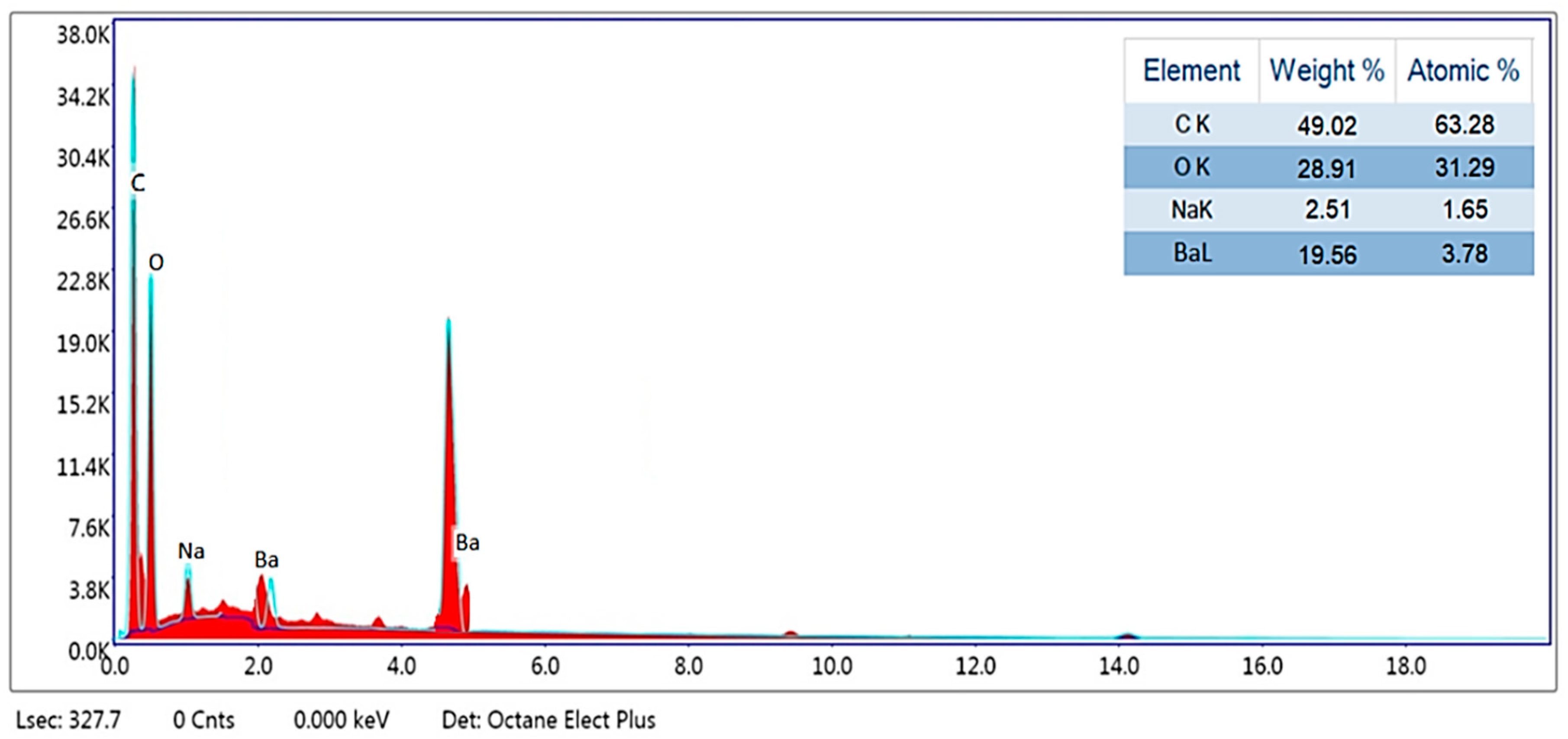
3.1.3. Characterization via EDS (Energy Dispersive Spectroscopy)
3.1.4. Characterization via XRD (Crystallinity Studies)
3.2. Chemistry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bijesh, P.; Selvaraj, V.; Andal, V. A review on synthesis and applications of nano metal oxide/porous carbon composite. Mater. Today Proc. 2022, 55, 212–219. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Khalil, K.D.; Bashal, A.H. Structural Properties and Catalytic Activity of Binary Poly(vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts 2020, 10, 100. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review, Renew. Sust. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Saadi, A.; Rassoul, Z.; Bettahar, M.M. Reduction of benzaldehyde on alkaline earth metal oxides. J. Mol. Catal. A Chem. 2006, 258, 59–67. [Google Scholar] [CrossRef]
- Frey, A.M.; Yang, J.; Feche, C.; Essayem, N.; Stellwagen, D.R.; Figueras, F.; Bitter, J.H. Influence of base strength on the catalytic performance of nano-sized alkaline earth metal oxides supported on carbon nanofibers. J. Catal. 2013, 305, 1–6. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G. Organic Reaction Pathways in the Nonaqueous Synthesis of Metal Oxide Nanoparticles. Chem. A Eur. J. 2006, 12, 7282–7302. [Google Scholar] [CrossRef]
- Seki, T.; Hattori, H. Tishchenko Reaction Over Solid Base Catalysts. Catal. Survey Asia 2003, 7, 145–156. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Sato, K.; Misu, S.; Yoshida, K. Synthesis of Solid Base Catalyst of BaO–Al2O3 Binary Oxide by Solid-liquid Interface Reaction of Hydrated Barium Hydroxide with Aluminum Alkoxide. J. Japan Petrol. Inst. 2020, 63, 20–27. [Google Scholar] [CrossRef]
- Patankar, S.C.; Yadav, G.D. Cascade engineered synthesis of 2-ethyl-1-hexanol from n-butanal and 2-methyl-1-pentanol from n-propanal using combustion synthesized Cu/Mg/Al mixed metal oxide trifunctional catalyst. Catal. Today 2017, 291, 223–233. [Google Scholar] [CrossRef]
- Aljuhani, A.; Riyadh, S.M.; Khalil, K.D. Chitosan/CuO nanocomposite films mediated regioselective synthesis of 1,3,4-trisubstituted pyrazoles under microwave irradiation. J. Saud. Chem. Soc. 2021, 25, 101276. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Alkayal, N.S.; Bashal, A.H.; Alharbi, K.H.; Alharbi, W. Chitosan-Strontium Oxide Nanocomposite: Preparation, Characterization, and Catalytic Potency in Thiadiazoles Synthesis. Polymers 2022, 14, 2827. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M. Synthesis of triazolo [4,3-b][1,2,4,5]tetrazines and triazolo [3,4-b][1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. Arkivoc 2009, 11, 58–68. [Google Scholar] [CrossRef]
- Abdul Rahman, N.; Abu Hanifah, S.; Mobarak, N.N.; Ahmad, A.; Ludin, N.A.; Bella, F.; Suait, M.S. Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer 2021, 231, 124092. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Aziz, S.B.; Kadir, M.F.Z. Environmentally friendly plasticized electrolyte based on chitosan (CS): Potato starch (PS) polymers for EDLC application: Steps toward the greener energy storage devices derived from biopolymers. J. Eng. Stor. 2023, 67, 107636. [Google Scholar] [CrossRef]
- Kolcsár, V.J.; Szőllősi, G. Chitosan as a chiral ligand and organocatalyst: Preparation conditions–property–catalytic performance relationships. Catal. Sci. Technol. 2021, 11, 7652–7666. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Hu, Y.; Zhang, X.; Chen, L.; Wang, C.; Zhang, Q. Synthesis of long chain alkanes via aldol condensation over modified chitosan catalyst and subsequent hydrodeoxygenation. Chem. Eng. J. 2022, 428, 131368. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Le, K.K.A.; Nguyen, T.V.; Le, N.T.H. Chitosan as a Renewable Heterogeneous Catalyst for the Knoevenagel Reaction in Ionic Liquid as Green Solvent. ISRN Org. Chem. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Ruiz-Pardo, C.; Silva-Gutiérrez, L.; Lizardi-Mendoza, J.; López-Franco, Y.; Peniche-Covas, C.; Argüelles-Monal, W. Chitosan Hydrogels Based on the Diels–Alder Click Reaction: Rheological and Kinetic Study. Polymers 2022, 14, 1202. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839. [Google Scholar] [CrossRef]
- De Tovar, J.; Rataboul, F.; Djakovitch, L. Heterogenization of Pd(II) complexes as catalysts for the Suzuki-Miyaura reaction. Appl. Catal. A Gen. 2021, 627, 118381, ISSN 0926-860X. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Lahcini, M.; Bousmina, M.; Garcia, H.; El Kadib, A. Pd embedded in chitosan microspheres as tunable soft-materials for Sonogashira cross-coupling in water–ethanol mixture. Green Chem. 2015, 17, 1893–1898. [Google Scholar] [CrossRef]
- Abbas, H.S.; Abd El-Karim, S.S. Design, synthesis and anticervical cancer activity of new benzofuran–pyrazol-hydrazono- thiazolidin-4-one hybrids as potential EGFR inhibitors and apoptosis inducing agents. Biorg. Chem. 2019, 89, 103035. [Google Scholar] [CrossRef]
- Mukhtar, S.; Al-Ahmdi, M.I.; Parveen, H. Synthesis, Stereochemistry and Antimicrobial Activity of Some Novel Flavanone-hydrazono-thiazolidin-4-ones from Flavanones. Asian J. Chem. 2016, 28, 2589–2595. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Brum, J.O.C.; França, T.C.C.; LaPlante, S.R.; Villar, J.D.F. Synthesis and biological activity of hydrazones and derivatives. Mini Rev. Med. Chem. 2020, 20, 342–368. [Google Scholar] [CrossRef]
- Popiołek, Ł. The bioactivity of benzenesulfonyl hydrazones: A short review. Biomed. Pharmacother. 2021, 141, 111851. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Gaonkar, S.L. A New Microwave-Assisted Method for the Synthesis of 2-Substituted-Thiazol-4(5H)-one via Hantzsch Condensation. Rasayan J. Chem. 2022, 15, 310–315. [Google Scholar] [CrossRef]
- Lv, P.-C.; Zhou, C.-F.; Chen, J.; Liu, P.-G.; Wang, K.R.; Mao, W.-J.; Li, H.-Q.; Yang, Y.; Xiong, J.; Zhu, H.-L. Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 kinase inhibitors. Bioorganic Med. Chem. 2010, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordon, C.; Jones-Brando, L. Design, synthesis and biological characterization of thiazolidin-4-one derivatives as promising inhibitors of Toxoplasma gondii. Eur. J. Med. Chem. 2014, 86, 17–30. [Google Scholar] [CrossRef]
- Ayyash, A.N.; Tomma, J.H. Synthesis and Characterization of Some Novel 4-Thiazolidinones and Isoxazolines Derived from Thiosemicarbazones. Am. J. Org. Chem. 2014, 4, 52–62. [Google Scholar]
- Abdel-Gawad, H.; Mohamed, H.A.; Dawood, K.M.; Badria, F.A. Synthesis and Antiviral Activity of New Indole-Based Heterocycles. Chem. Pharm. Bull. 2010, 58, 1529–1531. [Google Scholar] [CrossRef]
- Ansari, M.A.; Jahan, N. Structural and Optical Properties of BaO Nanoparticles Synthesized by Facile Co-precipitation Method. Mater. Highlights 2021, 2, 23–28. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures; John Wiley and Sons Inc.: New York, NY, USA, 1954; p. 633. [Google Scholar]
- Bi, J.; Ji, X.; Guo, M.; Guo, H.; Yang, F. A fluorescent sensor for thymine based on bis-BODIPY containing butanediamido bridges. New J. Chem. 2019, 43, 5890–5896. [Google Scholar] [CrossRef]
- Oniga, O.; Ndongo, J.T.; Moldovan, C.; Tiperciuc, B.; Oniga, S.; Pîrnău, A.; Vlase, L.; Verité, P. Synthesis and Antimicrobial Activity of Some New 2-Hydrazone-thiazoline-4-ones. Farmacia 2012, 60, 785–797. [Google Scholar]





| Entries | Substrates | Catalyst | Yield (%) |
|---|---|---|---|
| Entry 1 | 2a + 1a (molar ratio 2:1) | TEA | 85 |
| Entry 2 | 2a + 1a (molar ratio 2:1) | BaO-CS nanocomposite | 91 |
| Entry 3 | 2a + 1b (molar ratio 2:1) | TEA | 86 |
| Entry 4 | 2a + 1b (molar ratio 2:1) | BaO-CS nanocomposite | 92 |
| Entry 5 | 2a + 7 (molar ratio 1:1) | TEA | 80 |
| Entry 6 | 2a + 7 (molar ratio 1:1) | BaO-CS nanocomposite | 85 |
| Entries | Substrates | Fresh Catalyst Yield (%) | Recycled Catalyst | ||
|---|---|---|---|---|---|
| (1) Yield (%) | (2) Yield (%) | (3) Yield (%) | |||
| Entry 1 | 2a + 1a (molar ratio 2:1) | 91 | 91 | 89 | 89 |
| Entry 2 | 2a + 1b (molar ratio 2:1) | 92 | 92 | 90 | 90 |
| Entry 3 | 2a + 7 (molar ratio 1:1) | 85 | 84 | 84 | 83 |
| Compd. No. | R | Ar | Yield (%) | Ref. |
|---|---|---|---|---|
| 5a | H | C6H5 | 92 | [36] |
| 5b | H | 4-CH3OC6H4 | 91 | [37] |
| 5c | H | 4-ClC6H4 | 90 | [37] |
| 5d | H | 4-BrC6H4 | 88 | [37] |
| 5e | H | 4-NO2C6H4 | 89 | [37] |
| 5f | H | 4-HOC6H4 | 85 | [37] |
| 5g | H | 3-HOC6H4 | 84 | [37] |
| 5h | H | 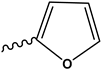 | [38] | |
| 5i | CH3 | C6H5 | 91 | [38] |
| 5j | CH3 | 4-BrC6H4 | 89 | [39] |
| 5k | CH3 |  | 87 | [38] |
| 5l | CH3 | 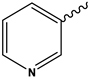 | 84 | [38] |
| 5m | CH3 | 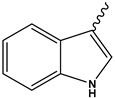 | 83 | [40] |
| 5n | CH3 |  | 80 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, K.D.; Riyadh, S.M.; Bashal, A.H.; Abolibda, T.Z.; Gomha, S.M. Green Synthetic Approaches of 2-Hydrazonothiazol-4(5H)-ones Using Sustainable Barium Oxide-Chitosan Nanocomposite Catalyst. Polymers 2023, 15, 3817. https://doi.org/10.3390/polym15183817
Khalil KD, Riyadh SM, Bashal AH, Abolibda TZ, Gomha SM. Green Synthetic Approaches of 2-Hydrazonothiazol-4(5H)-ones Using Sustainable Barium Oxide-Chitosan Nanocomposite Catalyst. Polymers. 2023; 15(18):3817. https://doi.org/10.3390/polym15183817
Chicago/Turabian StyleKhalil, Khaled D., Sayed M. Riyadh, Ali H. Bashal, Tariq Z. Abolibda, and Sobhi M. Gomha. 2023. "Green Synthetic Approaches of 2-Hydrazonothiazol-4(5H)-ones Using Sustainable Barium Oxide-Chitosan Nanocomposite Catalyst" Polymers 15, no. 18: 3817. https://doi.org/10.3390/polym15183817