Recent Developments on CO2 Hydrogenation Performance over Structured Zeolites: A Review on Properties, Synthesis, and Characterization
Abstract
:1. Introduction
2. Thermal CO2 Hydrogenation over Other Methods: A Brief Overview
3. Influential Parameters
3.1. Interplay of Composition and Preparation
3.2. Bimodal Mesoporous Structure and Surface Oxygen Vacancies
3.3. Si/Al Ratio
3.4. Porosity, Thermal Stability, and Structural Integrity
3.5. Electrical and Plasma Interactions
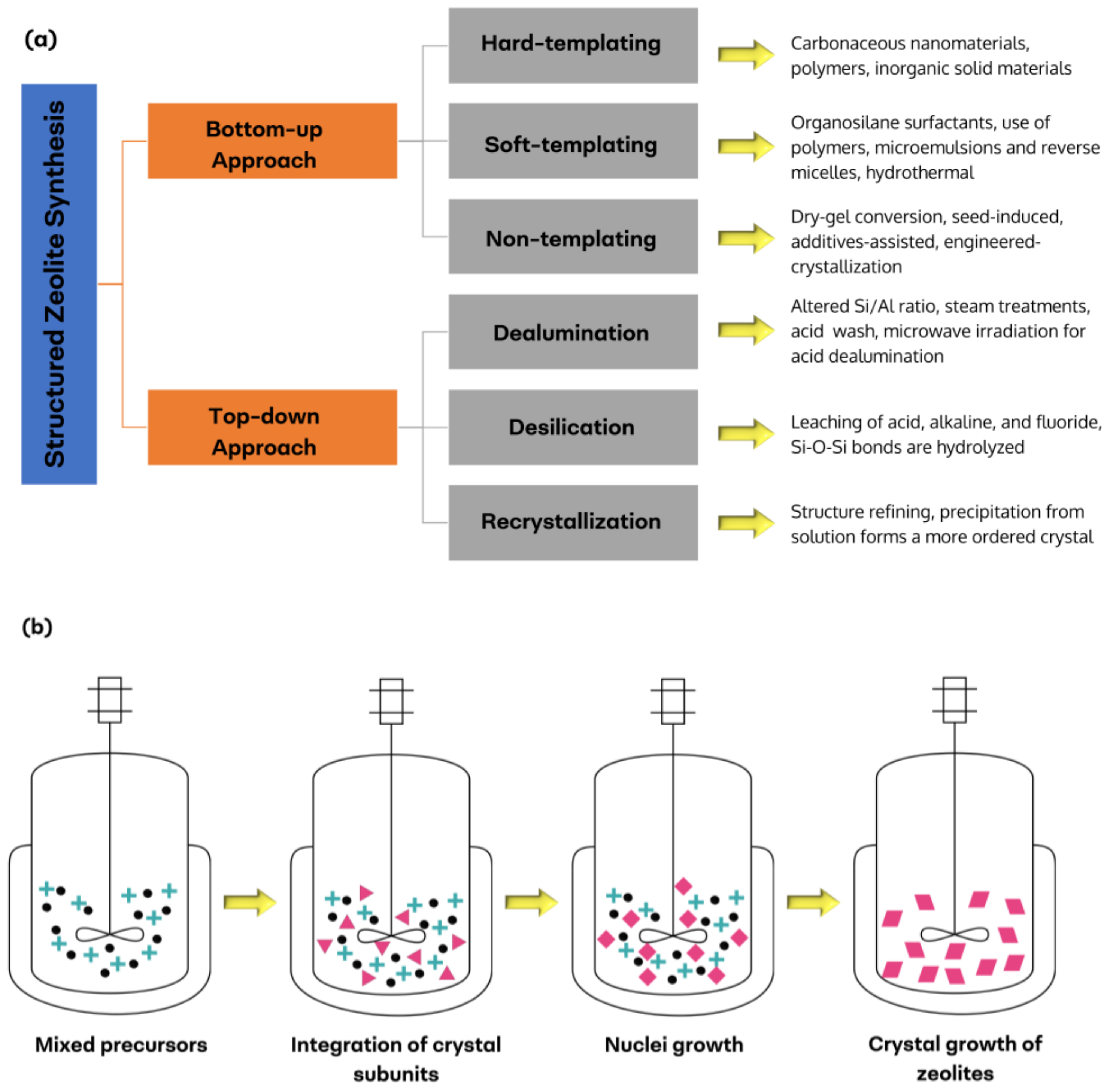
4. Synthesis Methods
4.1. Bottom-Up Approach
4.2. Top-Down Approach
5. Characterization Techniques
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- North, M.; Styring, P. Perspectives and visions on CO2capture and utilisation. Faraday Discuss. 2015, 183, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Tan, C.S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Nezam, I.; Zhou, W.; Gusmão, G.S.; Realff, M.J.; Wang, Y.; Medford, A.J.; Jones, C.W. Direct aromatization of CO2 via combined CO2 hydrogenation and zeolite-based acid catalysis. J. CO2 Util. 2021, 45, 101405. [Google Scholar] [CrossRef]
- Ramirez, A.; Gong, X.; Caglayan, M.; Nastase, S.-A.F.; Abou-Hamad, E.; Gevers, L.; Cavallo, L.; Chowdhury, A.D.; Gascon, J. Selectivity descriptors for the direct hydrogenation of CO2 to hydrocarbons during zeolite-mediated bifunctional catalysis. Nat. Commun. 2021, 12, 5914. [Google Scholar] [CrossRef] [PubMed]
- Murciano, R.; Serra, J.M.; Martínez, A. Direct hydrogenation of CO2 to aromatics via Fischer-Tropsch route over tandem K-Fe/Al2O3+H-ZSM-5 catalysts: Influence of zeolite properties. Catal. Today 2023, 427, 114404. [Google Scholar] [CrossRef]
- Dry, M.E.; Hoogendoorn, J.C. Technology of the Fischer-Tropsch Process. Catal. Rev. 1981, 23, 265–278. [Google Scholar] [CrossRef]
- Ibrahim, I.; Harrigan, F. Qatar’s economy: Past, present and future. QScience Connect. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Holm-Larsen, H. CO2 reforming for large scale methanol plants—An actual case. Stud. Surf. Sci. Catal 2001, 136, 441–446. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Ojelade, O.A.; Zaman, S.F. A review on CO2 hydrogenation to lower olefins: Understanding the structure-property relationships in heterogeneous catalytic systems. J. CO2 Util. 2021, 47, 101506. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Melaningtyas, G.S.A.; Krisnandi, Y.K.; Ekananda, R. Synthesis and characterization of NaY zeolite from Bayat natural zeolite: Effect of pH on synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012042. [Google Scholar] [CrossRef]
- Dimitar, G.; Bogdanov, B.; Angelova, K.; Markovska, I.; Hristov, Y. Synthetic Zeolites—Structure, Clasification, Current Trends in Zeolite Synthesis. International Science Conference. Available online: https://www.researchgate.net/profile/Irena-Markovska-2/publication/322211658_SYNTHETIC_ZEOLITES_-_STRUCTURE_CLASIFICATION_CURRENT_TRENDS_IN_ZEOLITE_SYNTHESIS_REVIEW/links/5a990a4f0f7e9ba4297769ff/SYNTHETIC-ZEOLITES-STRUCTURE-CLASIFICATION-CURRENT-TRENDS-IN-ZEOLITE-SYNTHESIS-REVIEW.pdf (accessed on 12 May 2024).
- Ruiz, A.Z.; Ban, T.; Calzaferri, G.; Brühwiler, D. Synthesis of Zeolite L. Tuning Size and Morphology. Monatshefte Fuer Chemie/Chem. Mon. 2005, 136, 77–89. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef]
- Tran, Y.T.; Lee, J.; Kumar, P.; Kim, K.-H.; Lee, S.S. Natural zeolite and its application in concrete composite production. Compos. Part B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Kalló, D. Applications of Natural Zeolites in Water and Wastewater Treatment. Rev. Miner. Geochem. 2001, 45, 519–550. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. The safety of synthetic zeolites used in detergents. Arch. Toxicol. 2008, 83, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wittcoff, H.A.; Reuben, B.G.; Plotkin, J.S. Industrial Organic Chemicals; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Overa, S.; Ko, B.H.; Zhao, Y.; Jiao, F. Electrochemical Approaches for CO2 Conversion to Chemicals: A Journey toward Practical Applications. Accounts Chem. Res. 2022, 55, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Leung, P.-K.; Wang, L.; de León, C.P. Developments on carbon dioxide reduction: Their promise, achievements, and challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Van Le, Q. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: Opportunities and challenges. Bioresour. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. J. Clean. Prod. 2020, 247, 119138. [Google Scholar] [CrossRef]
- Ribun, V.; Boichenko, S.; Kale, U. Advances in gas-to-liquid technology for environmentally friendly fuel synthesis: Analytical review of world achievements. Energy Rep. 2023, 9, 5500–5508. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Kakaras, E. Thermocatalytic CO2 hydrogenation for methanol and ethanol production: Process improvements. Int. J. Hydrogen Energy 2016, 41, 792–806. [Google Scholar] [CrossRef]
- Do, T.N.; Kim, J. Process development and techno-economic evaluation of methanol production by direct CO2 hydrogenation using solar-thermal energy. J. CO2 Util. 2019, 33, 461–472. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, G.; Liu, B.; Zhang, H.; Tu, X. Unraveling Temperature-Dependent Plasma-Catalyzed CO2 Hydrogenation. Ind. Eng. Chem. Res. 2023, 62, 19629–19637. [Google Scholar] [CrossRef] [PubMed]
- Chatzilias, C.; Martino, E.; Katsaounis, A.; Vayenas, C.G. Electrochemical promotion of CO2 hydrogenation in a monolithic electrochemically promoted reactor (MEPR). Appl. Catal. B Environ. 2021, 284, 119695. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef]
- Jhong, H.; Brushett, F.R.; Kenis, P.J.A. The Effects of Catalyst Layer Deposition Methodology on Electrode Performance. Adv. Energy Mater. 2013, 3, 589–599. [Google Scholar] [CrossRef]
- Kalaitzidou, I.; Makri, M.; Theleritis, D.; Katsaounis, A.; Vayenas, C. Comparative study of the electrochemical promotion of CO2 hydrogenation on Ru using Na+, K+, H+ and O2− conducting solid electrolytes. Surf. Sci. 2016, 646, 194–203. [Google Scholar] [CrossRef]
- Lo, A.-Y.; Taghipour, F. Review and prospects of microporous zeolite catalysts for CO2 photoreduction. Appl. Mater. Today 2021, 23, 101042. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Ji, X. Developing and Regenerating Cofactors for Sustainable Enzymatic CO2 Conversion. Processes 2022, 10, 230. [Google Scholar] [CrossRef]
- Long, N.V.D.; Lee, J.; Koo, K.-K.; Luis, P.; Lee, M. Recent Progress and Novel Applications in Enzymatic Conversion of Carbon Dioxide. Energies 2017, 10, 473. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel Heterogeneous Catalysts for CO2 Hydrogenation to Liquid Fuels. ACS Central Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Ojelade, O.A. CO2 Hydrogenation to Gasoline and Aromatics: Mechanistic and Predictive Insights from DFT, DRIFTS and Machine Learning. Chempluschem 2023, 88, e202300301. [Google Scholar] [CrossRef] [PubMed]
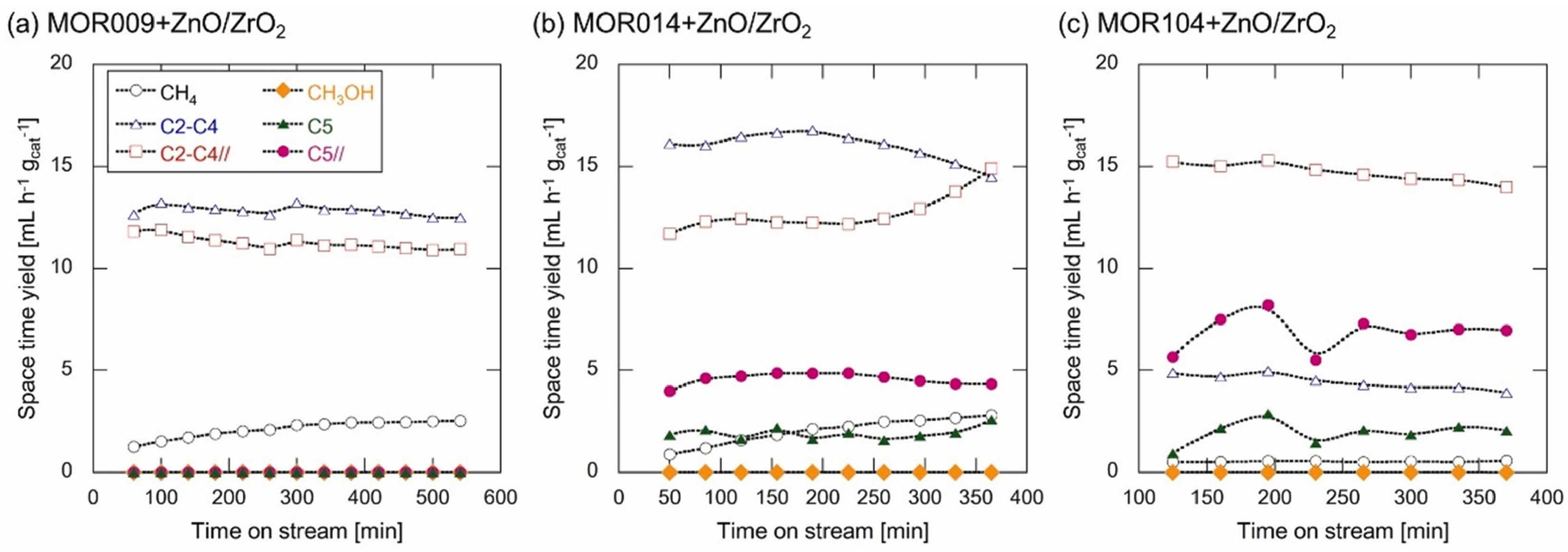
- Oishi, R.; Li, D.; Okazaki, M.; Kinoshita, H.; Ochiai, N.; Yamauchi, N.; Kobayashi, Y.; Wakihara, T.; Okubo, T.; Tada, S.; et al. Precise tuning of the properties of MOR-type zeolite nanoparticles to improve lower olefins selectivity in composite catalysts for CO2 hydrogenation. J. CO2 Util. 2023, 72, 102491. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Zhang, J.; Song, F.; Wu, Y.; Zhang, T.; Zhang, Q.; Tsubaki, N.; Tan, Y. Macroscopic assembly style of catalysts significantly determining their efficiency for converting CO2 to gasoline. Catal. Sci. Technol. 2019, 9, 5401–5412. [Google Scholar] [CrossRef]
- Azhari, N.J.; Nurdini, N.; Mardiana, S.; Ilmi, T.; Fajar, A.T.; Makertihartha, I.; Subagjo; Kadja, G.T. Zeolite-based catalyst for direct conversion of CO2 to C2+ hydrocarbon: A review. J. CO2 Util. 2022, 59, 101969. [Google Scholar] [CrossRef]
- Hölderich, W.; Hesse, M.; Näumann, F. Zeolites: Catalysts for Organic Syntheses. Angew. Chem. Int. Ed. 1988, 27, 226–246. [Google Scholar] [CrossRef]
- Yilmaz, B.; Müller, U. Catalytic Applications of Zeolites in Chemical Industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174, Erratum in 2017, 8, 16170. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Oykova, M.; Dimitrov, M.; Karashanova, D.; Kovacheva, D.; Atanasova, G.; Szegedi, Á. CO2 Hydrogenation to Renewable Methane on Ni/Ru Modified ZSM-5 Zeolites: The Role of the Preparation Procedure. Catalysts 2022, 12, 1648. [Google Scholar] [CrossRef]
- Bando, K.K.; Soga, K.; Kunimori, K.; Arakawa, H. Effect of Li additive on CO2 hydrogenation reactivity of zeolite supported Rh catalysts. Appl. Catal. A Gen. 1998, 175, 67–81. [Google Scholar] [CrossRef]
- Chan, B.; Radom, L. Design of Effective Zeolite Catalysts for the Complete Hydrogenation of CO2. J. Am. Chem. Soc. 2006, 128, 5322–5323. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Leshchev, D.; Stavitski, E.; Juneau, M.; Agwara, J.N.; Porosoff, M.D. Selective hydrogenation of CO2 and CO over potassium promoted Co/ZSM-5. Appl. Catal. B Environ. 2021, 284, 119787. [Google Scholar] [CrossRef]
- Ramirez, A.; Chowdhury, A.D.; Dokania, A.; Cnudde, P.; Caglayan, M.; Yarulina, I.; Abou-Hamad, E.; Gevers, L.E.; Ould-Chikh, S.; De Wispelaere, K.; et al. Effect of Zeolite Topology and Reactor Configuration on the Direct Conversion of CO2 to Light Olefins and Aromatics. ACS Catal. 2019, 9, 6320–6334. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Maghfirah, A.; Ilmi, M.; Fajar, A.; Kadja, G. A review on the green synthesis of hierarchically porous zeolite. Mater. Today Chem. 2020, 17, 100348. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Yan, P.; Nawaz, M.A.; Liu, D. High Conversion to Aromatics via CO2-FT over a CO-Reduced Cu-Fe2O3 Catalyst Integrated with HZSM-5. ACS Catal. 2020, 10, 11268–11279. [Google Scholar] [CrossRef]
- Tian, H.; Gao, P.; Yang, X.; Jiao, C.; Zha, F.; Chang, Y.; Chen, H. Tandem composite of M (Zn, Ga, In)-UIO-66/(HZSM-5)-palygorskite for hydrogenation of carbon dioxide to aromatics. Chem. Eng. J. 2023, 466, 143267. [Google Scholar] [CrossRef]
- Yan, P.; Peng, H.; Wu, X.; Rabiee, H.; Weng, Y.; Konarova, M.; Vogrin, J.; Rozhkovskaya, A.; Zhu, Z. Impact of varied zeolite materials on nickel catalysts in CO2 methanation. J. Catal. 2024, 432, 115439. [Google Scholar] [CrossRef]
- da Costa-Serra, J.F.; Cerdá-Moreno, C.; Chica, A. Zeolite-Supported Ni Catalysts for CO2 Methanation: Effect of Zeolite Structure and Si/Al Ratio. Appl. Sci. 2020, 10, 5131. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graca, I.; Lopes, J.M.; Henriques, C. Enhanced activity of CO2 hydrogenation to CH4 over Ni based zeolites through the optimization of the Si/Al ratio. Microporous Mesoporous Mater. 2018, 267, 9–19. [Google Scholar] [CrossRef]
- Tada, S.; Li, D.; Okazaki, M.; Kinoshita, H.; Nishijima, M.; Yamauchi, N.; Kobayashi, Y.; Iyoki, K. Influence of Si/Al ratio of MOR type zeolites for bifunctional catalysts specific to the one-pass synthesis of lower olefins via CO2 hydrogenation. Catal. Today 2023, 411–412, 113828. [Google Scholar] [CrossRef]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective Production of Aromatics Directly from Carbon Dioxide Hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B Environ. 2021, 283, 119648. [Google Scholar] [CrossRef]
- Xiang, M.; Shi, Z.; Zhang, X.; Gao, Z.; Guo, J.; Wu, Z.; Ma, S.; Bai, J.; Zhang, W.; Deng, Y.; et al. Facile synthesis of hierarchical SAPO-56 zeolite as a highly efficient catalyst for CO2 hydrogenation to methanol. Fuel 2024, 361, 130663. [Google Scholar] [CrossRef]
- Cui, W.-G.; Li, Y.-T.; Yu, L.; Zhang, H.; Hu, T.-L. Zeolite-Encapsulated Ultrasmall Cu/ZnOx Nanoparticles for the Hydrogenation of CO2 to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 18693–18703. [Google Scholar] [CrossRef] [PubMed]
- Ashford, B.; Poh, C.-K.; Ostrikov, K.; Chen, L.; Tu, X. Plasma-catalytic CO2 hydrogenation to ethane in a dielectric barrier discharge reactor. J. CO2 Util. 2022, 57, 101882. [Google Scholar] [CrossRef]
- Feliz, M.Q.; Polaert, I.; Ledoux, A.; Fernandez, C.; Azzolina-Jury, F. Influence of ionic conductivity and dielectric constant of the catalyst on DBD plasma-assisted CO2 hydrogenation into methanol. J. Phys. D Appl. Phys. 2021, 54, 334003. [Google Scholar] [CrossRef]
- Bacariza, M.; Biset-Peiró, M.; Graça, I.; Guilera, J.; Morante, J.; Lopes, J.; Andreu, T.; Henriques, C. DBD plasma-assisted CO2 methanation using zeolite-based catalysts: Structure composition-reactivity approach and effect of Ce as promoter. J. CO2 Util. 2018, 26, 202–211. [Google Scholar] [CrossRef]
- Chen, H.; Mu, Y.; Shao, Y.; Chansai, S.; Xu, S.; Stere, C.E.; Xiang, H.; Zhang, R.; Jiao, Y.; Hardacre, C.; et al. Coupling non-thermal plasma with Ni catalysts supported on BETA zeolite for catalytic CO2 methanation. Catal. Sci. Technol. 2019, 9, 4135–4145. [Google Scholar] [CrossRef]
- Jwa, E.; Lee, S.; Lee, H.; Mok, Y. Plasma-assisted catalytic methanation of CO and CO2 over Ni–zeolite catalysts. Fuel Process. Technol. 2013, 108, 89–93. [Google Scholar] [CrossRef]
- Da Costa, P.; Hasrack, G.; Bonnety, J.; Henriques, C. Ni-based catalysts for plasma-assisted CO2 methanation. Curr. Opin. Green Sustain. Chem. 2021, 32, 100540. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Henckel, D.; Saha, P.; Intia, F.; Taylor, A.K.; Baez-Cotto, C.; Hu, L.; Schellekens, M.; Simonson, H.; Miller, E.M.; Verma, S.; et al. Elucidation of Critical Catalyst Layer Phenomena toward High Production Rates for the Electrochemical Conversion of CO to Ethylene. ACS Appl. Mater. Interfaces 2024, 16, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Pinar, A.B.; Kenvin, J.; Crivelli, P.; Kärger, J.; Pérez-Ramírez, J. Structural analysis of hierarchically organized zeolites. Nat. Commun. 2015, 6, 8633. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.J.H.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous Zeolite Single Crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Schmidt, I.; Boisen, A.; Gustavsson, E.; Ståhl, K.; Pehrson, S.; Dahl, S.; Carlsson, A.; Jacobsen, C.J.H. Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.-S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal Synthesis of Zeolites with Three-Dimensionally Ordered Mesoporous-Imprinted Structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Wang, B.; Han, Y.; Xu, Y.; Liang, Z.; Wang, Z. Nickel Nanoparticles Encapsulated in Microporous Graphenelike Carbon (Ni@MGC) as Catalysts for CO2 Methanation. Ind. Eng. Chem. Res. 2019, 58, 20536–20542. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic–inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189–190, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Yilmaz, B.; Müller, U.; Bein, T. Hierarchical Zeolite Beta via Nanoparticle Assembly with a Cationic Polymer. Chem. Mater. 2011, 23, 4301–4310. [Google Scholar] [CrossRef]
- Kadja, G.T.; Suprianti, T.R.; Ilmi, M.M.; Khalil, M.; Mukti, R.R.; Subagjo. Sequential mechanochemical and recrystallization methods for synthesizing hierarchically porous ZSM-5 zeolites. Microporous Mesoporous Mater. 2020, 308, 110550. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.S.; Schlögl, R.; Yokoi, T.; et al. Catalytic Properties of Hierarchical Mesoporous Zeolites Templated with a Mixture of Small Organic Ammonium Salts and Mesoscale Cationic Polymers. Angew. Chem. 2006, 118, 3162–3165. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.-H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Yip, A.C.K. Advances in the Green Synthesis of Microporous and Hierarchical Zeolites: A Short Review. Catalysts 2019, 9, 274. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, G.; Tian, J.; Tan, K.B.; Guo, M.; Han, Y.; Fu, T.; Huang, J.; Li, Q. Direct CO2 hydrogenation to light olefins over ZnZrOx mixed with hierarchically hollow SAPO-34 with rice husk as green silicon source and template. Appl. Catal. B Environ. 2022, 315, 121572. [Google Scholar] [CrossRef]
- Din, I.U.; Din, I.U.; Alotaibi, M.A.; Alotaibi, M.A.; Alharthi, A.I.; Alharthi, A.I.; Al-Shalwi, M.N.; Al-Shalwi, M.N.; Alshehri, F.; Alshehri, F. Green synthesis approach for preparing zeolite based Co-Cu bimetallic catalysts for low temperature CO2 hydrogenation to methanol. Fuel 2022, 330, 125643. [Google Scholar] [CrossRef]
- Gourdon, A. On-Surface Covalent Coupling in Ultrahigh Vacuum. Angew. Chem. Int. Ed. 2008, 47, 6950–6953. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Le Bras, S.; Boissière, C.; Chaumonnot, A.; Sanchez, C. Aerosol processing: A wind of innovation in the field of advanced heterogeneous catalysts. Chem. Soc. Rev. 2018, 47, 4112–4155. [Google Scholar] [CrossRef] [PubMed]
- Thilakarajan, P. Synthesis and Evaluation of Nanocatalysts for CO2 Hydrogenation to Methanol: A Comprehensive Review. Int. J. High Sch. Res. 2024, 6, 118–126. [Google Scholar]
- Bahari, N.A.; Isahak, W.N.R.W.; Ba-Abbad, M.M. The Effectiveness of Various Bimetallic on Iron-Zeolite Catalyst by Carbon Dioxide Hydrogenation. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012110. [Google Scholar] [CrossRef]
- Gao, X.; Deng, S.; Kawi, S. Zeolite-based catalytic membrane reactors for thermo-catalytic conversion of CO2. iScience 2022, 25, 105343. [Google Scholar] [CrossRef] [PubMed]
- Maghfirah, A.; Susanti, Y.; Fajar, A.T.N.; Mukti, R.R.; Kadja, G.T.M. The role of tetraalkylammonium for controlling dealumination of zeolite Y in acid media. Mater. Res. Express 2019, 6, 094002. [Google Scholar] [CrossRef]
- Fajar, A.; Nurdin, F.; Mukti, R.; Subagjo; Rasrendra, C.; Kadja, G. Synergistic effect of dealumination and ceria impregnation to the catalytic properties of MOR zeolite. Mater. Today Chem. 2020, 17, 100313. [Google Scholar] [CrossRef]
- Le Hua, Z.; Zhou, J.; Shi, J.L. Recent advances in hierarchically structured zeolites: Synthesis and material performances. Chem. Commun. 2011, 47, 10536–10547. [Google Scholar] [CrossRef]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Pérez-Ramírez, J. Full Compositional Flexibility in the Preparation of Mesoporous MFI Zeolites by Desilication. J. Phys. Chem. C 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Wardani, M.K.; Kadja, G.T.M.; Fajar, A.T.N.; Subagjo, S.; Makertihartha, I.G.B.N.; Gunawan, M.L.; Suendo, V.; Mukti, R.R. Highly crystalline mesoporous SSZ-13 zeolite obtained via controlled post-synthetic treatment. RSC Adv. 2019, 9, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wakihara, T.; Tatami, J. Top-down Tuning of Nanosized Zeolites by Bead-milling and Recrystallization. J. Jpn. Pet. Inst. 2013, 56, 206–213. [Google Scholar] [CrossRef]
- Kurniawan, T.; Muraza, O.; Hakeem, A.S.; Al-Amer, A.M. Mechanochemical Route and Recrystallization Strategy To Fabricate Mordenite Nanoparticles from Natural Zeolites. Cryst. Growth Des. 2017, 17, 3313–3320. [Google Scholar] [CrossRef]
- Airi, A.; Signorile, M.; Bonino, F.; Quagliotto, P.; Bordiga, S.; Martens, J.A.; Crocellà, V. Insights on a Hierarchical MFI Zeolite: A Combined Spectroscopic and Catalytic Approach for Exploring the Multilevel Porous System Down to the Active Sites. ACS Appl. Mater. Interfaces 2021, 13, 49114–49127. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C. Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Design of Lewis-acid centres in zeolitic matrices for the conversion of renewables. Chem. Soc. Rev. 2015, 44, 7025–7043. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Shi, T.; Gu, J.; Cui, Y.; Zhang, Z.; Yang, C.; Chen, T.; Lin, M.; Wang, P.; Xue, N.; et al. CO2 Hydrogenation to Ethanol over Cu@Na-Beta. Chem 2020, 6, 2673–2689. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Westermann, A.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. CO2 Hydrogenation Over Ni-Based Zeolites: Effect of Catalysts Preparation and Pre-reduction Conditions on Methanation Performance. Top. Catal. 2016, 59, 314–325. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Tuning Zeolite Properties towards CO2 Methanation: An Overview. ChemCatChem 2019, 11, 2388–2400. [Google Scholar] [CrossRef]
- Verboekend, D.; Pérez-Ramírez, J. Design of hierarchical zeolite catalysts by desilication. Catal. Sci. Technol. 2011, 1, 879–890. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, Ł.; Podobiński, J.; Sulikowski, B.; Datka, J. Spectroscopic IR and NMR studies of hierarchical zeolites obtained by desilication of zeolite Y: Optimization of the desilication route. Microporous Mesoporous Mater. 2019, 281, 134–141. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Li, L.; Hou, K.; Ding, F.; Li, J.; Mu, D.; Song, C.; Liu, M.; Guo, X. Synthesis of Hollow Nanocubes and Macroporous Monoliths of Silicalite-1 by Alkaline Treatment. Chem. Mater. 2013, 25, 4197–4205. [Google Scholar] [CrossRef]
- Sharma, P.; Ho, P.H.; Di, W.; Creaser, D.; Olsson, L. Novel catalyst configuration to boost the yield of longer hydrocarbons from methanol-mediated CO2 hydrogenation. J. CO2 Util. 2023, 74, 102549. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Słowik, G.; Kuśmierz, M.; Dzwigaj, S. The state of BEA zeolite supported nickel catalysts in CO2 methanation reaction. Appl. Surf. Sci. 2021, 564, 150421. [Google Scholar] [CrossRef]
- Krachuamram, S.; Kidkhunthod, P.; Poo-Arporn, Y.; Kamonsutthipaijit, N.; Chanapattharapol, K.C. On the Optimization of Ni/A and Ni/X Synthesis Procedure toward Active and Selective Catalysts for the Production of CH4 from CO2. Catalysts 2022, 12, 823. [Google Scholar] [CrossRef]
- Velisoju, V.K.; Cerrillo, J.L.; Ahmad, R.; Mohamed, H.O.; Attada, Y.; Cheng, Q.; Yao, X.; Zheng, L.; Shekhah, O.; Telalovic, S.; et al. Copper nanoparticles encapsulated in zeolitic imidazolate framework-8 as a stable and selective CO2 hydrogenation catalyst. Nat. Commun. 2024, 15, 2045. [Google Scholar] [CrossRef]
- Ticali, P.; Salusso, D.; Ahmad, R.; Ahoba-Sam, C.; Ramirez, A.; Shterk, G.; Lomachenko, K.A.; Borfecchia, E.; Morandi, S.; Cavallo, L.; et al. CO2 hydrogenation to methanol and hydrocarbons over bifunctional Zn-doped ZrO2/zeolite catalysts. Catal. Sci. Technol. 2021, 11, 1249–1268. [Google Scholar] [CrossRef]
- Wang, X.; Jeong, S.Y.; Jung, H.S.; Shen, D.; Ali, M.; Zafar, F.; Chung, C.-H.; Bae, J.W. Catalytic activity for direct CO2 hydrogenation to dimethyl ether with different proximity of bifunctional Cu-ZnO-Al2O3 and ferrierite. Appl. Catal. B Environ. 2023, 327, 122456. [Google Scholar] [CrossRef]
- Wang, C.; Guan, E.; Wang, L.; Chu, X.; Wu, Z.; Zhang, J.; Yang, Z.; Jiang, Y.; Zhang, L.; Meng, X.; et al. Product Selectivity Controlled by Nanoporous Environments in Zeolite Crystals Enveloping Rhodium Nanoparticle Catalysts for CO2 Hydrogenation. J. Am. Chem. Soc. 2019, 141, 8482–8488. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]

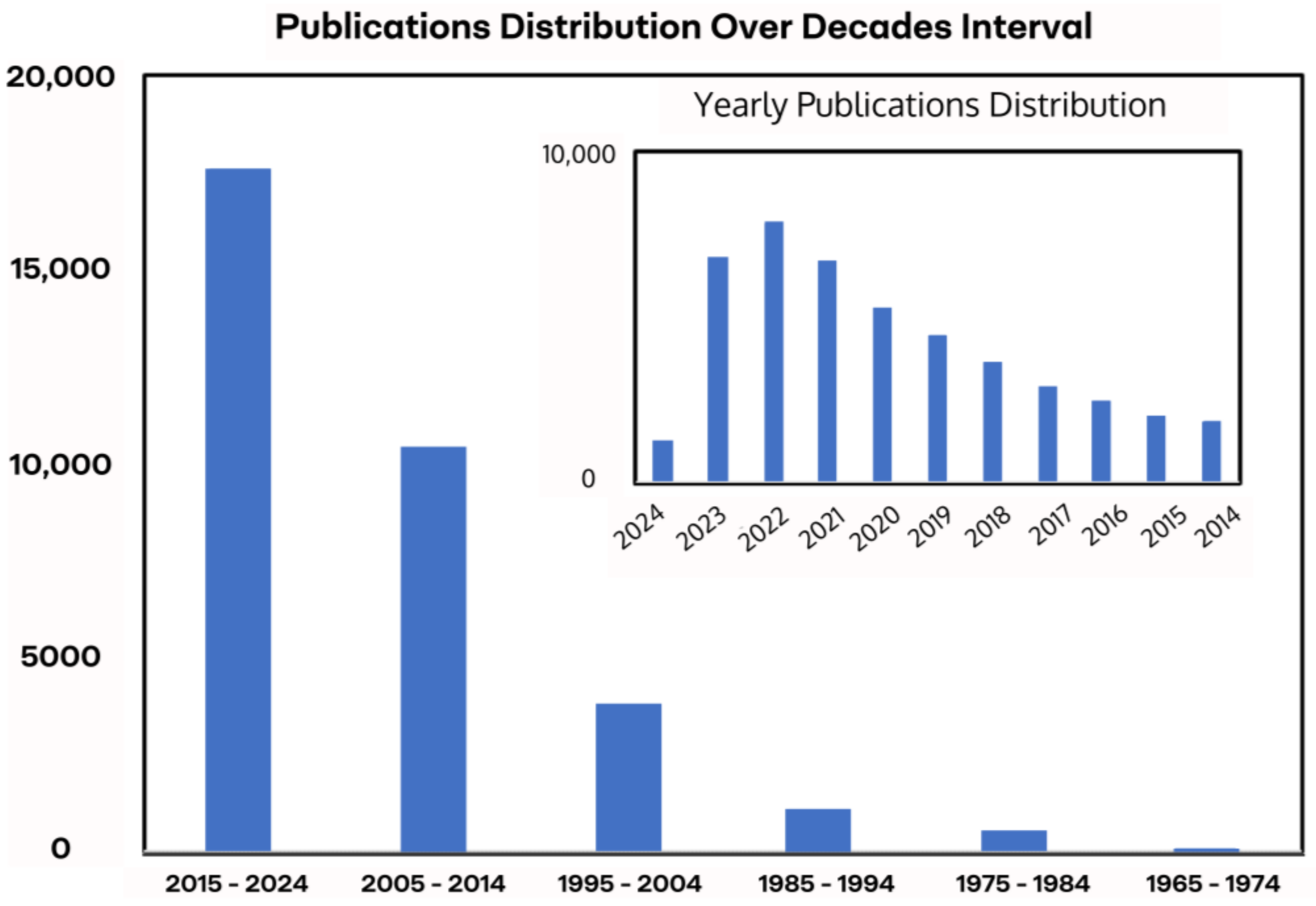

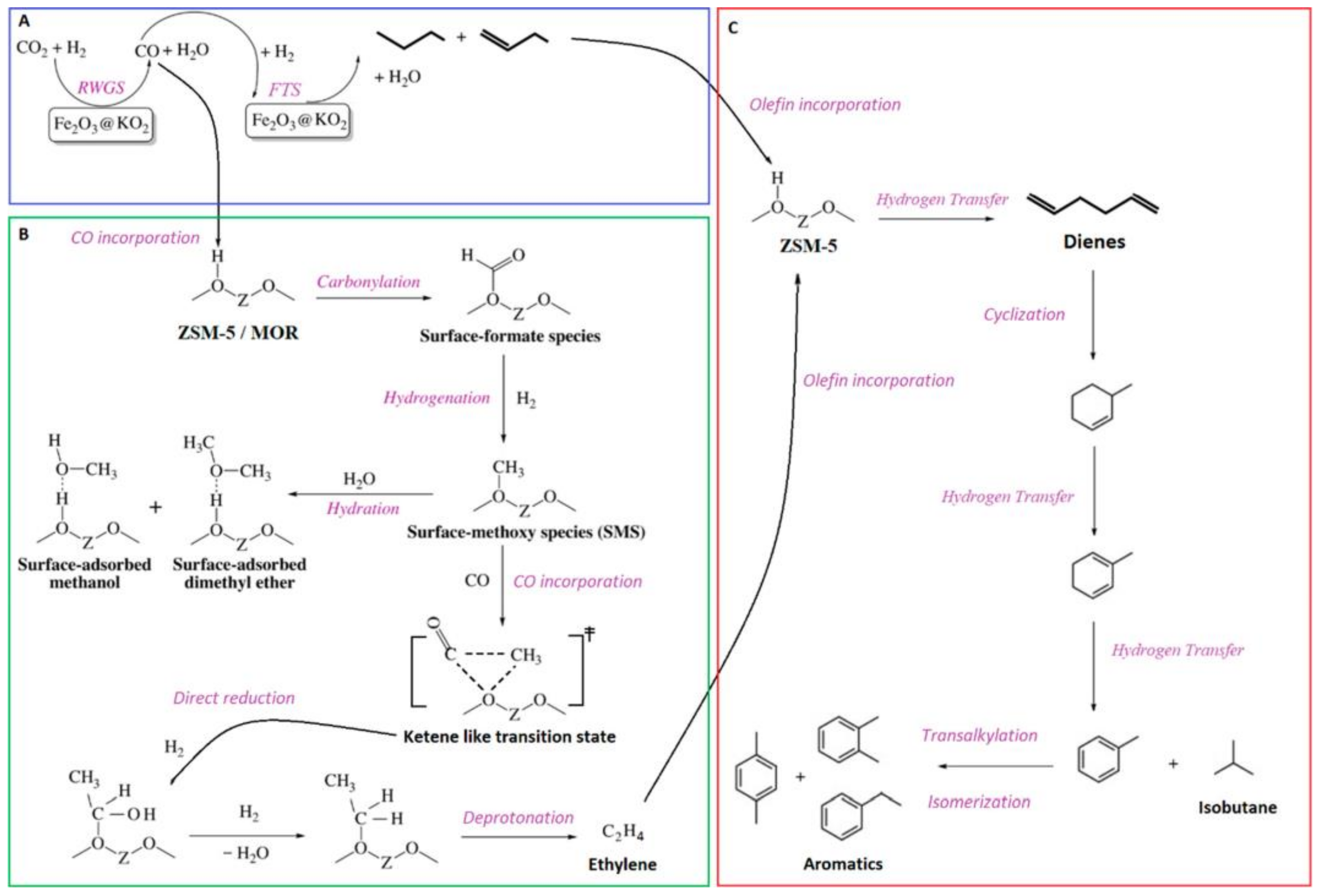
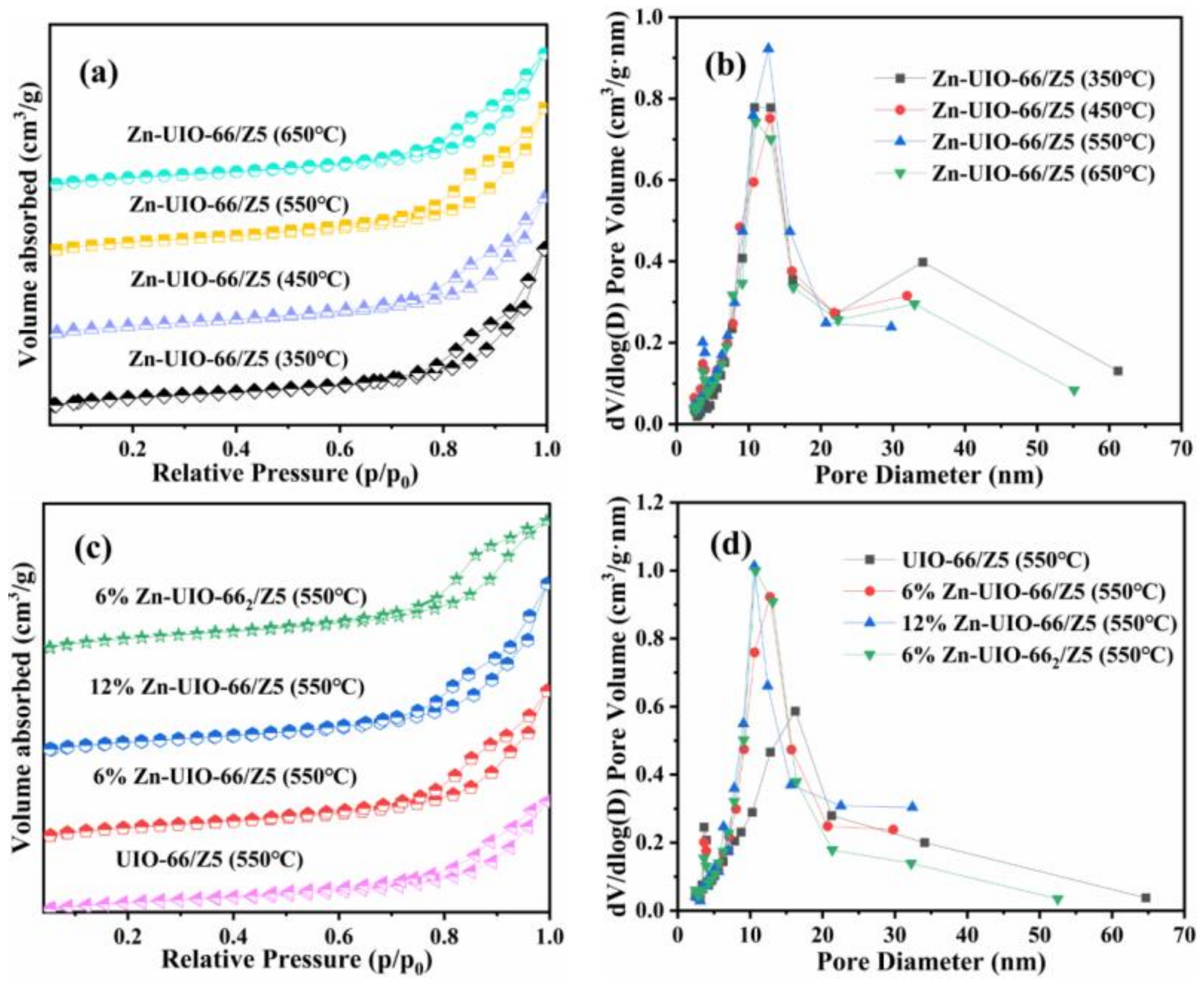
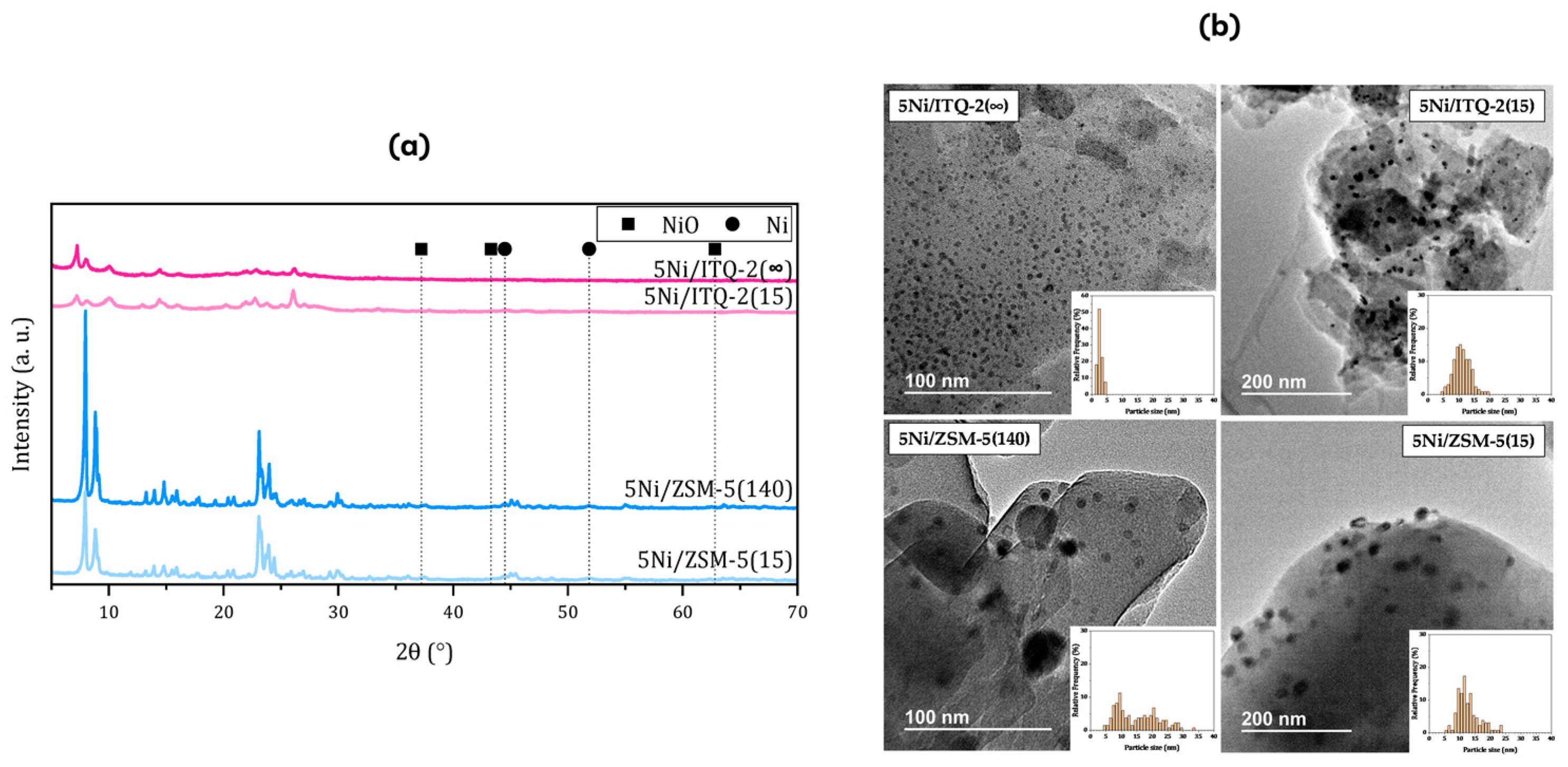



| Method | Advantages | Disadvantages |
|---|---|---|
| Thermal [10,11,12] | Proven scalability, high product yields, extensively used in industrial settings | Can be energy-intensive; less environmentally friendly without renewable energy integration; catalyst stability issues |
| Electrochemical [24,25] | Facilitates CO2 reduction using electricity, can be integrated with renewable electricity sources; scalable; stable long-term | Often a broad product distribution at high conversions; high energy demand; economically challenging |
| Photocatalytic [26,27] | Utilizes sunlight, environmentally friendly; no need for external energy inputs; economically feasible | Lower efficiency and stability, which hinders scalability; lower yields |
| Biochemical and Chemo-enzymatic [28,29,30] | High selectivity; operates under mild conditions; efficient for producing bulk chemicals | Slow reaction rates; complex maintenance of biocatalytic activity; high temperatures can inhibit enzyme activity |
| Hydrogenation Feature | Thermal Conversion [33,34,35] | Electrochemical Conversion [36,37,38,39] | Photocatalytic Conversion [37,40,41,42] | Biochemical and Chemo-Enzymatic Conversion [41,43,44,45] |
|---|---|---|---|---|
| Energy Efficiency | High, but varies based on the used catalytic materials | Energy-intensive with usually low catalytic material lifespan | Limited by light absorption efficiency | High cofactor costs impact overall efficiency |
| CO2 Conversion Rate | High at moderate to high temperatures | Can be high based on applied potential and choice of catalyst | Hampered by poor selectivity and product variety | Less productive, slow reaction rates |
| Catalyst Durability | Robust; choice of reducible supports enhances durability | Often short lifespan due to rigorous operational conditions | Challenges with catalyst degradation and by-product formation | Enzyme stability and recyclability need enhancement |
| Capital and Operational Costs | Relatively cost-effective due to higher efficiencies, and easy integration with reforming industries | Relatively high due to the need for optimizing efficiency and current density. Still requires technological maturity for CO2 conv. | Significant capital investment and energy required, especially for wide bandgap semiconductors | Costly due to the high price of enzymes and cofactors |
| Technological Maturity | Advanced | Remains limited in industry due to practical challenges | Processes are complex and mechanisms are not fully understood | Requires advancements in enzyme modification and immobilization |
| Reaction Mechanisms | Complex but widely studied on a variety of catalysts | Complex with limited understanding. Requires intricate tools to monitor reaction intermediates near electrodes in liquid medium | Involves intricate mechanisms that are poorly understood | Constrained by the complexity of biological systems |
| Selectivity | High and can be tailored for desired outcomes | Low; produces a mix of products. High conversion is often reported with broad product distribution | Often poor due to multiple possible reactions | Can be very selective. Dependent on the specificity of biological pathways |
| Scalability | Highly scalable and suitable for industrial scale-up | Faces practical challenges in scaling. Requires further technological development. | Limited scalability due to technical and efficiency constraints | Challenging due to the need to maintain microbial cultures and nutrient delivery |
| Downstream Processing | Simplified due to few by-products | Could require separation of multiple by-products | Complexities in separating the catalyst from the product increase expenses | Demands efficient cofactor regeneration and faces genetic knockout issues |
| Potential for improvement | Relatively well-established with room for incremental improvements based on catalyst and reactor design | Requires catalysts with fast electron transfer and robust transport features | Needs optimization of photocatalysts and treatment processes | Genetic engineering is suggested but leads to challenges like genetic knockout |
| Category | Method | Application |
|---|---|---|
| Molecular and Chemical Structure Analysis [5,114,115] | Fourier-transform infrared (FTIR) spectroscopy | Quantify absorption spectra in chemical bonds and functional groups in molecules |
| Raman spectroscopy | Postulate information about molecular vibrations, crystal structures, and phase transitions | |
| Nuclear Magnetic Resonance (NMR) spectroscopy | Provide detailed information on the framework aluminum distribution and the nature of acid sites | |
| Small-angle X-ray Scattering (SAXS) | Analyze the structural details of materials at the nanoscale level by detecting inhomogeneities and phase separation within | |
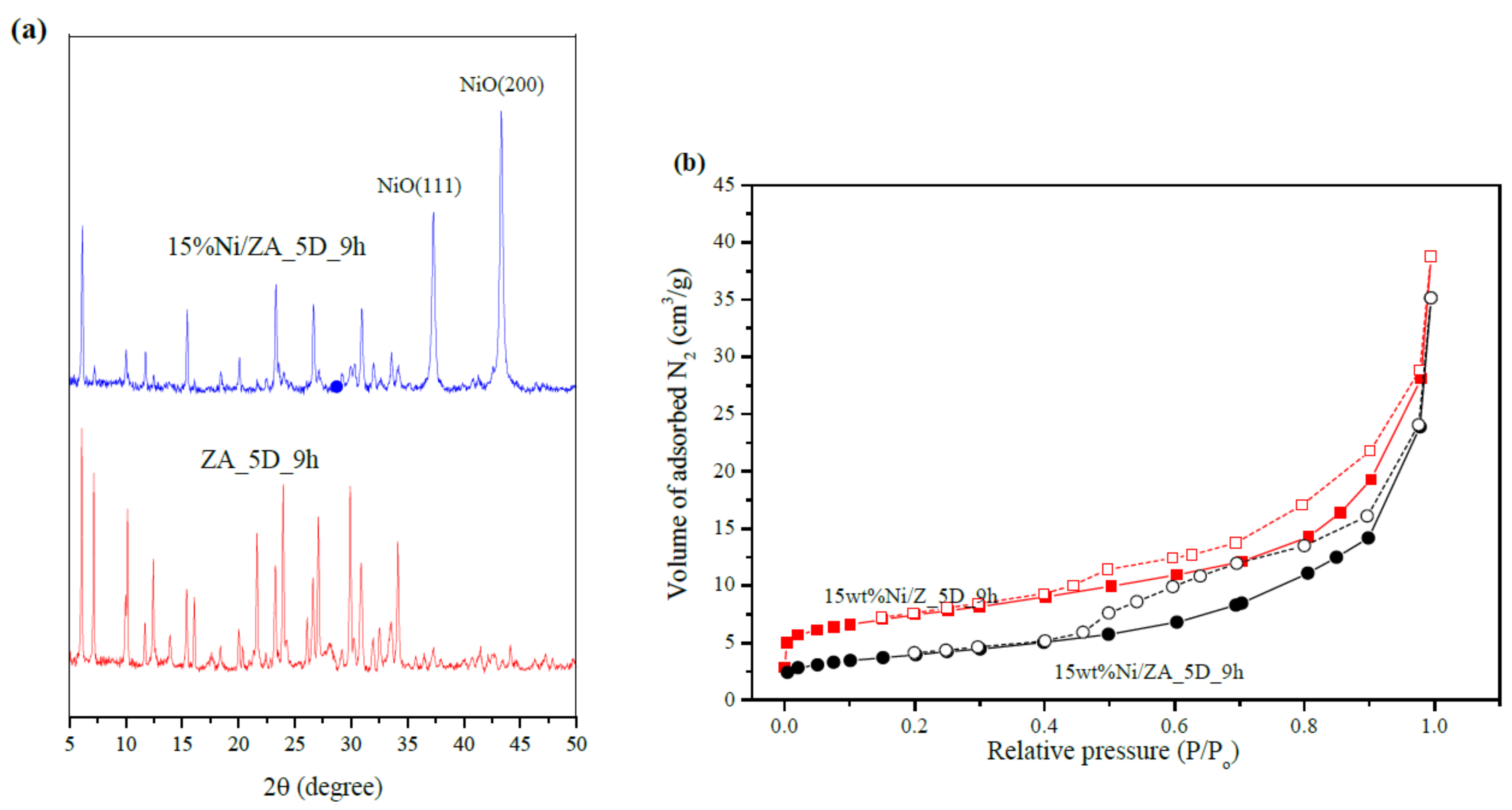
| Crystallographic and Phase Analysis [54,115,116] | X-ray diffraction (XRD) | Assess crystalline structures, crystal phases, and crystal defects |
| Powder X-ray Diffraction (PXRD) | Analyze powdered crystalline materials for crystal structure identification | |
| Selected Area Electron Diffraction (SAED) | Obtain crystallographic information from a sample area | |
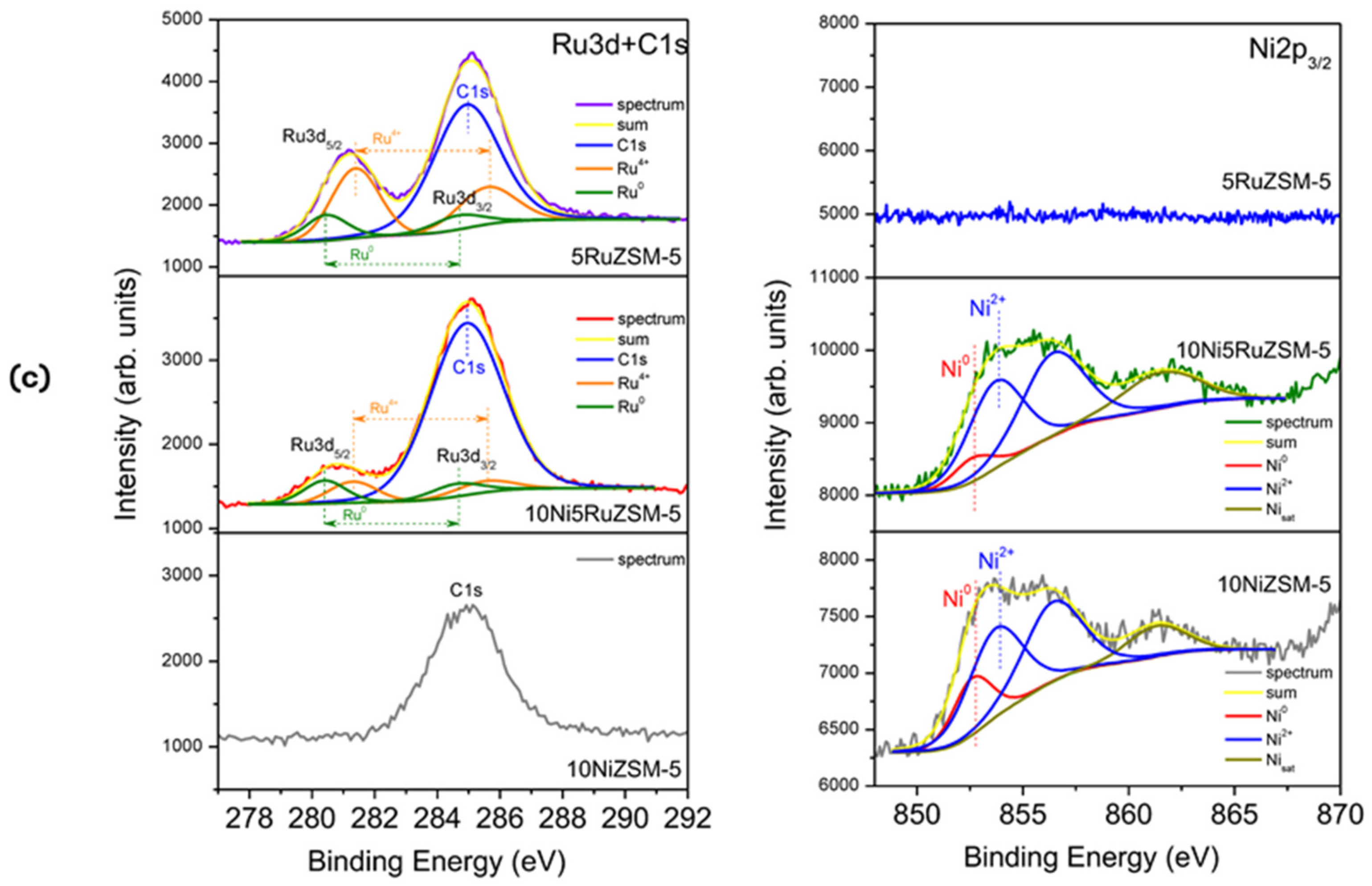
| Surface and Elemental Analysis [91,117,118] | X-ray photoelectron spectroscopy (XPS) | Examine the chemistry of the surface, including aspects such as elemental composition, chemical and empirical states, and the electronic state of elements |
| X-ray Absorption Spectroscopy (XAS) | Determine local geometric/electronic structural order | |
| Auger Electron Spectroscopy (AES) | Detect emitted energy of electrons from the catalyst surface | |
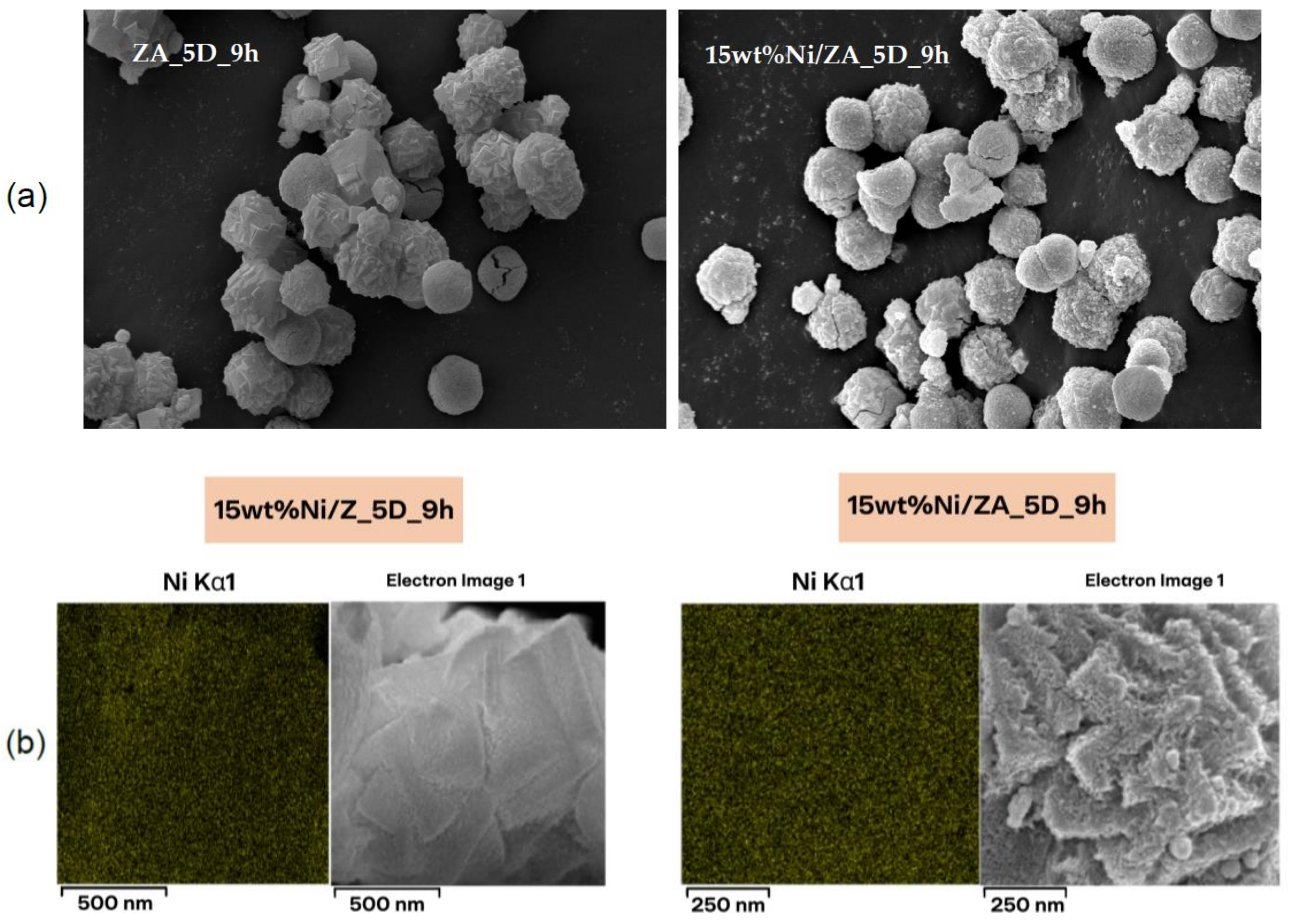
| Microscopy and Imaging [115,119] | Scanning Electron Microscopy (SEM) | Generate high-resolution images of the surface, internal structure, morphology, and crystallography of nanomaterials |
| Transmission Electron Microscopy (TEM) | ||
| Thermal Analysis [4,5,54,115,120] | Temperature-Programmed Reduction-Thermogravimetric Analysis (TPR-TGA) | TPR: Measure the change in chemical state upon heating TGA: Measure changes in physical and chemical states upon heating |
| Temperature-Programmed Desorption (TPD) | Investigate adsorption and desorption behaviors on surface interactions and binding energies | |
| Temperature-Programmed Oxidation (TPO) | Evaluate oxidation behaviors, particularly in carbonaceous materials, catalyst deactivation investigations | |
| Temperature-Programmed Reaction (TPRe) | Study reaction kinetics, and catalytic stability under different thermal environments | |
| Temperature-Programmed Surface Reaction (TPSR) | Focus on surface reactions; mechanisms of surface-mediated reactions | |
| Temperature-Programmed Reduction/Oxidation (TPR-O) | Explore redox properties for redox reactions | |
| Temperature Programmed Reduction-Differential Thermogravimetry (TPR-DTG) | Determine the temperatures at which reduction events occur and the quantitative aspects amount of oxygen removed from an oxide | |
| Temperature-Programmed Ammonia Desorption (TPAD) | Observe ammonia-desorption for acid catalysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutad, M.B.; Al-Marri, M.J.; Kumar, A. Recent Developments on CO2 Hydrogenation Performance over Structured Zeolites: A Review on Properties, Synthesis, and Characterization. Catalysts 2024, 14, 328. https://doi.org/10.3390/catal14050328
Cutad MB, Al-Marri MJ, Kumar A. Recent Developments on CO2 Hydrogenation Performance over Structured Zeolites: A Review on Properties, Synthesis, and Characterization. Catalysts. 2024; 14(5):328. https://doi.org/10.3390/catal14050328
Chicago/Turabian StyleCutad, Methene Briones, Mohammed J. Al-Marri, and Anand Kumar. 2024. "Recent Developments on CO2 Hydrogenation Performance over Structured Zeolites: A Review on Properties, Synthesis, and Characterization" Catalysts 14, no. 5: 328. https://doi.org/10.3390/catal14050328









