Diagnostic Biomarkers in Renal Cell Tumors According to the Latest WHO Classification: A Focus on Selected New Entities
Abstract
Simple Summary
Abstract
1. Introduction
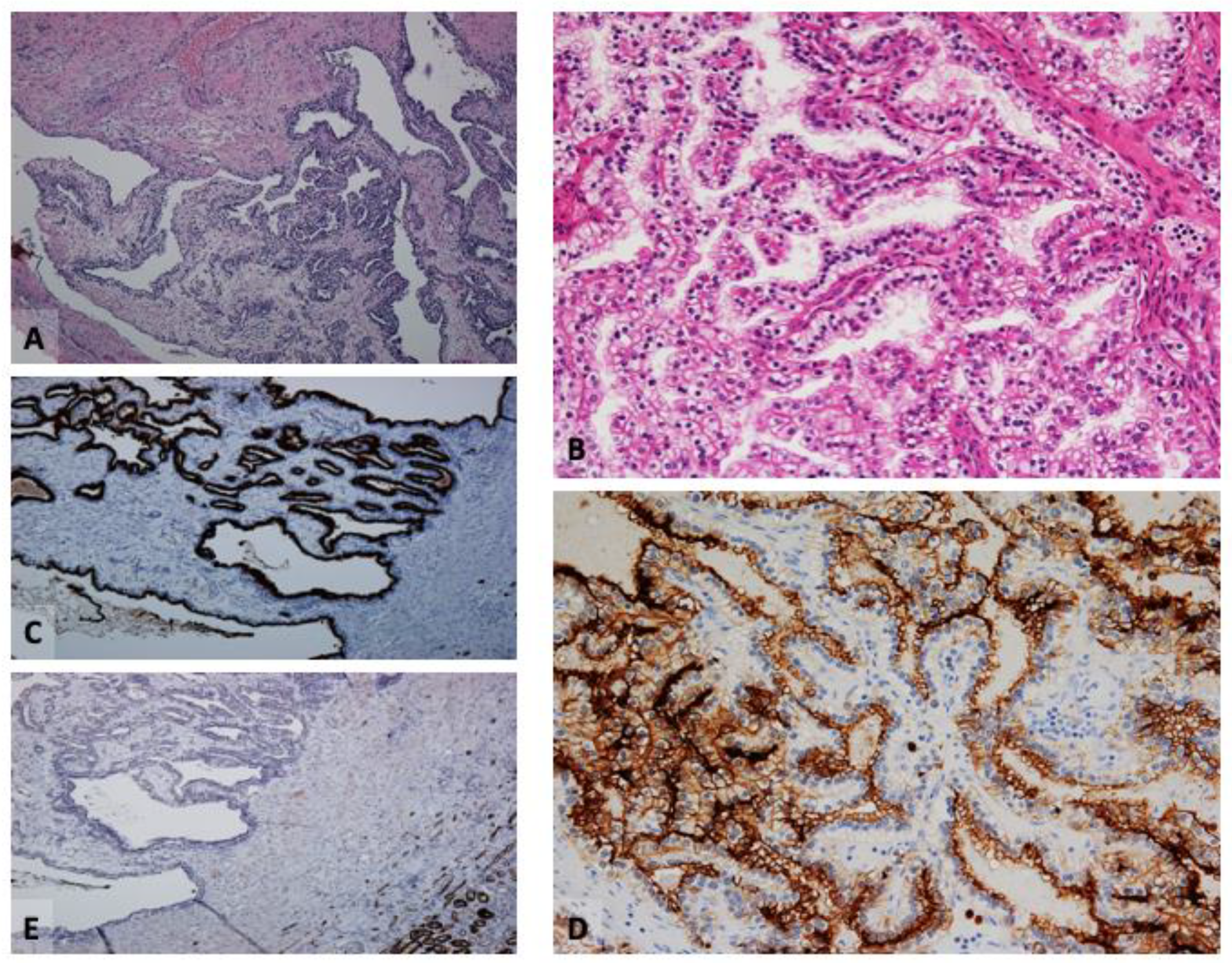
2. Clear Cell Papillary Renal Cell Tumor (CCPRCT)
2.1. Immunophenotype
2.2. Critical Topics and Insights
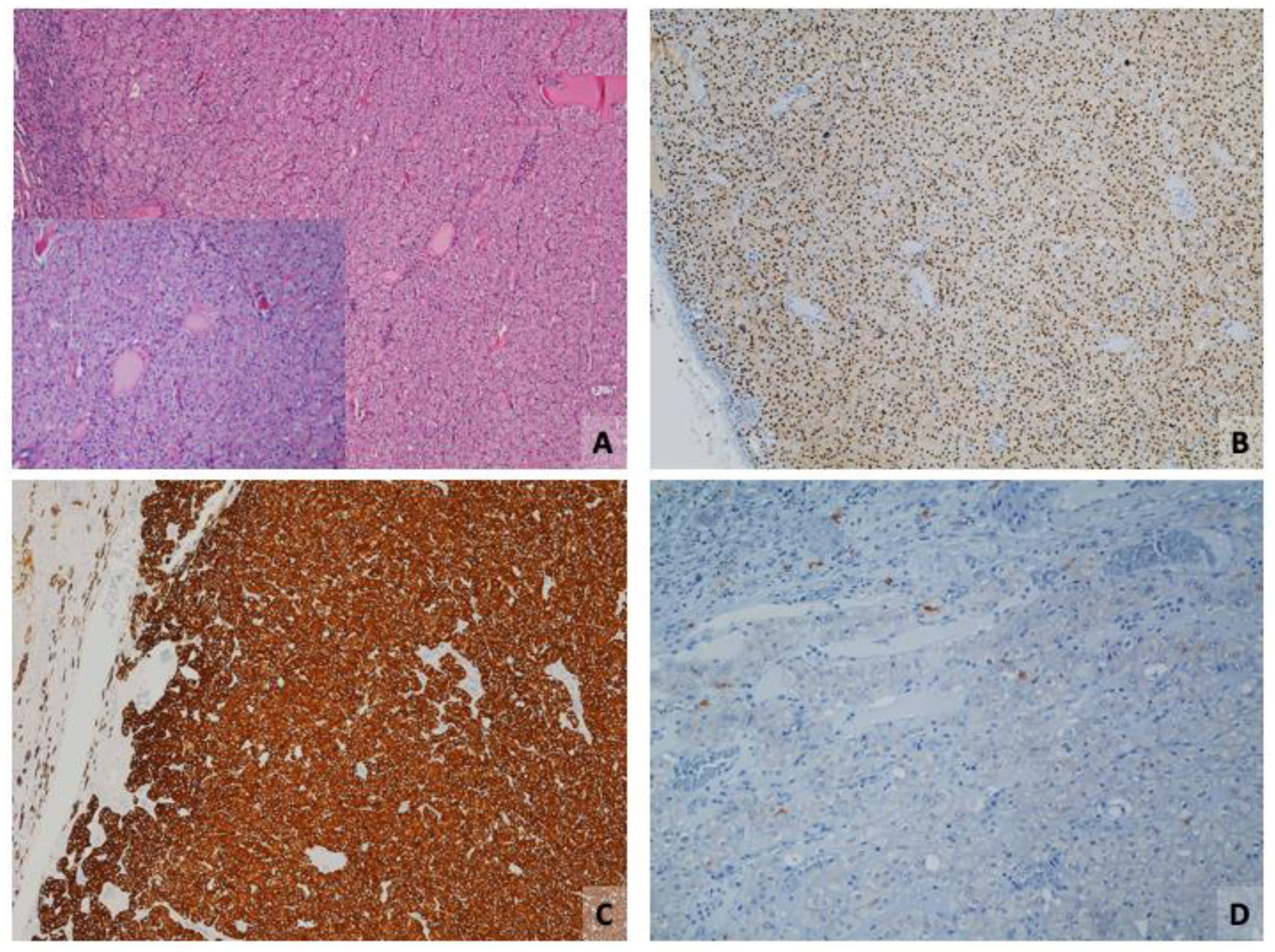
3. Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC-RCC)
3.1. Immunophenotype
3.2. Critical Topics and Insights
4. Oncocytic Tumors
4.1. Eosinophilic Vacuolated Tumor (EVT)
4.1.1. Immunophenotype
4.1.2. Critical Topics and Insights
4.2. Low-Grade Oncocytic Tumor (LOT)
4.2.1. Immunophenotype
4.2.2. Critical Topics and Insights
5. Immunohistochemical Biomarkers in Selected Renal Tumors: A Primer
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMACR | α-Methylacyl coenzyme A racemase |
| CAIX | carbonic anhydrase IX |
| CCPRCT | clear cell papillary renal cell tumor |
| ChrRCC | chromophobe renal cell carcinoma |
| CK | cytokeratins |
| ESC-RCC | eosinophilic solid and cystic renal cell carcinoma |
| EVT | eosinophilic vacuolated tumor |
| IHC | immunohistochemistry |
| ISUP | International Society of Urological Pathology |
| LOT | low-grade oncocytic tumor |
| PRCC | papillary renal cell carcinoma |
| RCC | renal cell carcinoma |
| TSC | tuberous sclerosis complex |
| VHL | von Hippel Lindau |
| WHO | World Health Organization |
References
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Alaghehbandan, R.; Siadat, F.; Trpkov, K. What’s new in the WHO 2022 classification of kidney tumours? Pathologica 2022, 115, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wilkerson, M.L.; Deng, F.M.; Liu, H. The Application and Pitfalls of Immunohistochemical Markers in Challenging Diagnosis of Genitourinary Pathology. Arch. Pathol. Lab. Med. 2024, 148, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Tickoo, S.K.; de Peralta-Venturina, M.N.; Harik, L.R.; Worcester, H.D.; Salama, M.E.; Young, A.N.; Moch, H.; Amin, M.B. Spectrum of epithelial neoplasms in end-stage renal disease: An experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am. J. Surg. Pathol. 2006, 30, 141–153. [Google Scholar] [CrossRef]
- Gobbo, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; MacLennan, G.T.; Shah, R.B.; Zhang, S.; Brunelli, M.; Cheng, L. Clear cell papillary renal cell carcinoma: A distinct histopathologic and molecular genetic entity. Am. J. Surg. Pathol. 2008, 32, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R. Clear cell papillary renal cell carcinoma: An update after 15 years. Pathology 2021, 53, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Ciccarese, C.; Hes, O.; Michal, M.; Caliò, A.; Fiorentino, M.; Giunchi, F.; D’Amuri, A.; Sanguedolce, F.; Sabbatini, R.; et al. The Tumor Entity Denominated “clear cell-papillary renal cell carcinoma” According to the WHO 2016 new Classification, have the Clinical Characters of a Renal Cell Adenoma as does Harbor a Benign Outcome. Pathol. Oncol. Res. 2018, 24, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Inwards, C.Y.; Van Dyke, D.L.; Jimenez, R.E.; Cheville, J.C. Defining clear cell papillary renal cell carcinoma in routine clinical practice. Histopathology 2020, 76, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Reznik, E.; Lee, H.J.; Gundem, G.; Jonsson, P.; Sarungbam, J.; Bialik, A.; Sanchez-Vega, F.; Creighton, C.J.; Hoekstra, J.; et al. Abnormal oxidative metabolism in a quiet genomic background underlies clear cell papillary renal cell carcinoma. Elife 2019, 8, e38986. [Google Scholar] [CrossRef]
- Mantilla, J.G.; Antic, T.; Tretiakova, M. GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics. Hum. Pathol. 2017, 66, 152–158. [Google Scholar] [CrossRef]
- Martignoni, G.; Brunelli, M.; Segala, D.; Munari, E.; Gobbo, S.; Cima, L.; Borze, I.; Wirtanen, T.; Sarhadi, V.K.; Atanesyan, L.; et al. Validation of 34betaE12 immunoexpression in clear cell papillary renal cell carcinoma as a sensitive biomarker. Pathology 2017, 49, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williamson, S.R.; Gill, A.J.; Argani, P.; Chen, Y.B.; Egevad, L.; Kristiansen, G.; Grignon, D.J.; Hes, O. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: III: Molecular Pathology of Kidney Cancer. Am. J. Surg. Pathol. 2020, 44, e47–e65. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Joo, J.W.; Lee, S.J.; Cho, Y.A.; Park, C.K.; Cho, N.H. Comprehensive Immunoprofiles of Renal Cell Carcinoma Subtypes. Cancers 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.B.; Lin, X. Cytomorphologic analysis of clear cell papillary renal cell carcinoma: Distinguishing diagnostic features. Cancer Cytopathol. 2021, 129, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Akgul, M.; Williamson, S.R. Immunohistochemistry for the diagnosis of renal epithelial neoplasms. Semin. Diagn. Pathol. 2022, 39, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reuter, V.E.; Argani, P.; Zhou, M.; Delahunt, B.; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: Report from the International Society of Urologic Pathology consensus conference. Am. J. Surg. Pathol. 2014, 38, e35–e49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, P.; Wang, L.; Wang, J.; Ji, X.; Li, Y.; Shi, H.; Li, Y.; Zhang, W.; Jiang, Y. Analysis of clinicopathological and molecular features of ELOC(TCEB1)-mutant renal cell carcinoma. Pathol. Res. Pract. 2022, 235, 153960. [Google Scholar] [CrossRef] [PubMed]
- Argani, P. A Molecular Marker for Eosinophilic Solid and Cystic Renal Cell Carcinoma. Eur. Urol. 2018, 74, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Williamson, S.R.; Gill, A.J.; Adeniran, A.J.; Agaimy, A.; Alaghehbandan, R.; Amin, M.B.; Argani, P.; Chen, Y.B.; Cheng, L.; et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 2021, 34, 1167–1184. [Google Scholar] [CrossRef]
- Trpkov, K.; Hes, O.; Agaimy, A.; Bonert, M.; Martinek, P.; Magi-Galluzzi, C.; Kristiansen, G.; Lüders, C.; Nesi, G.; Compérat, E.; et al. Fumarate hydratase-deficient renal cell carcinoma is strongly correlated with fumarate hydratase mutation and hereditary Leiomyomatosis and renal cell carcinoma syndrome. Am. J. Surg. Pathol. 2016, 40, 865–875. [Google Scholar] [CrossRef]
- Guo, J.; Tretiakova, M.S.; Troxell, M.L.; Osunkoya, A.O.; Fadare, O.; Sangoi, A.R.; Shen, S.S.; Lopez-Beltran, A.; Mehra, R.; Heider, A.; et al. Tuberous sclerosis-associated renal cell carcinoma: A clinicopathologic study of 57 separate carcinomas in 18 patients. Am. J. Surg. Pathol. 2014, 38, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, A.; Daneshmand, S.; Bayne, A.; Countryman, G.; Corless, C.L.; Troxell, M.L. Distinctive morphology of renal cell carcinomas in tuberous sclerosis. Int. J. Surg. Pathol. 2010, 18, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Palsgrove, D.N.; Li, Y.; Pratilas, C.A.; Lin, M.T.; Pallavajjalla, A.; Gocke, C.; De Marzo, A.M.; Matoso, A.; Netto, G.J.; Epstein, J.I.; et al. Eosinophilic solid and cystic (ESC) renal cell carcinomas harbor TSC mutations: Molecular analysis supports an expanding clinicopathologic spectrum. Am. J. Surg. Pathol. 2018, 42, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Przybycin, C.G.; Trpkov, K.; Magi-Galluzzi, C. Eosinophilic solid and cystic renal cell carcinomas have metastatic potential. Histopathology 2018, 72, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, M.S. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: Series of 4 primary tumors and 2 metastases. Hum. Pathol. 2018, 80, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Abou-Ouf, H.; Hes, O.; Lopez, J.I.; Nesi, G.; Comperat, E.; Sibony, M.; Osunkoya, A.O.; Zhou, M.; Gokden, N.; et al. Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC RCC): Further Morphologic and Molecular Characterization of ESC RCC as a Distinct Entity. Am. J. Surg. Pathol. 2017, 41, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Ohashi, R.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Cree, I.A.; Gill, A.J.; Hartmann, A.; Menon, S.; Netto, G.J.; et al. WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology 2022, 81, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Siadat, F.; Trpkov, K. ESC, ALK, HOT and LOT: Three Letter Acronyms of Emerging Renal Entities Knocking on the Door of the WHO Classification. Cancers 2020, 12, 168. [Google Scholar] [CrossRef]
- Li, Y.; Reuter, V.E.; Matoso, A.; Netto, G.J.; Epstein, J.I.; Argani, P. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology 2018, 72, 588–600. [Google Scholar] [CrossRef]
- Akgul, M.; Cheng, L. Immunophenotypic and pathologic heterogeneity of unclassified renal cell carcinoma: A study of 300 cases. Hum. Pathol. 2020, 102, 70–78. [Google Scholar] [CrossRef]
- Pivovarcikovam, K.; Alaghehbandan, R.; Vanecek, T.; Ohashi, R.; Pitra, T.; Hes, O. TSC/ mTOR pathway mutation associated eosinophilic/oncocytic renal neoplasms: A heterogeneous group of tumors with distinct morphology, immunohistochemical profile, and similar genetic background. Biomedicine 2022, 10, 322. [Google Scholar]
- Hartmann, A.; Agaimy, A. Eosinophiles solide und zystisches Nierenzellkarzinom (ESC-NZK) [Eosinophilic, solid, and cystic renal cell carcinoma (ESC-RCC)]. Pathologe 2021, 42, 565–570, Erratum in Pathologe 2021, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Caliò, A.; Marletta, S.; Brunelli, M.; Martignoni, G. WHO 2022 Classification of Kidney Tumors: What is relevant? An update and future novelties for the pathologist. Pathologica 2022, 115, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Siadat, F.; Trpkov, K. Low-grade oncocytic tumor (LOT)—A new renal entity ready for a prime time: An updated review. Histol. Histopathol. 2022, 37, 405–413. [Google Scholar]
- He, H.; Trpkov, K.; Martinek, P.; Isikci, O.T.; Maggi-Galuzzi, C.; Alaghehbandan, R.; Gill, A.J.; Tretiakova, M.; Lopez, J.I.; Williamson, S.R.; et al. “High-grade oncocytic renal tumor”: Morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch. 2018, 473, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Mirsadraei, L.; Jayakumaran, G.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; Reuter, V.E. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am. J. Surg. Pathol. 2019, 43, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.Y.; Wang, X.T.; Zhao, M.; He, H.Y.; Fang, R.; Ye, S.B.; Li, R.; Wang, X.; Zhang, R.S.; Lu, Z.F.; et al. TSC/MTOR-associated eosinophilic renal tumors exhibit a heterogeneous clinicopathologic spectrum: A targeted next-generation sequencing and gene expression profiling study. Am. J. Surg. Pathol. 2022, 46, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Farcaş, M.; Gatalica, Z.; Trpkov, K.; Swensen, J.; Zhou, M.; Alaghehbandan, R.; Williamson, S.R.; Magi-Galluzzi, C.; Gill, A.J.; Tretiakova, M.; et al. Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: Next-generation sequencing multi-institutional study of 19 cases. Mod. Pathol. 2022, 35, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lerma, L.A.; Schade, G.R.; Tretiakova, M.S. Co-existence of ESC-RCC, EVT, and LOT as synchronous and metachronous tumors in six patients with multifocal neoplasia but without clinical features of tuberous sclerosis complex. Hum. Pathol. 2021, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.; Gao, M.; Zhong, H.; Rakheja, D.; Cai, Q.; Pedrosa, I.; Margulis, V.; Xu, L.; Kinch, L.; Brugarolas, J. Eosinophilic vacuolated tumor of the kidney: A review of evolving concepts in this novel subtype with additional insights from a case with MTOR mutation and concomitant chromosome 1 loss. Adv. Anat. Pathol. 2021, 28, 251–257. [Google Scholar] [CrossRef]
- Hes, O.; Trpkov, K. Do we need an updated classification of oncocytic renal tumors? Emergence of low-grade oncocytic tumor (LOT) and eosinophilic vacuolated tumor (EVT) as novel renal entities. Mod. Pathol. 2022, 35, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Williamson, S.R.; Gao, Y.; Martinek, P.; Cheng, L.; Sangoi, A.R.; Yilmaz, A.; Wang, C.; Fraile, P.S.M.; Montiel, D.M.P.; et al. Low-grade oncocytic tumor of kidney (CD117 Negative, Cytokeratin 7 Positive): A distinct entity? Histopathology 2019, 75, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, O.; Gupta, S.; Cheville, J.C.; Sukov, W.R.; Rowsey, R.; Herrera-Hernandez, L.P.; Lohse, C.M.; Knudson, R.; Leibovich, B.C.; Jimenez, R.E. Low-grade oncocytic tumor of kidney (CK7-positive, CD117-negative): Incidence in a single institutional experience with clinicopathological and molecular characteristics. Hum. Pathol. 2021, 114, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.; Gao, M.; Zhong, H.; Chintalapati, S.; Mitui, M.; Barnes, S.D.; Zhou, Q.; Miyata, J.; Carrillo, D.; Malladi, V.S.; et al. Germline and sporadic mTOR pathway mutations in low-grade oncocytic tumor of the kidney. Mod. Pathol. 2022, 35, 333–343. [Google Scholar] [CrossRef]
- Morini, A.; Drossart, T.; Timsit, M.-O.; Sibony, M.; Vasiliu, V.; Gimenez-Roqueplo, A.-P.; Favier, J.; Badoual, C.; Mejean, A.; Burnichon, N.; et al. Low-grade oncocytic renal tumor (LOT): Mutations in mTOR pathway genes and low expression of FOXI1. Mod. Pathol. 2022, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Akgul, M.; Al-Obaidy, I.K.; Cheng, L.; Idrees, M.T. Low-grade oncocytic tumour expands the spectrum of renal oncocytic tumours and deserves separate classification: A review of 23 cases from a single tertiary institute. J. Clin. Pathol. 2021, 75, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Hes, O.; Trpkov, K.; Aggarwal, A.; Satapathy, A.; Mishra, S.; Sharma, S.; Sangoi, A.; Cheng, L.; Akgul, M.; et al. Low-grade oncocytic tumour of the kidney is characterised by genetic alterations of TSC1, TSC2, MTOR or PIK3CA and consistent GATA3 positivity. Histopathology 2023, 82, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rowsey, R.A.; Cheville, J.C.; Jimenez, R.E. Morphologic overlap between low-grade oncocytic tumor and eosinophilic variant of chromophobe renal cell carcinoma. Hum. Pathol. 2022, 119, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Athanazio, D.A.; Amorim, L.S.; da Cunha, I.W.; Leite, K.R.M.; da Paz, A.R.; Gomes, R.d.P.X.; Tavora, F.R.F.; Faraj, S.F.; Cavalcanti, M.S.; Bezerra, S.M. Classification of renal cell tumors—Current concepts and use of ancillary tests: Recommendations of the Brazilian Society of Pathology. Surg. Exp. Pathol. 2021, 4, 4. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Satapathy, A.; Aggarwal, A.; Mishra, S.K.; Sampat, N.Y.; Sharma, S.; Williamson, S. Oncocytic renal neoplasms with diffuse keratin 7 immunohistochemistry harbor frequent alterations in the mammalian target of rapamycin pathway. Mod. Pathol. 2022, 35, 361–375. [Google Scholar] [CrossRef]
- Hes, O.; Michal, M.; Kuroda, N.; Martignoni, G.; Brunelli, M.; Lu, Y.; Adley, B.P.; Alvarado-Cabrero, I.; Yang, X.J. Vimentin reactivity in renal oncocytoma: Immunohistochemical study of 234 cases. Arch. Pathol. Lab. Med. 2007, 131, 1782–1788. [Google Scholar] [CrossRef]
- Ricci, C.; Ambrosi, F.; Franceschini, T.; Giunchi, F.; Grillini, A.; Franchini, E.; Grillini, M.; Schiavina, R.; Massari, F.; Mollica, V.; et al. Evaluation of an institutional series of low-grade oncocytic tumor (LOT) of the kidney review of the mutational landscape of LOT. Virchows Arch. 2023, 483, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Truong, L.D.; Shen, S.S. Immunohistochemical diagnosis of renal neoplasms. Arch. Pathol. Lab. Med. 2011, 135, 92–109. [Google Scholar] [CrossRef]
- Tuffaha, M.S.A.; Guski, H.; Kristiansen, G. Markers and Immunoprofile of Renal and Urinary Tract Tumors. In Immunohistochemistry in Tumor Diagnostics; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Netto, G.J.; Epstein, J.I. Immunohistology of the Bladder, Kidney, and Testis. In Diagnostic Immunohistochemistry Theranostic and Genomic Applications, 5th ed.; Dabbs, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Baniak, N.; Flood, T.A.; Buchanan, M.; Dal Cin, P.; Hirsch, M.S. Carbonic anhydrase IX (CA9) expression in multiple renal epithelial tumour subtypes. Histopathology 2020, 77, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Sawczuk, I.S.; Pastorek, J.; Wiernik, P.H.; Dutcher, J.P. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur. J. Cancer 2005, 41, 2935–2947. [Google Scholar] [CrossRef]
- Farber, N.J.; Kim, C.J.; Modi, P.K.; Hon, J.D.; Sadimin, E.T.; Singer, E.A. Renal cell carcinoma: The search for a reliable biomarker. Transl. Cancer Res. 2017, 6, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Truong, L.D.; Scarpelli, M.; Lopez-Beltran, A. Role of immunohistochemistry in diagnosing renal neoplasms: When is it really useful? Arch. Pathol. Lab. Med. 2012, 136, 410–417. [Google Scholar] [CrossRef]
- Caliò, A.; Brunelli, M.; Gobbo, S.; Argani, P.; Munari, E.; Netto, G.; Martignoni, G. Cathepsin K: A Novel Diagnostic and Predictive Biomarker for Renal Tumors. Cancers 2021, 13, 2441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iakymenko, O.A.; Delma, K.S.; Jorda, M.; Kryvenko, O.N. Cathepsin K (Clone EPR19992) Demonstrates Uniformly Positive Immunoreactivity in Renal Oncocytoma, Chromophobe Renal Cell Carcinoma, and Distal Tubules. Int. J. Surg. Pathol. 2021, 29, 600–605. [Google Scholar] [CrossRef]
- Rotterova, P.; Alaghehbandan, R.; Skopal, J.; Rogala, J.; Slisarenko, M.; Strakova Peterikova, A.; Michalova, K.; Montiel, D.P.; Farcas, M.; Ulamec, M.; et al. Alpha-methyl CoA racemase (AMACR) reactivity across the spectrum of clear cell renal cell neoplasms. Ann. Diagn. Pathol. 2024, 71, 152297. [Google Scholar] [CrossRef] [PubMed]
| WHO 2022 | WHO 2016 | Reason |
|---|---|---|
| Clear cell papillary renal cell tumor (CCPRCT) | Clear cell papillary renal cell carcinoma (CCPRCC) | Benign behavior |
| Eosinophilic solid and cystic renal cell carcinoma (ESC-RCC) | Not present | New entity in the subgroup of “Other renal tumours” |
| Low-grade oncocytic tumor (LOT) | Not present | Emerging entity in the subgroup of “Other oncocytic tumours of the kidney” |
| Eosinophilic vacuolated tumor (EVT) | Not present | Emerging entity in the subgroup of “Other oncocytic tumours of the kidney” |
| Markers | CCRCC | CCPRCT | PRCC | ||
|---|---|---|---|---|---|
| CAIX | + |  | + |  | − |
| CK7 | −/+ |  | + |  | + |
| AMACR | −/+ |  | −/+ |  | + |
| CD10 | + |  | −/+ |  | + |
| 34βE12 | − |  | +/− |  | − |
| GATA3 | − |  | + |  | − |
| CK7 | CD117 | Cathepsin K | CK20 | |
|---|---|---|---|---|
| RO | − | + | − | − |
| ChrRCC, eos | + | + | − | − |
| EVT | −/+ | + | + | − |
| LOT | + | − | − | − |
| ESC-RCC | − | − | + | + |
| Biomarker | Staining Pattern | Main Expression |
|---|---|---|
| CAIX | membranous/cytoplasmic | CCRCC, CCPRCT |
| CK7 | cytoplasmic | PRCC, ChrRCC, CDC, CCRPCT, MTSCC, LOT |
| CK20 | cytoplasmic | ESC-RCC |
| 34βE12 | cytoplasmic | CDC, RMC, CCPRCT |
| CD10 | membranous | CRCC, PRCC, CDC |
| CD117 | membranous/cytoplasmic | ChrRCC, RO, EVT |
| cathepsin K | cytoplasmic | EVT, ESC-RCC, AML |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanguedolce, F.; Mazzucchelli, R.; Falagario, U.G.; Cormio, A.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Eccher, A.; Brunelli, M.; Galosi, A.B.; et al. Diagnostic Biomarkers in Renal Cell Tumors According to the Latest WHO Classification: A Focus on Selected New Entities. Cancers 2024, 16, 1856. https://doi.org/10.3390/cancers16101856
Sanguedolce F, Mazzucchelli R, Falagario UG, Cormio A, Zanelli M, Palicelli A, Zizzo M, Eccher A, Brunelli M, Galosi AB, et al. Diagnostic Biomarkers in Renal Cell Tumors According to the Latest WHO Classification: A Focus on Selected New Entities. Cancers. 2024; 16(10):1856. https://doi.org/10.3390/cancers16101856
Chicago/Turabian StyleSanguedolce, Francesca, Roberta Mazzucchelli, Ugo Giovanni Falagario, Angelo Cormio, Magda Zanelli, Andrea Palicelli, Maurizio Zizzo, Albino Eccher, Matteo Brunelli, Andrea Benedetto Galosi, and et al. 2024. "Diagnostic Biomarkers in Renal Cell Tumors According to the Latest WHO Classification: A Focus on Selected New Entities" Cancers 16, no. 10: 1856. https://doi.org/10.3390/cancers16101856
APA StyleSanguedolce, F., Mazzucchelli, R., Falagario, U. G., Cormio, A., Zanelli, M., Palicelli, A., Zizzo, M., Eccher, A., Brunelli, M., Galosi, A. B., Carrieri, G., & Cormio, L. (2024). Diagnostic Biomarkers in Renal Cell Tumors According to the Latest WHO Classification: A Focus on Selected New Entities. Cancers, 16(10), 1856. https://doi.org/10.3390/cancers16101856








