Understanding the Effect of Dispersant Rheology and Binder Decomposition on 3D Printing of a Solid Oxide Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Requirements of the Slurry for Direct Writing
Need for Dispersant
2.2. Experiments
2.2.1. Cathode Slurry Materials
2.2.2. Binder Concentration
2.2.3. Variations in Dispersant and Solid Loading Concentrations
2.2.4. Slurry Preparation Process
2.2.5. YSZ Substrate Preparation
2.2.6. Testing Procedures
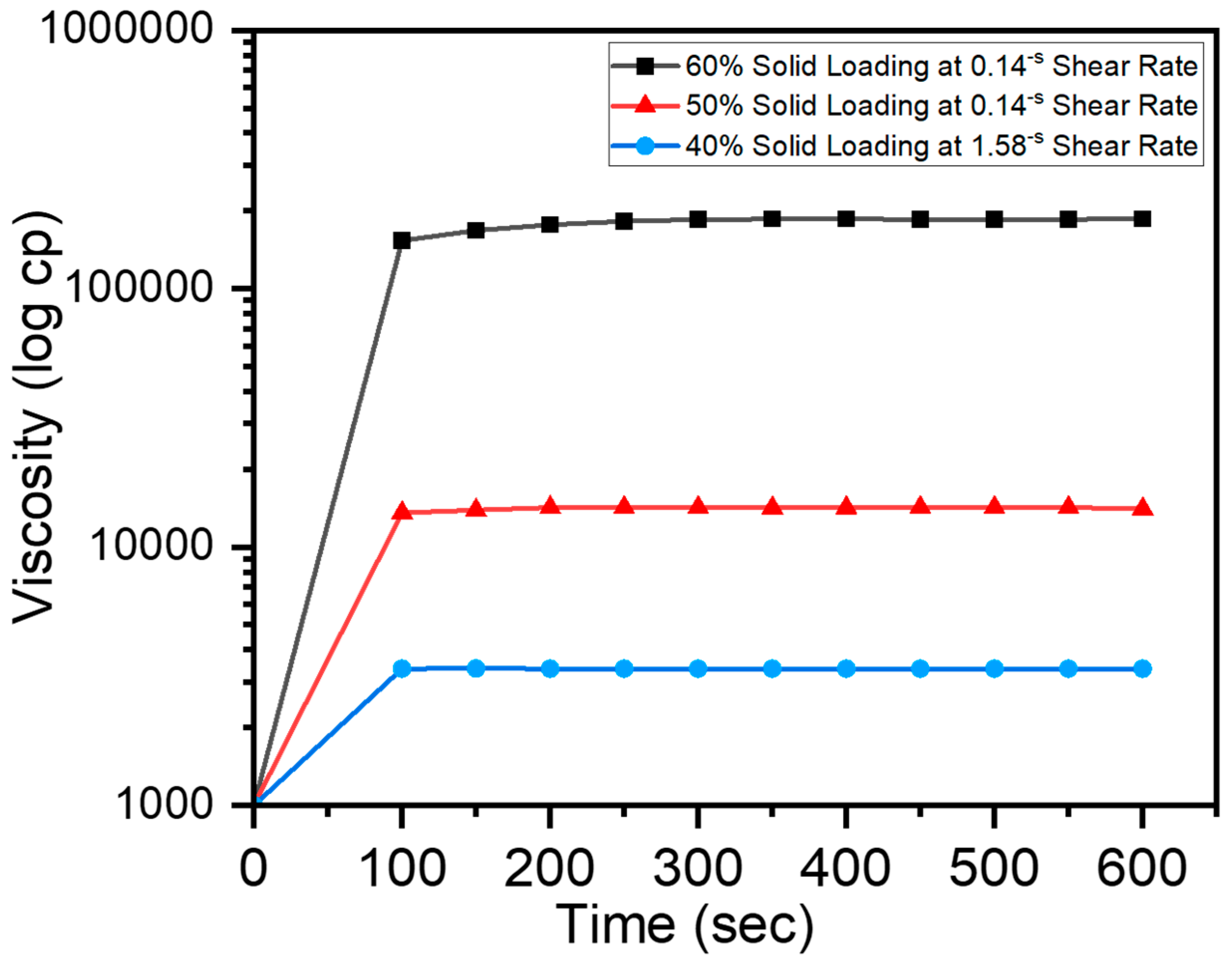
Stability of Slurry Viscosity with Respect to Time
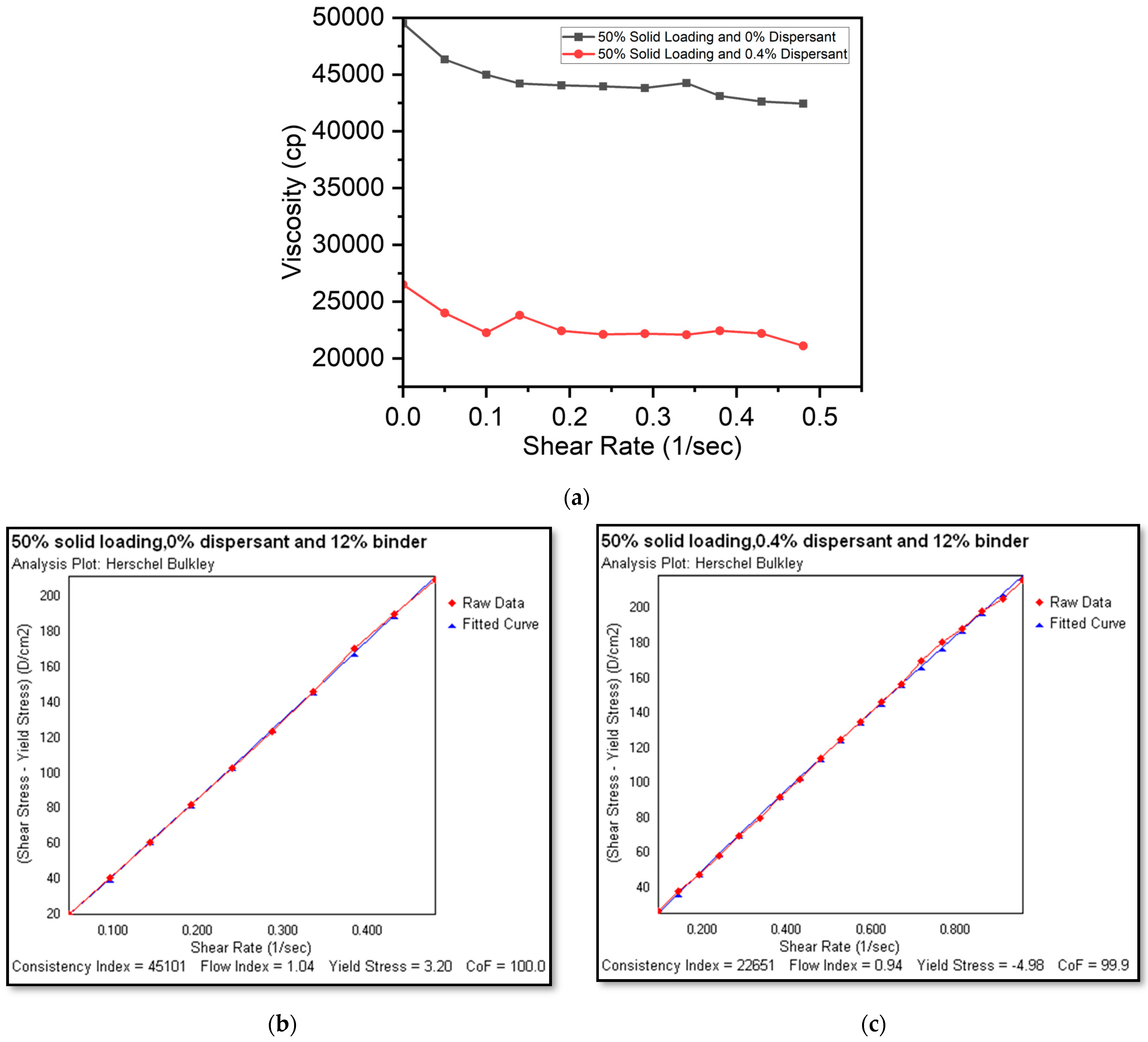
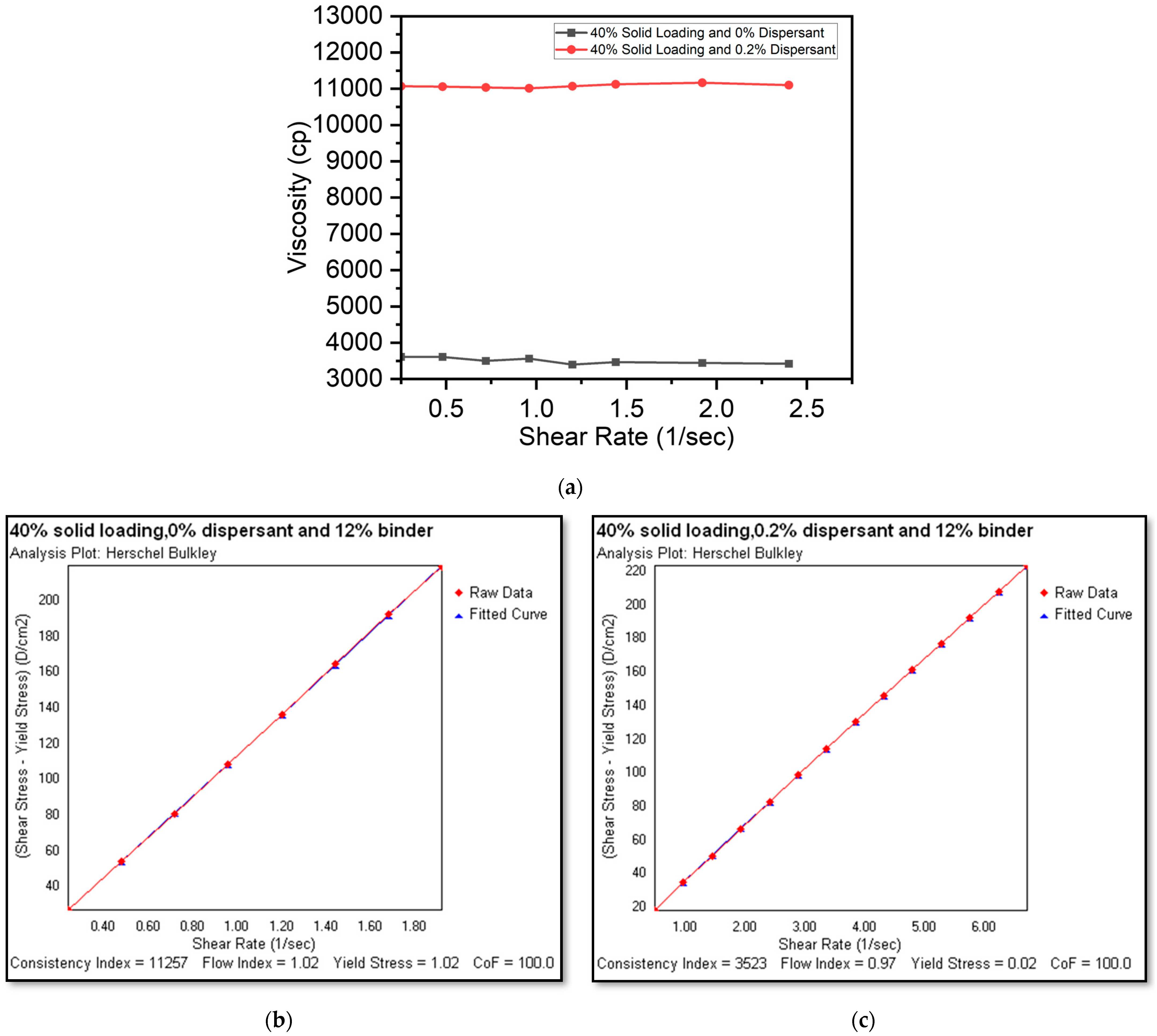
Effect of Shear Rate and Shear Stress Variation on Slurry Viscosity
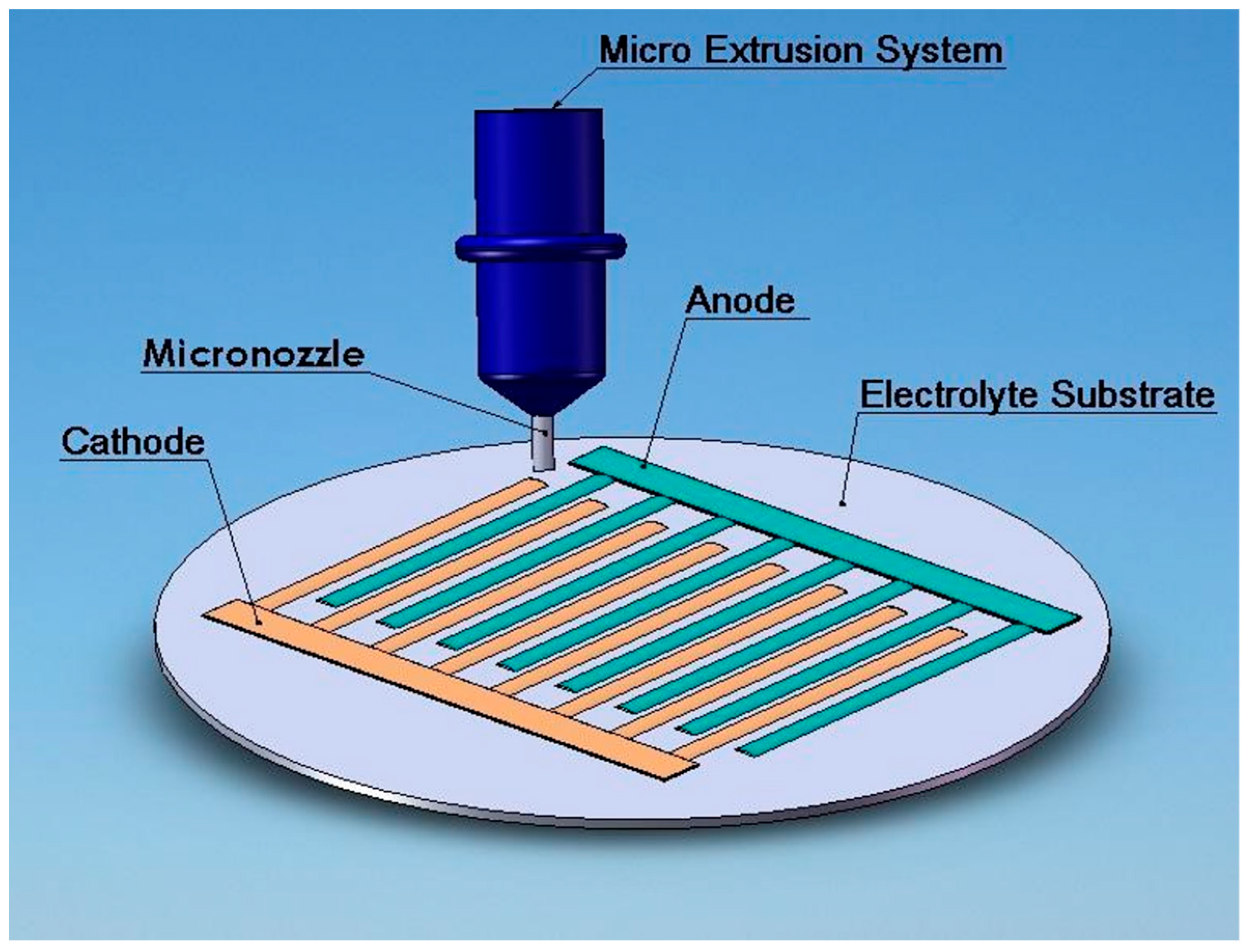
2.2.7. Direct Writing Cathode Lines on the YSZ Substrate
Evaluating Width and Height of the Line
3. Results
3.1. Stability of Slurries with Respect to Time
3.2. Rheological Characteristics of Slurries
- When = 0 and n = 1, the slurry is a Newtonian fluid.
- When = 0 and n < 1, the slurry is a pseudoplastic fluid.
- When = 0 and n > 1, the slurry is a dilatant fluid (shear-thickening).
- When ≠ 0 and n = 1, the slurry is a Bingham fluid.
- When ≠ 0 and n < 1, the slurry is a viscoplastic fluid.
3.3. Evaluating the Optimal Dispersant Concentration
3.4. Evaluating Width and Height of Lines
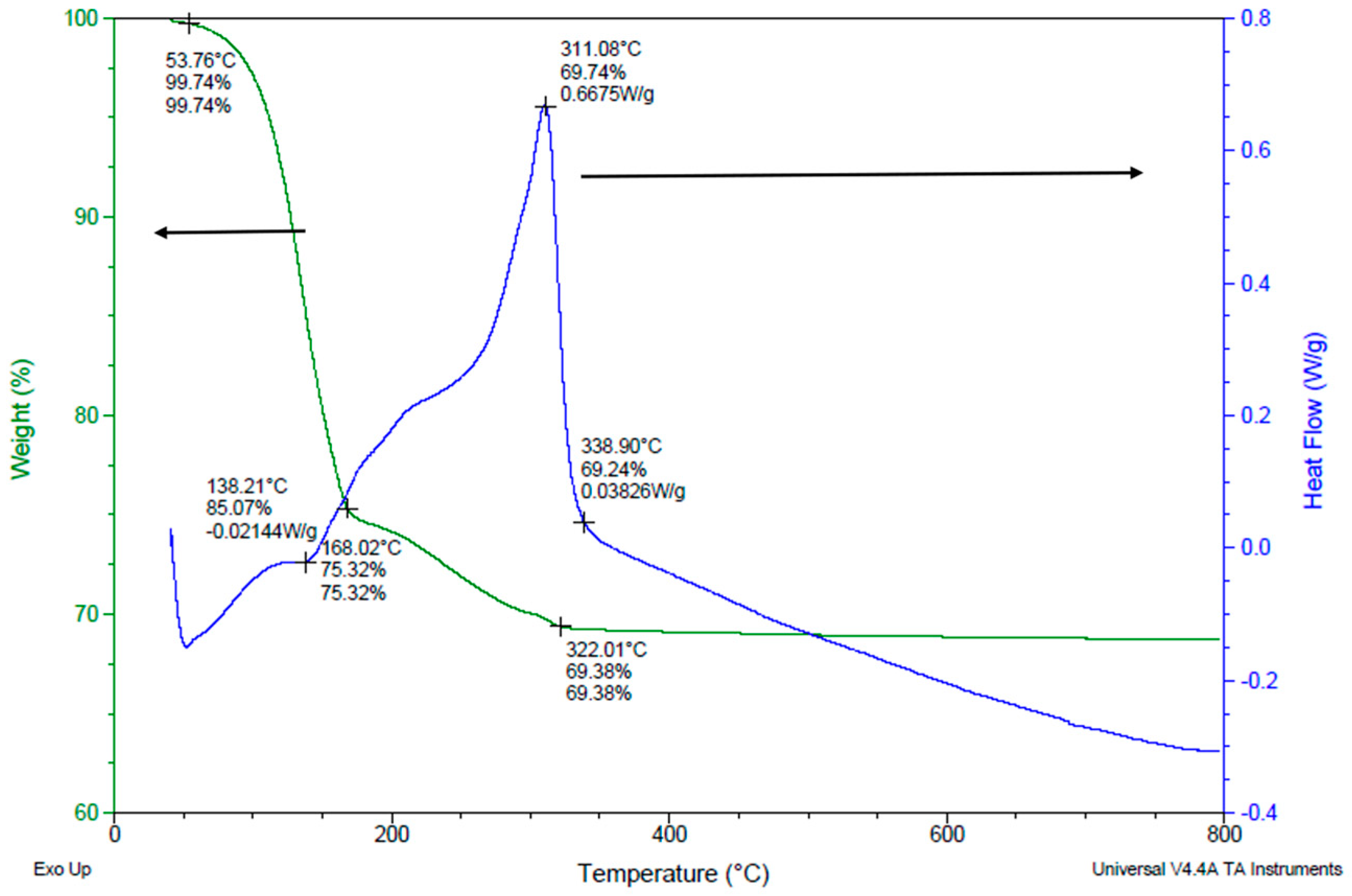
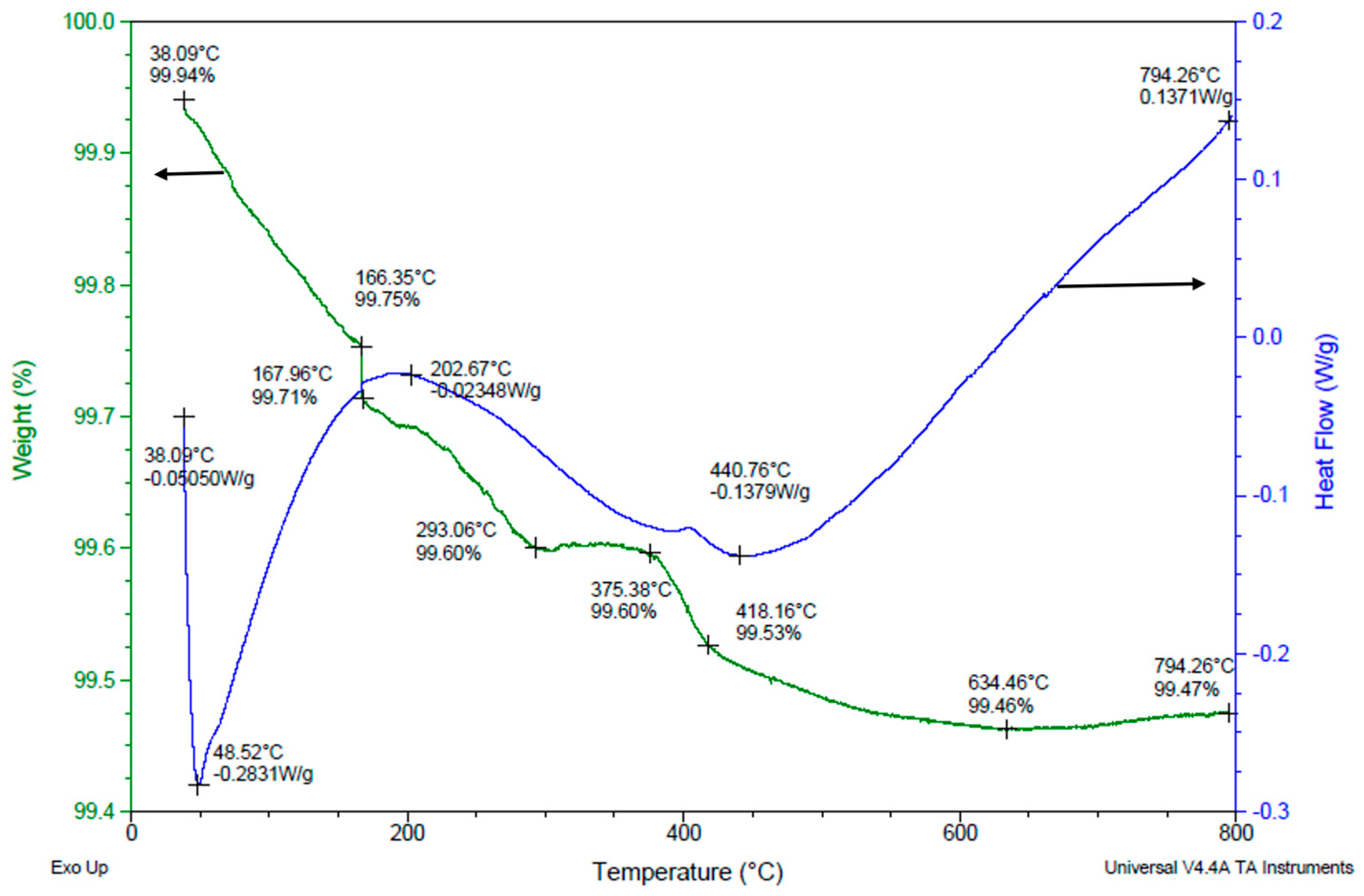
3.5. Binder Removal Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Bentley, Y. Modelling world natural gas production. Energy Rep. 2020, 6, 1363–1372. [Google Scholar] [CrossRef]
- Cichon, K.; Kliks, A.; Bogucka, H. Energy-efficient cooperative spectrum sensing: A survey. IEEE Commun. Surv. Tutor. 2016, 18, 1861–1886. [Google Scholar] [CrossRef]
- Midilli, A.; Dincer, I.; Ay, M. Green energy strategies for sustainable development. Energy Policy 2006, 34, 3623–3633. [Google Scholar] [CrossRef]
- Shafiq, R.R.G.; ElHalawany, B.M.; Ali, N.; Elsherbini, M.M. A power source for E-devices based on green energy. Energy Harvest. Syst. 2024, 11, 20230078. [Google Scholar] [CrossRef]
- Omer, A.M. Green energies and the environment. Renew. Sustain. Energy Rev. 2008, 12, 1789–1821. [Google Scholar] [CrossRef]
- Hayat, M.B.; Ali, D.; Monyake, K.C.; Alagha, L.; Ahmed, N. Solar energy—A look into power generation, challenges, and a solar-powered future. Int. J. Energy Res. 2019, 43, 1049–1067. [Google Scholar] [CrossRef]
- Bartle, A. Hydropower potential and development activities. Energy Policy 2002, 30, 1231–1239. [Google Scholar] [CrossRef]
- Gipe, P. Wind power. Wind Eng. 2004, 28, 629–631. [Google Scholar] [CrossRef]
- Toth, F.L.; Rogner, H.-H. Oil and nuclear power: Past, present, and future. Energy Econ. 2006, 28, 1–25. [Google Scholar] [CrossRef]
- Williamson, K.; Gunderson, R.; Hamblin, G.; Gallup, D.; Kitz, K. Geothermal power technology. Proc. IEEE 2001, 89, 1783–1792. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Grove, W. XXIV. On voltaic series and the combination of gases by platinum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1839, 14, 127–130. [Google Scholar] [CrossRef]
- Bacon, F.T. Fuel cells, past, present and future. Electrochim. Acta 1969, 14, 569–585. [Google Scholar] [CrossRef]
- Choi, W. New Approaches to Improve the Performance of the PEM Based Fuel Cell Power Systems; Texas A&M University: College Station, TX, USA, 2004. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells. Fundamentals, Technology, Applications; PBD: Gateshead, UK, 2003. [Google Scholar]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Meda, U.S.; Rajyaguru, Y.V.; Pandey, A. Generation of green hydrogen using self-sustained regenerative fuel cells: Opportunities and challenges. Int. J. Hydrogen Energy 2023, 48, 28289–28314. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- O’Hayre, R.; Colella, W.; Cha, S.-W.; Prinz, F.B. Fuel Cell Fundamentals, 2nd ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kobayashi, Y.; Ando, Y.; Kabata, T.; Nishiura, M.; Tomida, K.; Matake, N. Extremely high-efficiency thermal power system-solid oxide fuel cell (SOFC) triple combined-cycle system. Mitsubishi Heavy Ind. Tech. Rev. 2011, 48, 9–15. [Google Scholar]
- Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Evans, A.; Bieberle-Hütter, A.; Rupp, J.L.; Gauckler, L.J. Review on microfabricated micro-solid oxide fuel cell membranes. J. Power Sources 2009, 194, 119–129. [Google Scholar] [CrossRef]
- Beckel, D.; Bieberle-Hütter, A.; Harvey, A.; Infortuna, A.; Muecke, U.; Prestat, M.; Rupp, J.; Gauckler, L. Thin films for micro solid oxide fuel cells. J. Power Sources 2007, 173, 325–345. [Google Scholar] [CrossRef]
- Evans, A.; Bieberle-Hütter, A.; Galinski, H.; Rupp, J.L.; Ryll, T.; Scherrer, B.; Tölke, R.; Gauckler, L.J. Micro-solid oxide fuel cells: Status, challenges, and chances. Monatshefte für Chemie-Chem. Mon. 2009, 140, 975–983. [Google Scholar] [CrossRef]
- Kwon, C.; Son, J.; Lee, J.; Kim, H.; Lee, H.; Kim, K. High-performance micro-solid oxide fuel cells fabricated on nanoporous anodic aluminum oxide templates. Adv. Funct. Mater. 2011, 21, 1154–1159. [Google Scholar] [CrossRef]
- Jasinski, P. Micro solid oxide fuel cells and their fabrication methods. Microelectron. Int. 2008, 25, 42–48. [Google Scholar] [CrossRef]
- Bieberle-Hütter, A.; Beckel, D.; Infortuna, A.; Muecke, U.P.; Rupp, J.L.; Gauckler, L.J.; Rey-Mermet, S.; Muralt, P.; Bieri, N.R.; Hotz, N.; et al. A micro-solid oxide fuel cell system as battery replacement. J. Power Sources 2008, 177, 123–130. [Google Scholar] [CrossRef]
- Patil, T.C.; Duttagupta, S.P. Micro-Solid Oxide Fuel Cell: A multi-fuel approach for portable applications. Appl. Energy 2016, 168, 534–543. [Google Scholar] [CrossRef]
- Zoupalis, K.; Amiri, A.; Sugden, K.; Kendall, M.; Kendall, K. Micro solid oxide fuel cell thermal dynamics: Incorporation of experimental measurements and model-based estimations for a multidimensional thermal analysis. Energy Convers. Manag. 2023, 277, 116650. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the technology of solid oxide fuel cell (SOFC) energy systems for stationary power generation: A review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. Understanding Hybrid Additive Manufacturing of Functional Devices. Am. J. Eng. Appl. Sci. 2017, 10, 264–271. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.; Meunier, M.; Vengallatore, S.; Therriault, D. Direct-write microfabrication of single-chamber micro solid oxide fuel cells. J. Micromech. Microeng. 2007, 18, 015005. [Google Scholar] [CrossRef]
- Chiabrera, F.; Garbayo, I.; Alayo, N.; Tarancón, A. Micro solid oxide fuel cells: A new generation of micro-power sources for portable applications. Smart Sens. Actuators MEMS VIII 2017, 10246, 188–198. [Google Scholar]
- Evans, A.; Karalić, S.; Martynczuk, J.; Prestat, M.; Tölke, R.; Yáng, Z.; Gauckler, L.J. La0.6Sr0.4CoO3-δ Thin Films Prepared by Pulsed Laser Deposition as Cathodes for Micro-Solid Oxide Fuel Cells. ECS Trans. 2012, 45, 333. [Google Scholar] [CrossRef]
- Hibino, T.; Ushiki, K.; Kuwahara, Y. New concept for simplifying SOFC system. Solid State Ionics 1996, 91, 69–74. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chung, Y.-C. Performance characteristics of micro single-chamber solid oxide fuel cell: Computational analysis. J. Power Sources 2006, 154, 35–41. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Inoue, T.; Tokuno, J.-I.; Yoshida, S.-I.; Sano, M. Single-chamber solid oxide fuel cells at intermediate temperatures with various hydrocarbon-air mixtures. J. Electrochem. Soc. 2000, 147, 2888–2892. [Google Scholar] [CrossRef]
- Shao, Z.; Mederos, J.; Chueh, W.C.; Haile, S. High power-density single-chamber fuel cells operated on methane. J. Power Sources 2006, 162, 589–596. [Google Scholar] [CrossRef]
- McKenzie, J.; Parupelli, S.; Martin, D.; Desai, S. Additive manufacturing of multiphase materials for electronics. In IIE Annual Conference. Proceedings; Institute of Industrial and Systems Engineers (IISE): Norcross, GA, USA, 2017; pp. 1133–1138. [Google Scholar]
- Parupelli, S.K.; Desai, S. Hybrid additive manufacturing (3D printing) and characterization of functionally gradient materials via in situ laser curing. Int. J. Adv. Manuf. Technol. 2020, 110, 543–556. [Google Scholar] [CrossRef]
- Rajaram, G.; Desai, S.; Xu, Z.; Pai, D.M.; Sankar, J. Systematic studies on NI-YSZ anode material for Solid Oxide Fuel Cell (SOFCs) applications. Int. J. Manuf. Res. 2008, 3, 350–359. [Google Scholar] [CrossRef]
- Tohver, V.; Morissette, S.L.; Lewis, J.A.; Tuttle, B.A.; Voigt, J.A.; Dimos, D.B. Direct-Write Fabrication of Zinc Oxide Varistors. J. Am. Ceram. Soc. 2002, 85, 123–128. [Google Scholar] [CrossRef]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct ink writing of three-dimensional ceramic structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- Zhao, X.; Evans, J.R.G.; Edirisinghe, M.J.; Song, J. Direct ink-jet printing of vertical walls. J. Am. Ceram. Soc. 2002, 85, 2113–2115. [Google Scholar] [CrossRef]
- Parupelli, S.; Desai, S. A comprehensive review of additive manufacturing (3D printing): Processes, applications and future potential. Am. J. Appl. Sci. 2019, 16, 244–272. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Ahn, S.-J.; Moon, J.; Kim, J.; Lee, H.-W. Direct-write fabrication of integrated planar solid oxide fuel cells. J. Electroceram. 2006, 17, 683–687. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Kim, Y.-B.; Moon, J.; Lee, J.-H.; Kim, J. Co-planar type single chamber solid oxide fuel cell with micro-patterned electrodes. J. Electroceram. 2006, 17, 689–693. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.; Meunier, M.; Therriault, D.; Vengallatore, S. Fabrication and testing of coplanar single-chamber micro solid oxide fuel cells with geometrically complex electrodes. J. Power Sources 2008, 177, 148–153. [Google Scholar] [CrossRef]
- Desai, S.; Yang, M.; Xu, Z.; Sankar, J. Direct write manufacturing of solid oxide fuel cells for green energy. J. Environ. Res. Dev. 2014, 8, 477. [Google Scholar]
- Yang, M.; Xu, Z.; Desai, S.; Kumar, D.; Sankar, J. Fabrication of novel single-chamber solid oxide fuel cells towards green technology. ASME Int. Mech. Eng. Congr. Expo. 2009, 43871, 61–66. [Google Scholar]
- Rajaram, G.; Xu, Z.; Jiang, X.S.; Pai, D.M.; Desai, S.; Sankar, J. A statistical approach to the design and fabrication of anode material for solid oxide fuel cells-A case study. Int. J. Ind. Eng. Theory Appl. Pract. 2006, 13, 349–356. [Google Scholar]
- Ali, S.A.M.; Anwar, M.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Enhanced electrochemical performance of LSCF cathode through selection of optimum fabrication parameters. J. Solid State Electrochem. 2017, 22, 263–273. [Google Scholar]
- Yang, M.; Xu, Z.; Desai, S.; Kumar, D.; Sankar, J. Fabrication of micro single chamber solid oxide fuel cell using photolithography and pulsed laser deposition. J. Fuel Cell Sci. Technol. 2015, 12, 021004. [Google Scholar] [CrossRef]
- Renger, C.; Kuschel, P.; Kristoffersson, A.; Clauss, B.; Oppermann, W.; Sigmund, W. Rheology studies on highly filled nano-zirconia suspensions. J. Eur. Ceram. Soc. 2007, 27, 2361–2367. [Google Scholar] [CrossRef]
- Loh, N.H.; Tor, S.B.; Tay, B.Y.; Murakoshi, Y.; Maeda, R. Fabrication of micro gear by micro powder injection molding. Microsyst. Technol. 2007, 14, 43–50. [Google Scholar] [CrossRef]
- Çetinel, F.A.; Bauer, W.; Müller, M.; Knitter, R.; Haußelt, J. Influence of dispersant, storage time and temperature on the rheological properties of zirconia–paraffin feedstocks for LPIM. J. Eur. Ceram. Soc. 2010, 30, 1391–1400. [Google Scholar] [CrossRef]
- Zürcher, S.; Graule, T. Influence of dispersant structure on the rheological properties of highly-concentrated zirconia dispersions. J. Eur. Ceram. Soc. 2005, 25, 863–873. [Google Scholar] [CrossRef]
- Seo, J.; Kuk, S.; Kim, K. Thermal decomposition of PVB (polyvinyl butyral) binder in the matrix and electrolyte of molten carbonate fuel cells. J. Power Sources 1997, 69, 61–68. [Google Scholar] [CrossRef]
- Lee, E.; Kim, B.; Choi, S. Hand-held, automatic capillary viscometer for analysis of Newtonian and non-Newtonian fluids. Sens. Actuators A Phys. 2020, 313, 112176. [Google Scholar] [CrossRef]
- Chinyoka, T. Comparative response of Newtonian and Non-Newtonian fluids subjected to exothermic reactions in shear flow. Int. J. Appl. Comput. Math. 2021, 7, 75. [Google Scholar] [CrossRef]
- Yang, M. Investigating Fabrication Methods for Micro Single-Chamber Solid Oxide Fuel Cells. Ph.D. Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2010. [Google Scholar]




| Solid Loading (wt%/v) | Dispersant (% of Solid Loading wt) | Binder (% of Solid Loading wt) |
|---|---|---|
| 40% | 0.0 | 12% |
| 40% | 0.2 | 12% |
| 40% | 0.4 | 12% |
| 40% | 0.6 | 12% |
| 40% | 1.0 | 12% |
| 40% | 1.5 | 12% |
| 50% | 0.0 | 12% |
| 50% | 0.2 | 12% |
| 50% | 0.4 | 12% |
| 50% | 0.6 | 12% |
| 50% | 1.0 | 12% |
| 50% | 1.5 | 12% |
| 60% | 0.0 | 12% |
| 60% | 0.2 | 12% |
| 60% | 0.4 | 12% |
| 60% | 0.6 | 12% |
| 60% | 1.0 | 12% |
| 60% | 1.5 | 12% |
| Pressure (kPa) | Viscosity (kcp) | Line Width before Sintering (µm) | Line Height before Sintering (µm) |
|---|---|---|---|
| 200 | 150–220 | 180–260 | 20–36 |
| 200 | 80–150 | 280–330 | 30–40 |
| 200 | 20–45 | 360–500 | 23–40 |
| 200 | 10–20 | >600 | Around 100 |
| 100 | 10–15 | Around 600 | Around 190 |
| 100 | 3.2–5 | >800 | 40–100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Parupelli, S.K.; Xu, Z.; Desai, S. Understanding the Effect of Dispersant Rheology and Binder Decomposition on 3D Printing of a Solid Oxide Fuel Cell. Micromachines 2024, 15, 636. https://doi.org/10.3390/mi15050636
Yang M, Parupelli SK, Xu Z, Desai S. Understanding the Effect of Dispersant Rheology and Binder Decomposition on 3D Printing of a Solid Oxide Fuel Cell. Micromachines. 2024; 15(5):636. https://doi.org/10.3390/mi15050636
Chicago/Turabian StyleYang, Man, Santosh Kumar Parupelli, Zhigang Xu, and Salil Desai. 2024. "Understanding the Effect of Dispersant Rheology and Binder Decomposition on 3D Printing of a Solid Oxide Fuel Cell" Micromachines 15, no. 5: 636. https://doi.org/10.3390/mi15050636







