Baby Food Pouches, Baby-Led Weaning, and Iron Status in New Zealand Infants: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Eligibility and Recruitment
2.3. Procedure
2.4. Questionnaire Data
2.5. Anthropometric Assessment
2.6. Assessment of Infant Formula Intake
2.7. Blood Collection and Laboratory Methods
2.8. Statistical Analysis
3. Results
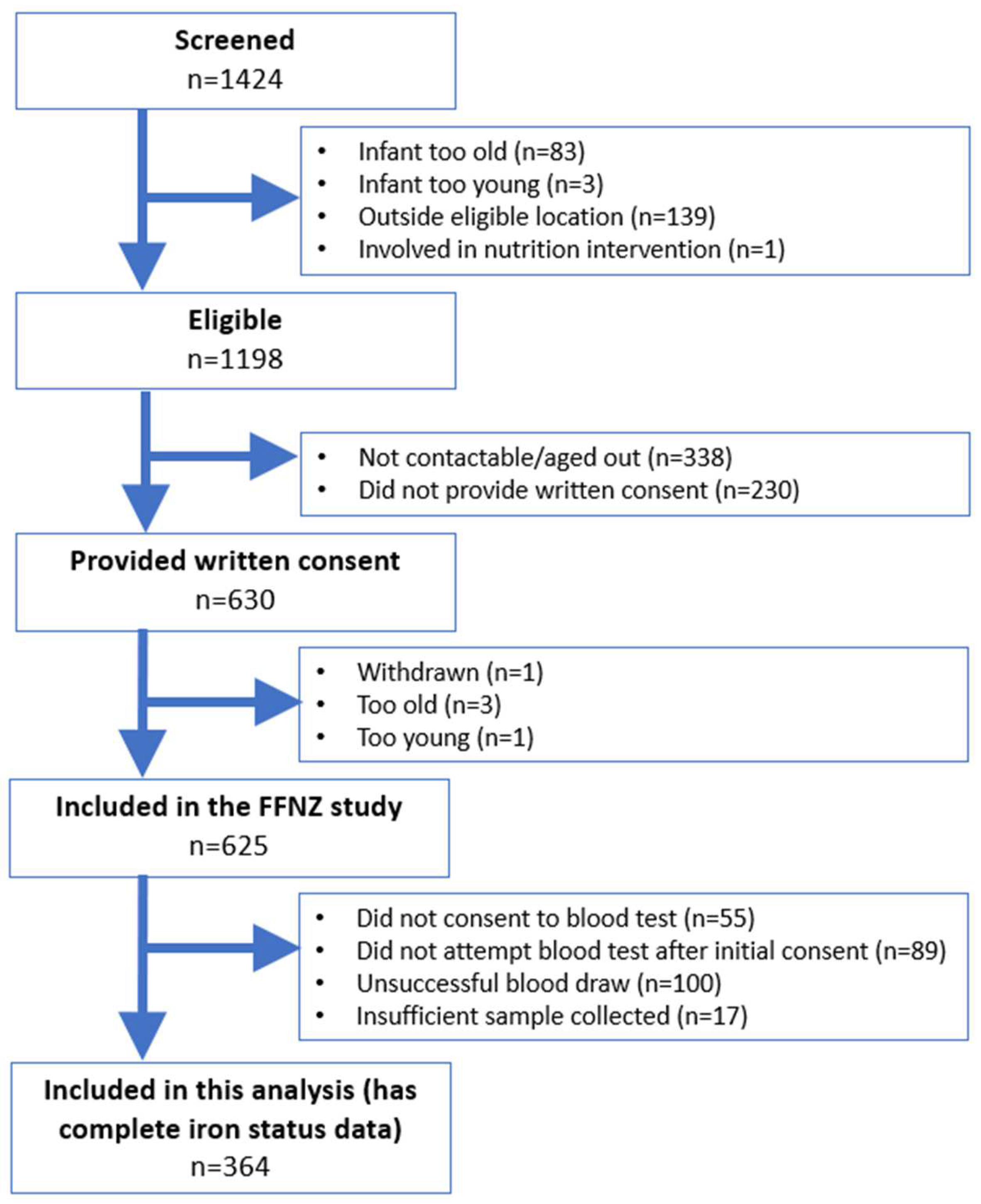
3.1. Participant Recruitment and Inclusion
3.2. Participant Characteristics
3.3. Iron Status of New Zealand Infants
3.4. Baby Food Pouch Use as a Predictor of Iron Status
3.5. Baby-Led Weaning as a Predictor of Iron Status
3.6. Other Feeding Characteristics at 6 Months of Age and Current Iron Status
3.7. Demographic and Later Feeding Characteristics and Current Iron Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beauregard, J.L.; Bates, M.; Cogswell, M.E.; Nelson, J.M.; Hamner, H.C. Nutrient Content of Squeeze Pouch Foods for Infants and Toddlers Sold in the United States in 2015. Nutrients 2019, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Lee, M. A descriptive study investigating the use and nature of baby-led weaning in a UK sample of mothers. Matern. Child Nutr. 2011, 7, 34–47. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, E.; Jenkins, K.; Mathews, M.; Roebothan, B. Baby-led Weaning: A Preliminary Investigation. Can. J. Diet. Pract. Res. 2016, 77, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Conlon, C.A.; Haszard, J.J.; Beck, K.L.; von Hurst, P.R.; Taylor, R.W.; Heath, A.M. Food fussiness and early feeding characteristics of infants following Baby-Led Weaning and traditional spoon-feeding in New Zealand: An internet survey. Appetite 2018, 130, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Katiforis, I.; Fleming, E.A.; Haszard, J.J.; Hape-Cramond, T.; Taylor, R.W.; Heath, A.-L.M. Energy, Sugars, Iron, and Vitamin B12 Content of Commercial Infant Food Pouches and Other Commercial Infant Foods on the New Zealand Market. Nutrients 2021, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- The Nielsen Company. Global Baby Care Report: Oh, Baby! Trends in the Baby Food and Diaper Markets around the World. Nielsen. 2015. Available online: https://www.lemoci.com/wp-content/uploads/2015/09/Global-Baby-Care-Report-Nielsen-ao%C3%BBt-2015.pdf (accessed on 19 December 2023).
- Cameron, S.L.; Heath, A.-L.M.; Taylor, R.W. Healthcare professionals’ and mothers’ knowledge of, attitudes to and experiences with, Baby-Led Weaning: A content analysis study. BMJ Open 2012, 2, e001542. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bührer, C.; Ensenauer, R.; Jochum, F.; Kalhoff, H.; Lawrenz, B.; Körner, A.; Mihatsch, W.; Rudloff, S.; Zimmer, K.-P. Complementary foods in baby food pouches: Position statement from the Nutrition Commission of the German Society for Pediatrics and Adolescent Medicine (DGKJ, e.V.). Mol. Cell. Pediatr. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Theurich, M.A. Perspective: Novel Commercial Packaging and Devices for Complementary Feeding. Adv. Nutr. 2018, 9, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Theurich, M.A.; Fewtrell, M.; Baumgartner, J.; Perkin, M.R.; Breda, J.; Wickramansinghe, K.; Weber, M.W.; Koletzko, B. Moving Complementary Feeding Forward: Report on a Workshop of the Federation of International Societies for Pediatric Gastroenterology, Hepatology and Nutrition (FISPGHAN) and the World Health Organization Regional Office for Europe. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.L.; Westerlund, A.; Algarin, C.R.; Peirano, P.D.; Gregas, M.; Lozoff, B.; Nelson, C.A. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J. Pediatr. 2012, 160, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43. discussion S72–S91. [Google Scholar] [CrossRef] [PubMed]
- Morison, B.J.; Taylor, R.W.; Haszard, J.J.; Schramm, C.J.; Williams Erickson, L.; Fangupo, L.J.; Fleming, E.A.; Luciano, A.; Heath, A.-L.M. How different are baby-led weaning and conventional complementary feeding? A cross-sectional study of infants aged 6–8 months. BMJ Open 2016, 6, e010665. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Langley-Evans, S.C. Comparison of food and nutrient intake in infants aged 6-12 months, following baby-led or traditional weaning: A cross-sectional study. J. Hum. Nutr. Diet. 2022, 35, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Rowan, H.; Lee, M.; Brown, A. Estimated energy and nutrient intake for infants following baby-led and traditional weaning approaches. J. Hum. Nutr. Diet. 2022, 35, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Taylor, R.W.; Williams, S.M.; Gibson, R.S.; Fleming, E.A.; Wheeler, B.J.; Taylor, B.J.; Haszard, J.J.; Heath, A.-L.M. Impact of a modified version of baby-led weaning on iron intake and status: A randomised controlled trial. BMJ Open 2018, 8, e019036. [Google Scholar] [CrossRef]
- Dogan, E.; Yilmaz, G.; Caylan, N.; Turgut, M.; Gokcay, G.; Oguz, M.M. Baby-led complementary feeding: Randomized controlled study. Pediatr. Int. 2018, 60, 1073–1080. [Google Scholar] [CrossRef]
- Grant, C.C.; Wall, C.R.; Brunt, D.; Crengle, S.; Scragg, R. Population prevalence and risk factors for iron deficiency in Auckland, New Zealand. J. Paediatr. Child Health 2007, 43, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Soh, P.; Ferguson, E.L.; McKenzie, J.E.; Homs, M.Y.; Gibson, R.S. Iron deficiency and risk factors for lower iron stores in 6-24-month-old New Zealanders. Eur. J. Clin. Nutr. 2004, 58, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Healthy Eating Guidelines for New Zealand Babies and Toddlers (0–2 Years Old). New Zealand Ministry of Health. 2021. Available online: https://www.health.govt.nz/publication/healthy-eating-guidelines-new-zealand-babies-and-toddlers-0-2-years-old (accessed on 19 December 2023).
- Taylor, R.W.; Conlon, C.A.; Beck, K.L.; von Hurst, P.R.; Te Morenga, L.A.; Daniels, L.; Haszard, J.J.; Meldrum, A.M.; McLean, N.H.; Cox, A.M.; et al. Nutritional Implications of Baby-Led Weaning and Baby Food Pouches as Novel Methods of Infant Feeding: Protocol for an Observational Study. JMIR Res. Protoc. 2021, 10, e29048. [Google Scholar] [CrossRef] [PubMed]
- Statistics New Zealand. Stats NZ DataInfo+. Available online: https://datainfoplus.stats.govt.nz/Item/nz.govt.stats/7079024d-6231-4fc4-824f-dd8515d33141?_ga=2.60256877.1738686287.1671397721-676869378.1671397721 (accessed on 19 December 2023).
- Atkinson, J.; Salmond, C.; Crampton, P. NZDep2018 Index of Deprivation: Interim Research Report; University of Otago: Wellington, New Zealand, 2019. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Van den Broeck, J.; Chumlea, W.C.; Martorell, R. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr. Bull. 2004, 25, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Anthro Software—Stata Macro. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 19 December 2023).
- University of Otago and Ministry of Health. Methodology Report for the 2008/09 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011. [Google Scholar]
- The New Zealand Institute for Plant and Food Research; New Zealand Ministry of Health. New Zealand Food Composition Database. Available online: https://www.foodcomposition.co.nz/foodfiles/ (accessed on 19 December 2023).
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Luo, H.; Geng, J.; Zeiler, M.; Nieckula, E.; Sandalinas, F.; Williams, A.; Young, M.F.; Suchdev, P.S. A Practical Guide to Adjust Micronutrient Biomarkers for Inflammation Using the BRINDA Method. J. Nutr. 2023, 153, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Namaste, S.M.; Aaron, G.J.; Raiten, D.J.; Brown, K.H.; Flores-Ayala, R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv. Nutr. 2016, 7, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Looker, A.C.; Pfeiffer, C.M.; Cook, J.D.; Lacher, D.A.; Beard, J.L.; Lynch, S.R.; Grummer-Strawn, L.M. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am. J. Clin. Nutr. 2009, 89, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef]
- Southern Community Laboratories Ltd. Biochemistry: Paediatric Reference Intervals; Southern Community Laboratories Ltd.: Dunedin, New Zealand, 2017. [Google Scholar]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Haszard, J.J.; Taylor, R.W.; Taylor, B.J. Prevalence of low and high BMI during the first 3 years of life: Using New Zealand national electronic health data. Pediatr. Obes. 2023, 18, e13013. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Understanding Excess Body Weight: New Zealand Health Survey; Ministry of Health: Wellington, New Zealand, 2008. [Google Scholar]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- Gupta, P.M.; Hamner, H.C.; Suchdev, P.S.; Flores-Ayala, R.; Mei, Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am. J. Clin. Nutr. 2017, 106, 1640S–1646S. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Standard 2.9.1—Infant Formula Products; Department of Health and Aged Care: Canberra, Australia, 2022. [Google Scholar]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- Moumin, N.A.; Grieger, J.A.; Netting, M.J.; Makrides, M.; Green, T.J. Iron-Fortified Foods Are Needed to Meet the Estimated Average Requirement for Iron in Australian Infants Aged 6 to 12 Months. J. Nutr. 2023, 153, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- McLean, N.H.; Taylor, R.W.; Haszard, J.J.; Daniels, L.; Conlon, C.A.; Beck, K.L.; von Hurst, P.R.; Te Morenga, L.A.; Paul, R.; Cox, A.M.; et al. Baby Food Pouch Use in Infants Aged 7–10 Months in New Zealand: Findings from the First Foods New Zealand Observational Study. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4517318 (accessed on 27 March 2023).
- Ministry of Health. Baby-Led Weaning—Ministry Position Statement. Available online: https://www.health.govt.nz/our-work/preventative-health-wellness/nutrition/baby-led-weaning-ministry-position-statement (accessed on 27 March 2023).
- Karr, M.; Alperstein, G.; Causer, J.; Mira, M.; Lammi, A.; Fett, M.J. Iron status and anaemia in preschool children in Sydney. Aust. N. Z. J. Public Health 1996, 20, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Thane, C.W.; Walmsley, C.M.; Bates, C.J.; Prentice, A.; Cole, T.J. Risk factors for poor iron status in British toddlers: Further analysis of data from the National Diet and Nutrition Survey of children aged 1.5–4.5 years. Public Health Nutr. 2000, 3, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, I.; Gunnarsson, B.S.; Atladottir, H.; Michaelsen, K.F.; Palsson, G. Iron status at 12 months of age—Effects of body size, growth and diet in a population with high birth weight. Eur. J. Clin. Nutr. 2003, 57, 505–513. [Google Scholar] [CrossRef] [PubMed]
| Iron Status Category | Criteria Used |
|---|---|
| Iron-sufficient a | Plasma ferritin ≥ 15 μg/L b, in the absence of early “functional” iron deficiency, iron deficiency anaemia, and iron depletion |
| Iron-depleted | Plasma ferritin < 15 μg/L b, in the absence of early “functional” iron deficiency and iron deficiency anaemia |
| Early “functional” iron deficiency | Body iron < 0 mg/kg c and haemoglobin ≥ 105 g/L d |
| Iron deficiency anaemia | Body iron < 0 mg/kg c and haemoglobin < 105 g/L d |
| Whole Sample (n = 625) | Sample with Iron Status Data a (n = 364) | |
|---|---|---|
| n (%), Unless Otherwise Stated | ||
| Infant characteristics | ||
| Age (months), mean (SD) | 8.4 (0.8) | 8.3 (0.8) |
| Female sex b, | 289 (46.2) | 161 (44.2) |
| Ethnicity c | ||
| Māori | 131 (21.0) | 62 (17.0) |
| Pacific | 44 (7.0) | 14 (3.9) |
| Asian | 90 (14.4) | 39 (10.7) |
| Others | 16 (2.6) | 10 (2.8) |
| NZ and other European | 344 (55.0) | 239 (65.7) |
| Born at term d | 578 (92.6) | 340 (93.4) |
| BMI z-score e, mean (SD) | 0.30 (1.0) | 0.31 (1.0) |
| Respondent characteristics | ||
| Mother | 617 (98.7) | 359 (98.6) |
| Age (years), mean (SD) f | 32.7 (4.9) | 33.0 (4.7) |
| Highest level of education completed | ||
| School (primary or secondary) | 94 (15.1) | 49 (13.5) |
| Polytechnic or similar tertiary institution | 125 (20.0) | 71 (19.6) |
| University | 405 (64.9) | 243 (66.9) |
| Maternal primiparous | 303 (48.6) | 178 (48.9) |
| Current employment status | ||
| Employed full time | 70 (11.2) | 45 (12.4) |
| Employed part time | 137 (21.9) | 84 (23.1) |
| Other g | 418 (66.9) | 235 (64.6) |
| BMI (kg/m2), mean (SD) h | 27.8 (6.4) | 27.8 (6.1) |
| Overweight or obese, n (%) h | 348 (58.4) | 217 (61.7) |
| Childcare use i | 109 (17.4) | 66 (18.1) |
| Household factors | ||
| Number of children living in household | ||
| One | 283 (45.4) | 165 (45.5) |
| Two | 200 (32.1) | 124 (34.2) |
| Three or more | 141 (22.6) | 74 (20.4) |
| Number of adults living in household | ||
| One | 25 (4.0) | 16 (4.4) |
| Two | 517 (82.7) | 311 (85.4) |
| Three or more | 83 (13.3) | 37 (10.2) |
| Household deprivation decile j | ||
| 1–3 (low) | 180 (28.8) | 111 (30.5) |
| 4–7 | 282 (45.1) | 165 (45.3) |
| 8–10 (high) | 163 (26.1) | 88 (24.2) |
| Infant feeding characteristics | ||
| Ever breastfed | 612 (97.9) | 355 (97.5) |
| Currently breastfed | 414 (66.2) | 249 (68.4) |
| Current infant formula intake k | ||
| No formula | 319 (51.0) | 191 (52.5) |
| <1000 kJ/d | 73 (11.7) | 49 (13.5) |
| 1000 to <2500 kJ/d | 167 (26.7) | 82 (22.5) |
| ≥2500 kJ/d | 66 (10.6) | 42 (11.5) |
| Age when solids introduced | ||
| ≤4 months | 140 (22.4) | 70 (19.2) |
| 5 months | 230 (36.8) | 150 (41.2) |
| 6 months | 241 (38.6) | 134 (36.8) |
| ≥7 months | 14 (2.2) | 10 (2.8) |
| Current iron supplement l | 4 (0.6) | 3 (0.8) |
| Sample with Iron Status Data (n = 364) | Weighted Estimate of Mean or Percentage as Specified (95% CI) a | |
|---|---|---|
| Haemoglobin (g/L), mean (SD) | 115 (9) | 115 (114 to 116) |
| Plasma ferritin b (μg/L), geometric mean (95% CI) | 23.2 (21.4 to 25.1) | 22.8 (21.0 to 24.8) |
| Soluble transferrin receptor b (mg/L), mean (SD) | 4.3 (1.3) | 4.3 (4.2 to 4.4) |
| Body iron (mg/kg), mean (SD) | 3.1 (3.2) | 3.0 (2.7 to 3.4) |
| C-reactive protein, % (n) c | ||
| ≤5 mg/L | 95.3 (347) | 95.6 (93.0 to 97.3) |
| >5 mg/L | 4.7 (17) | 4.4 (2.7 to 7.0) |
| α1-acid glycoprotein, % (n) c | ||
| ≤1 g/L | 89.0 (324) | 89.2 (85.5 to 92.1) |
| >1 g/L | 11.0 (40) | 10.8 (7.9 to 14.5) |
| Iron status categories, % (n) | ||
| Iron-sufficient d | 77.8 (283) | 77.1 (72.3 to 81.3) |
| Iron-depleted e | 9.1 (33) | 8.9 (6.3 to 12.3) |
| Early “functional” iron deficiency f | 10.4 (38) | 11.1 (8.1 to 15.1) |
| Iron deficiency anaemia g | 2.8 (10) | 2.9 (1.5 to 5.5) |
| Non-Pouch User | Non-Frequent Pouch User | Frequent Pouch User | ||
|---|---|---|---|---|
| n | 189 | 113 | 62 | |
| Current plasma ferritin concentration | ||||
| Geometric mean (95% CI), μg/L | 21.0 (18.6, 23.7) | 26.3 (23.1, 29.9) | 24.9 (20.7, 30.1) | |
| Percent mean difference (95% CI), % | Reference | 25.2 (4.5, 50.0) | 15.2 (−3.5, 35.6) | |
| Adjusted c percent mean difference (95% CI), % | Reference | 15.8 (−3.0, 38.1) | 5.2 (−15.8, 31.3) | |
| Current body iron | ||||
| Mean (SD), mg/kg | 2.63 (3.47) | 3.67 (2.77) | 3.65 (2.87) | |
| Mean difference (95% CI), mg/kg | Reference | 1.04 (0.30, 1.78) | 0.74 (0.01, 1.47) | |
| Adjusted c mean difference (95% CI), mg/kg | Reference | 0.75 (0.02, 1.48) | 0.58 (−0.33, 1.50) | |
| Current iron sufficiency d | ||||
| n (%) | 134 (70.9) | 97 (85.8) | 52 (83.9) | |
| Odds ratio (95% CI) | Reference | 2.49 (1.35, 4.60) | 2.13 (1.01, 4.50) | |
| Adjusted c odds ratio (95% CI) | Reference | 2.20 (1.15, 4.21) | 1.50 (0.67, 3.39) | |
| Traditional Spoon-Feeding | Partial Baby-Led Weaning | Full Baby-Led Weaning | ||
|---|---|---|---|---|
| n | 269 | 42 | 53 | |
| Current plasma ferritin concentration | ||||
| Geometric mean (95% CI), μg/L | 23.9 (21.8, 26.1) | 20.3 (15.7, 26.3) | 22.2 (17.3, 28.5) | |
| Percent mean difference (95% CI), % | Reference | −15.0 (−34.1, 9.6) | −7.0 (−26.1, 17.0) | |
| Adjusted c percent mean difference (95% CI), % | Reference | −14.0 (−32.9, 10.3) | 3.5 (−17.6, 30.0) | |
| Current body iron | ||||
| Mean (SD), mg/kg | 3.23 (3.07) | 2.84 (3.22) | 2.84 (3.84) | |
| Mean difference (95% CI), mg/kg | Reference | −0.39 (−1.43, 0.66) | −0.39 (−1.34, 0.56) | |
| Adjusted c mean difference (95% CI), mg/kg | Reference | −0.34 (−1.37, 0.69) | 0.02 (−0.92, 0.97) | |
| Current iron sufficiency d | ||||
| n (%) | 217 (80.7) | 28 (66.7) | 38 (71.7) | |
| Odds ratio (95% CI) | Reference | 0.48 (0.24, 0.97) | 0.61 (0.31, 1.19) | |
| Adjusted c odds ratio (95% CI) | Reference | 0.52 (0.24, 1.13) | 0.91 (0.45, 1.87) | |
| Feeding Practices | |||||
|---|---|---|---|---|---|
| Exclusively Breastfeeding up to around 6 Months b | No | Yes | |||
| n | 223 | 141 | |||
| Current plasma ferritin concentration | |||||
| Geometric mean (95% CI), μg/L | 24.1 (21.8, 26.6) | 21.8 (19.0, 24.9) | |||
| Percent mean difference (95% CI), % | Reference | −9.6 (−23.3, 6.6) | |||
| Adjusted c percent mean difference (95% CI), % | Reference | 9.2 (−8.8, 30.9) | |||
| Current body iron | |||||
| Mean (SD), mg/kg | 3.34 (3.10) | 2.80 (3.34) | |||
| Mean difference (95% CI), mg/kg | Reference | −0.54 (−1.22, 0.14) | |||
| Adjusted c mean difference (95% CI), mg/kg | Reference | 0.13 (−0.62, 0.88) | |||
| Current iron sufficiency d | |||||
| n (%) | 179 (80.3) | 104 (73.8) | |||
| Odds ratio (95% CI) | Reference | 0.69 (0.42, 1.14) | |||
| Adjusted c odds ratio (95% CI) | Reference | 1.41 (0.79, 2.51) | |||
| Age when solids introduced | ≤4 months | 5 months | 6 months | ≥7 months | |
| n | 70 | 150 | 134 | 10 | |
| Current plasma ferritin concentration | |||||
| Geometric mean (95% CI), μg/L | 24.4 (20.5, 29.0) | 25.5 (22.5, 28.9) | 21.1 (18.5, 24.1) | 13.4 (6.7, 26.9) | |
| Percent mean difference (95% CI), % | 15.6 (−7.5, 44.6) | 20.7 (0.8, 44.6) | Reference | −36.4 (−61.3, 4.6) | |
| Adjusted c percent mean difference (95% CI), % | 5.7 (−15.4, 32.0) | 10.8 (−7.0, 32.0) | Reference | −41.8 (−64.6, −3.8) | |
| Current body iron | |||||
| Mean (SD), mg/kg | 3.36 (3.09) | 3.48 (3.12) | 2.79 (2.17) | 0.80 (4.50) | |
| Mean difference (95% CI), mg/kg | 0.57 (−0.35, 1.49) | 0.68 (−0.06, 1.43) | Reference | −2.00 (−4.04, 0.05) | |
| Adjusted c mean difference (95% CI), mg/kg | 0.25 (−0.67, 1.17) | 0.35 (−0.37, 1.08) | Reference | −2.19 (−4.20, −0.18) | |
| Current iron sufficiency d | |||||
| n (%) | 57 (81.4) | 121 (80.7) | 100 (74.6) | 5 (50.0) | |
| Odds ratio (95% CI) | 1.49 (0.73, 3.05) | 1.42 (0.81, 2.49) | Reference | 0.34 (0.09, 1.25) | |
| Adjusted c odds ratio (95% CI) | 1.13 (0.52, 2.46) | 1.17 (0.65, 2.14) | Reference | 0.21 (0.05, 0.96) | |
| Red meat consumption e | Never | Less than weekly | Weekly | Daily | |
| n | 150 | 23 | 167 | 24 | |
| Current plasma ferritin concentration | |||||
| Geometric mean (95% CI), μg/L | 22.5 (19.8, 25.6) | 25.7 (20.8, 31.9) | 23.0 (20.4, 26.1) | 26.2 (19.5, 35.1) | |
| Percent mean difference (95% CI), % | Reference | 14.3 (−18.9, 61.2) | 2.3 (−13.9, 21.6) | 16.3 (−17.0, 62.9) | |
| Adjusted c percent mean difference (95% CI), % | Reference | 12.3 (−19.4, 56.4) | 1.3 (−14.2, 19.5) | 24.1 (−10.3, 71.8) | |
| Current body iron | |||||
| Mean (SD), mg/kg | 2.93 (3.34) | 3.88 (2.34) | 3.11 (3.24) | 3.81 (2.69) | |
| Mean difference (95% CI), mg/kg | Reference | 0.96 (−0.45, 2.37) | 0.18 (−0.53, 0.89) | 0.88 (−0.50, 2.27) | |
| Adjusted c mean difference (95% CI), mg/kg | Reference | 0.93 (−0.43, 2.30) | 0.14 (−0.54, 0.83) | 1.15 (−0.19, 2.49) | |
| Current iron sufficiency d | |||||
| n (%) | 115 (76.7) | 21 (91.3) | 127 (76.1) | 20 (83.3) | |
| Odds ratio (95% CI) | Reference | 3.20 (0.71, 14.31) | 0.97 (0.58, 1.62) | 1.52 (0.49, 4.75) | |
| Adjusted c odds ratio (95% CI) | Reference | 3.40 (0.71, 16.36) | 0.96 (0.55, 1.68) | 1.73 (0.51, 5.84) | |
| Iron-fortified rice cereal consumption e | Never | Less than weekly | Weekly | Daily | |
| n | 190 | 10 | 72 | 92 | |
| Current plasma ferritin concentration | |||||
| Geometric mean (95% CI), μg/L | 23.4 (20.9, 26.2) | 24.3 (12.3, 47.9) | 24.1 (19.9, 29.3) | 21.9 (18.9, 25.3) | |
| Percent mean difference (95% CI), % | Reference | 3.6 (−37.1, 70.4) | 3.0 (−16.7, 27.4) | −6.7 (−13.2, 13.4) | |
| Adjusted c percent mean difference (95% CI), % | Reference | −0.2 (−39.5, 64.6) | −0.5 (−18.8, 22.0) | −13.3 (−28.5, 5.1) | |
| Current body iron | |||||
| Mean (SD), mg/kg | 3.16 (3.31) | 3.23 (3.79) | 3.38 (3.09) | 2.86 (3.02) | |
| Mean difference (95% CI), mg/kg | Reference | 0.08 (−1.97, 2.13) | 0.23 (−0.65, 1.10) | −0.30 (−1.10, 0.51) | |
| Adjusted c mean difference (95% CI), mg/kg | Reference | −0.03 (−2.09, 2.04) | 0.09 (−0.75, 0.93) | −0.58 (−1.37, 0.22) | |
| Current iron sufficiency d | |||||
| n (%) | 143 (75.3) | 8 (80.0) | 58 (80.6) | 74 (80.4) | |
| Odds ratio (95% CI) | Reference | 1.31 (0.27, 6.41) | 1.36 (0.70, 2.66) | 1.35 (0.73, 2.49) | |
| Adjusted c odds ratio (95% CI) | Reference | 1.14 (0.21, 6.23) | 1.25 (0.55, 2.56) | 1.08 (0.55, 2.10) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLean, N.H.; Haszard, J.J.; Daniels, L.; Taylor, R.W.; Wheeler, B.J.; Conlon, C.A.; Beck, K.L.; von Hurst, P.R.; Te Morenga, L.A.; McArthur, J.; et al. Baby Food Pouches, Baby-Led Weaning, and Iron Status in New Zealand Infants: An Observational Study. Nutrients 2024, 16, 1494. https://doi.org/10.3390/nu16101494
McLean NH, Haszard JJ, Daniels L, Taylor RW, Wheeler BJ, Conlon CA, Beck KL, von Hurst PR, Te Morenga LA, McArthur J, et al. Baby Food Pouches, Baby-Led Weaning, and Iron Status in New Zealand Infants: An Observational Study. Nutrients. 2024; 16(10):1494. https://doi.org/10.3390/nu16101494
Chicago/Turabian StyleMcLean, Neve H., Jillian J. Haszard, Lisa Daniels, Rachael W. Taylor, Benjamin J. Wheeler, Cathryn A. Conlon, Kathryn L. Beck, Pamela R. von Hurst, Lisa A. Te Morenga, Jenny McArthur, and et al. 2024. "Baby Food Pouches, Baby-Led Weaning, and Iron Status in New Zealand Infants: An Observational Study" Nutrients 16, no. 10: 1494. https://doi.org/10.3390/nu16101494







