The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of 25(OH)D, 25(OH)D2, and 25(OH)D3
2.3. Assessment of SUA and Higher SUA Status
2.4. Other Covariates
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association between Serum 25(OH)D, Log-25(OH)D2, 25(OH)D3, and SUA Levels
3.3. Stratified Analyses of the Association between Serum 25(OH)D and SUA Levels
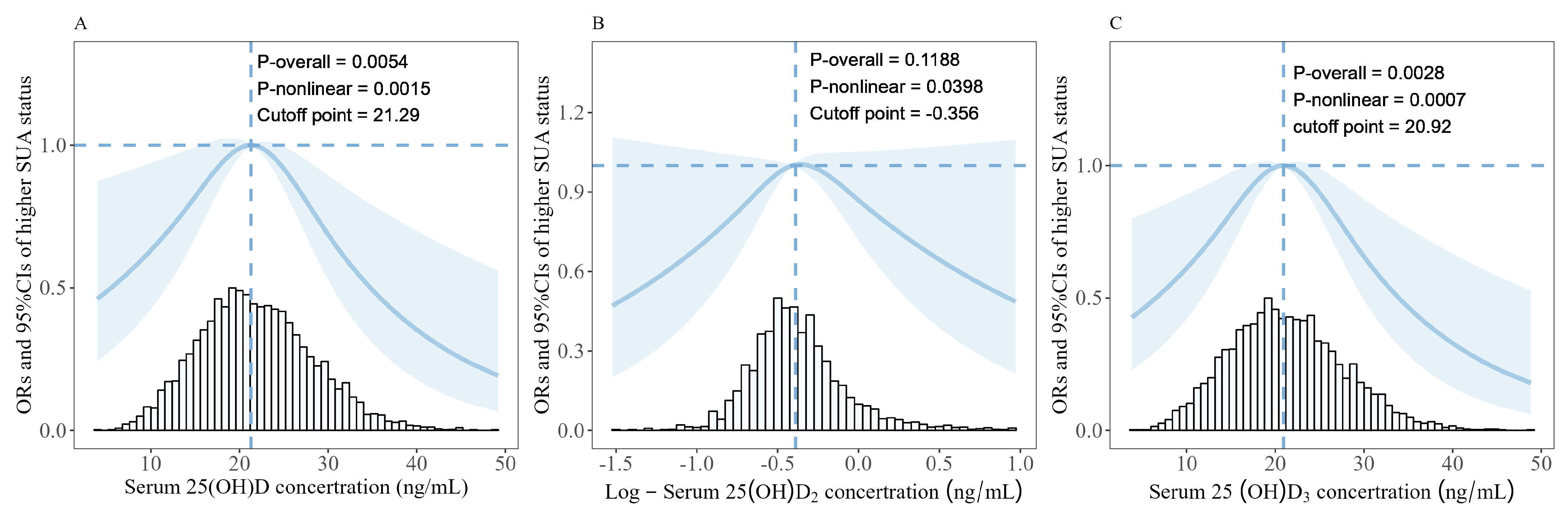
3.4. Associations between Serum 25(OH)D, Log-25(OH)D2, 25(OH)D3, and Risk of Higher SUA Status
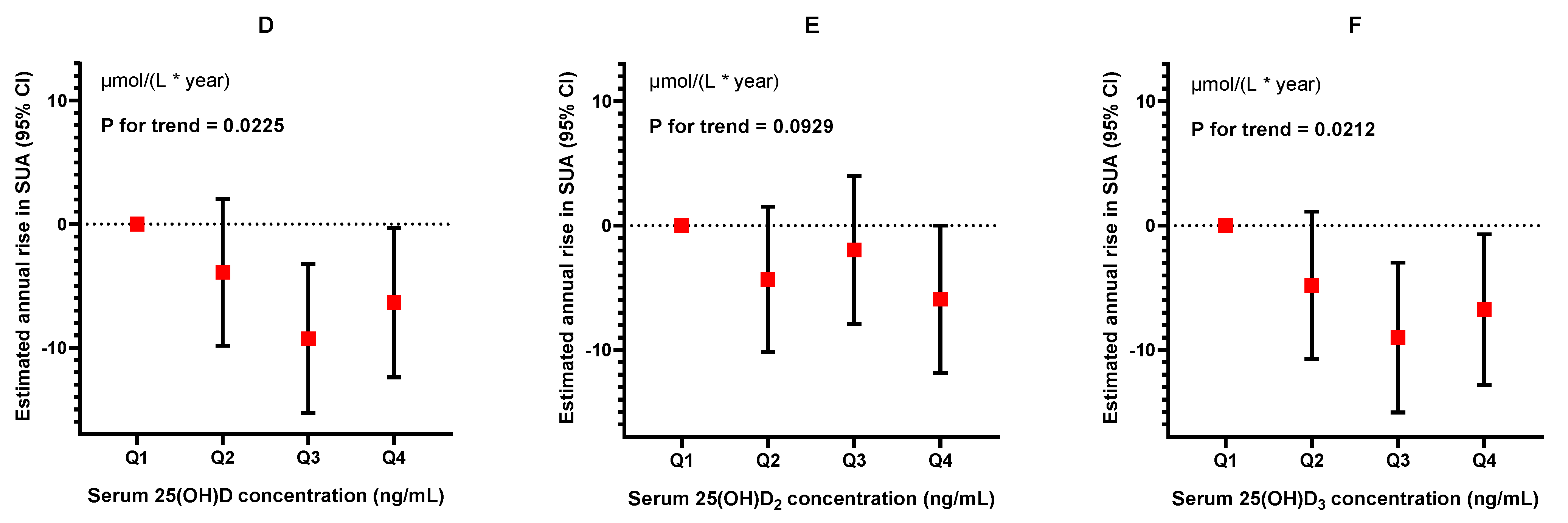
3.5. Longitudinal Analysis of the Association between Serum 25(OH)D, 25(OH)D2, 25(OH)D3, and SUA Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasalic, D.; Marinkovic, N.; Feher-Turkovic, L. Uric acid as one of the important factors in multifactorial disorders--facts and controversies. Biochem. Medica 2012, 22, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Smith, T.; Nicolson, B.; Clark, B.; Callon, K.; Naot, D.; Haskard, D.O.; McQueen, F.M.; Reid, I.R.; Cornish, J. Enhanced osteoclastogenesis in patients with tophaceous gout: Urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum. 2008, 58, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, K.; Han, X.; Yin, Y.; Di, H.; Wu, J.; Zhang, Y.; Zeng, X. Serum uric acid might be positively associated with hypertension in Chinese adults: An analysis of the China health and nutrition survey. Front. Med. 2022, 8, 755509. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, X.; Yin, Y.; Cao, Y.; Di, H.; Wu, J.; Zhang, Y.; Zeng, X. Dose-response relationship of uric acid with fasting glucose, insulin, and insulin resistance in a united states cohort of 5,148 non-diabetic people. Front. Med. 2022, 9, 905085. [Google Scholar] [CrossRef]
- Rho, Y.H.; Zhu, Y.; Choi, H.K. The Epidemiology of Uric Acid and Fructose. Semin. Nephrol. 2011, 31, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Ye, P.; Lu, J.; Chen, B.; Li, N.; Zhang, H.; Bo, H.; Chen, X.; Liu, H.; Zhang, C.; et al. Prevalence and related factors of hyperuricaemia in Chinese children and adolescents: A pooled analysis of 11 population-based studies. Ann. Med. 2022, 54, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef]
- Luciano, R.; Shashaj, B.; Spreghini, M.; Del Fattore, A.; Rustico, C.; Wietrzykowska Sforza, R.; Morino, G.S.; Dallapiccola, B.; Manco, M. Percentiles of serum uric acid and cardiometabolic abnormalities in obese Italian children and adolescents. Ital. J. Pediatr. 2017, 43, 3. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689s–1696s. [Google Scholar] [CrossRef] [PubMed]
- Zella, J.B.; DeLuca, H.F. Vitamin D and autoimmune diabetes. J. Cell. Biochem. 2003, 88, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.; Tikkanen, E.; Eriksson, J.; et al. Association of vitamin D status with arterial blood pressure and hypertension risk: A mendelian randomisation study. Lancet. Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Ghosh, S.; Pandit, K.; Chatterjee, P.; Majhi, B.; Chowdhury, S. Uric Acid and Its Correlation with Various Metabolic Parameters: A Population-Based Study. Indian J. Endocrinol. Metab. 2019, 23, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.Y.; Choi, J.W.; Mount, D.B.; Zhu, Y.; Zhang, Y.; Choi, H.K. The independent association between parathyroid hormone levels and hyperuricemia: A national population study. Arthritis Res. Ther. 2012, 14, R56. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.Y.; Gordon, C.M. Vitamin D deficiency in children and adolescents: Epidemiology, impact and treatment. Rev. Endocr. Metab. Disord. 2008, 9, 161–170. [Google Scholar] [CrossRef]
- Saintonge, S.; Bang, H.; Gerber, L.M. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: The National Health and Nutrition Examination Survey III. Pediatrics 2009, 123, 797–803. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Ginde, A.A.; Camargo, C.A., Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics 2009, 124, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, S.; Lu, J.; Yang, Z.; Yang, X.; Yang, L. Vitamin D Status for Chinese Children and Adolescents in CNNHS 2016-2017. Nutrients 2022, 14, 4928. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, K.; Zhang, Y.; Zeng, X. Serum 25-hydroxyvitamin D might be negatively associated with hyperuricemia in U.S. adults: An analysis of the National Health and Nutrition Examination Survey 2007–2014. J. Endocrinol. Investig. 2022, 45, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Takir, M.; Solak, Y.; Erek, A.; Kostek, O.; Oral, A.; Elcioglu, O.C.; Bakan, A.; Afsar, B.; Ozkok, A.; Jalal, D. Association between elevated serum uric acid and vitamin D insufficiency among the middle-aged and elderly population. Turk Nefroloji Dializ Ve Transplant. Derg. 2016, 25, 182–186. [Google Scholar] [CrossRef]
- Nabipour, I.; Sambrook, P.N.; Blyth, F.M.; Janu, M.R.; Waite, L.M.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Cumming, R.G.; Seibel, M.J. Serum uric acid is associated with bone health in older men: A cross-sectional population-based study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, S.; Acikgoz, S.B.; Genc, A.B.; Yildirim, M.; Solak, Y.; Tamer, A. The Association of Vitamin D Status and Vitamin D Replacement Therapy with Glycemic Control, Serum Uric Acid Levels, and Microalbuminuria in Patients with Type 2 Diabetes and Chronic Kidney Disease. Med. Princ. Pract. 2016, 26, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. Nutrition in adolescents: Physiology, metabolism, and nutritional needs. Ann. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef]
- Baldree, L.A.; Stapleton, F.B. Uric Acid Metabolism in Children. Pediatr. Clin. N. Am. 1990, 37, 391–418. [Google Scholar] [CrossRef]
- Xiong, T.; Wu, Y.; Li, Y.; Chen, W.; Zhang, Z.; Lu, W.; He, M.; Chen, L.; Tang, Y.; Yao, P.; et al. Association of serum 25-hydroxyvitamin D with bone health measured by calcaneal quantitative ultrasound: A large cross-sectional analysis in children and adolescents. Food Funct. 2024, 15, 1379–1389. [Google Scholar] [CrossRef]
- WS/T456-2014; Screening Standard for Malnutrition of School-Age Children and Adolescents. National Health Commission of People’s Republic of China: Beijing, China, 2014.
- WS/T 586-2018; Screening for Overweight and Obesity among School-Age Children and Adolescents. National Health Commission of People’s Republic of China: Beijing, China, 2018.
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, X.; Gasevic, D.; Flores, A.B.; Yu, Z. BMI, Waist Circumference Reference Values for Chinese School-Aged Children and Adolescents. Int. J. Environ. Res. Public Health 2016, 13, 589. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M. Hyperuricemia in Children and Adolescents: Present Knowledge and Future Directions. J. Nutr. Metab. 2019, 2019, 3480718. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Elkhashab, M.; Trinh, H.; Tak, W.Y.; Ma, X.; Chuang, W.L.; Kim, Y.J.; Martins, E.B.; Lin, L.; Dinh, P.; et al. Association of baseline vitamin D levels with clinical parameters and treatment outcomes in chronic hepatitis B. J. Hepatol. 2015, 63, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Li, C.; Chao, X.; Zhang, Q.; Zhang, Y. Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS ONE 2013, 8, e61159. [Google Scholar] [CrossRef] [PubMed]
- El, K.; Abo Khosheim, K.; Elsayed, M.; Sabry, R.; Bedewy, E. MARSLAND PRESS Multidisciplinary Academic Journal Publisher Relation between Serum Uric Acid and Vitamin D among Elderly Egyptians. J. Am. Sci. 2014, 12, 984. [Google Scholar]
- Zhang, Y.Y.; Qiu, H.B.; Tian, J.W. Association Between Vitamin D and Hyperuricemia Among Adults in the United States. Front. Nutr. 2020, 7, 592777. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef]
- Gu, F.; Luo, X.; Jin, X.; Cai, C.; Zhao, W. Association of Total Calcium With Serum Uric Acid Levels Among United States Adolescents Aged 12-19 Years: A Cross-Sectional Study. Front. Med. 2022, 9, 915371. [Google Scholar] [CrossRef] [PubMed]
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone 1995, 17, 107s–111s. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Liu, B.; Wang, T.; Zhao, H.N.; Yue, W.W.; Yu, H.P.; Liu, C.X.; Yin, J.; Jia, R.Y.; Nie, H.W. The prevalence of hyperuricemia in China: A meta-analysis. BMC Public Health 2011, 11, 832. [Google Scholar] [CrossRef]

| Total | Q1 | Q2 | Q3 | Q4 | p-Value | |
|---|---|---|---|---|---|---|
| N | 4777 | 1190 | 1198 | 1194 | 1195 | |
| Age, years | 11.94 (9.2–14.7) | 14.61 (12.2–16.2) | 12.53 (10.2–14.8) | 10.91 (8.8–13.4) | 9.45 (7.9–12.1) | <0.001 |
| Female, n (%) | 2125 (44.5) | 701 (58.9) | 545 (45.5) | 457 (38.3) | 422 (35.3) | <0.001 |
| Waist, cm | 63.00 (55.80, 70.80) | 66.00 (61.00, 72.72) | 64.00 (57.70, 71.10) | 61.00 (54.00, 69.58) | 58.10 (52.50, 66.50) | <0.001 |
| BMI, kg/m2 | 18.07 (15.8–20.8) | 19.16 (17.2–21.7) | 18.54 (16.2–21.3) | 17.52 (15.4–20.4) | 16.81 (15.0–19.6) | <0.001 |
| Weight status, n (%) | 0.019 | |||||
| Underweight | 420 (8.8) | 82 (6.9) | 99 (8.3) | 115 (9.6) | 124 (10.4) | |
| Normal | 3270 (68.5) | 868 (72.9) | 816 (68.1) | 1072 (89.8) | 789 (66.0) | |
| Overweight | 643 (13.5) | 141 (11.8) | 167 (13.9) | 39 (3.3) | 171 (14.3) | |
| Obesity | 444 (9.3) | 99 (8.3) | 116 (9.7) | 83 (7.0) | 111 (9.3) | |
| Puberty stage, n (%) | <0.001 | |||||
| Pre-puberty | 1494 (31.3) | 113 (9.5) | 281 (23.5) | 450 (37.7) | 650 (54.4) | |
| Mid-puberty | 1060 (22.2) | 179 (15.0) | 271 (22.6) | 340 (28.5) | 270 (22.6) | |
| Post-puberty | 2223 (46.5) | 898 (75.5) | 646 (53.9) | 404 (33.8) | 275 (23.0) | |
| Household income, n (%) | <0.001 | |||||
| <CNY 120,000 per year | 1254 (26.3) | 377 (31.7) | 342 (28.5) | 270 (22.6) | 265 (22.2) | |
| ~CNY 250,000 per year | 1603 (33.6) | 398 (33.4) | 399 (33.3) | 416 (34.8) | 390 (32.6) | |
| ≥CNY 250,000 per year | 1885 (39.5) | 404 (33.9) | 446 (37.2) | 500 (41.9) | 535 (44.8) | |
| Missing | 35 (0.7) | 11 (0.9) | 11 (0.9) | 8 (0.7) | 5 (0.4) | |
| Paternal education level, n (%) | <0.001 | |||||
| ≤9 years | 768 (16.1) | 138 (11.5) | 151 (12.6) | 205 (17.1) | 274 (23.0) | |
| ~12 years | 1073 (22.5) | 223 (18.7) | 266 (22.3) | 292 (24.4) | 292 (24.5) | |
| ~15 years | 1333 (27.9) | 347 (29.0) | 323 (27.1) | 336 (28.0) | 327 (27.5) | |
| ≥16 years | 1556 (32.6) | 475 (39.7) | 446 (37.4) | 352 (29.4) | 283 (23.8) | |
| Missing | 47 (1.0) | 10 (0.8) | 8 (0.7) | 13 (1.1) | 14 (1.2) | |
| Maternal education level, n (%) | <0.001 | |||||
| ≤9 years | 930 (19.5) | 168 (14.1) | 202 (16.9) | 240 (20.0) | 320 (26.9) | |
| ~12 years | 1241 (26.0) | 273 (22.8) | 293 (24.5) | 328 (27.4) | 347 (29.2) | |
| ~15 years | 1351 (28.3) | 377 (31.5) | 365 (30.6) | 337 (28.1) | 272 (22.9) | |
| ≥16 years | 1223 (25.6) | 367 (30.7) | 329 (27.6) | 290 (24.2) | 237 (19.9) | |
| Missing | 32 (0.7) | 3 (0.3) | 5 (0.4) | 3 (0.3) | 14 (1.2) | |
| Vitamin D supplement, n (%) | <0.001 | |||||
| No | 4078 (85.4) | 1089 (91.5) | 1032 (86.1) | 998 (83.6) | 959 (80.3) | |
| Yes | 686 (14.4) | 96 (8.1) | 165 (13.8) | 191 (16.0) | 234 (19.6) | |
| Missing | 13 (0.3) | 5 (0.4) | 1 (0.1) | 5 (0.4) | 2 (0.2) | |
| Multivitamin/mineral supplement, n (%) | 0.420 | |||||
| No | 4321 (90.5) | 1095 (92.0) | 1077 (89.9) | 1069 (89.5) | 1080 (90.4) | |
| Yes | 425 (8.9) | 87 (7.3) | 115 (9.6) | 116 (9.7) | 107 (9.0) | |
| Missing | 31 (0.6) | 8 (0.7) | 6 (0.5) | 9 (0.8) | 8 (0.7) | |
| Smoking status, n (%) | 0.469 | |||||
| Never | 4674 (97.8) | 1162 (97.6) | 1170 (97.7) | 1167 (97.7) | 1175 (98.3) | |
| <1 cigarette per month | 32 (0.7) | 13 (1.1) | 8 (0.7) | 6 (0.5) | 5 (0.4) | |
| ≥1 cigarette per month | 21 (0.4) | 6 (0.5) | 6 (0.5) | 6 (0.5) | 3 (0.3) | |
| e-Cigarette | 8 (0.2) | 2 (0.2) | 2 (0.2) | 4 (0.3) | 0 (0.0) | |
| Missing | 42 (0.9) | 7 (0.6) | 12 (1.0) | 11 (0.9) | 12 (1.0) | |
| Drinking status, n (%) | <0.001 | |||||
| Never | 4223 (88.4) | 991 (83.3) | 1036 (86.5) | 1076 (90.1) | 1120 (93.7) | |
| <1 standard drink per month | 410 (8.6) | 141 (11.8) | 128 (10.7) | 84 (7.0) | 57 (4.8) | |
| ≥1 standard drink per month | 120 (2.5) | 52 (4.4) | 27 (2.3) | 26 (2.2) | 15 (1.3) | |
| Missing | 24 (0.5) | 6 (0.5) | 7 (0.6) | 8 (0.7) | 3 (0.3) | |
| Levels of MVPA, n (%) | <0.001 | |||||
| <0.5 h per d | 1322 (27.7) | 426 (35.8) | 310 (25.9) | 280 (23.5) | 306 (25.6) | |
| ~1 h per d | 2162 (45.3) | 532 (44.7) | 534 (44.6) | 558 (46.7) | 538 (45.0) | |
| ~3 h per d | 1089 (22.8) | 192 (16.1) | 304 (25.4) | 296 (24.8) | 297 (24.9) | |
| >3 h per d | 161 (3.4) | 32 (2.7) | 40 (3.3) | 47 (3.9) | 42 (3.5) | |
| Missing | 43 (0.9) | 8 (0.7) | 10 (0.8) | 13 (1.1) | 12 (1.0) | |
| SUA, μmol/L | 343.0 (289.0–415.0) | 363.0 (302.0–428.0) | 353.0 (297.0–432.0) | 337.5 (284.0–412.0) | 324.0 (275.0–385.0) | <0.001 |
| 25(OH)D, ng/mL | 21.44 (17.4–26.0) | 14.61 (12.5–16.1) | 19.44 (18.4–20.4) | 23.64 (22.5–24.7) | 29.47 (27.5–32.2) | <0.001 |
| 25(OH)D2, ng/mL | 0.39 (0.27–0.58) | 0.37 (0.25–0.56) | 0.38 (0.26–0.58) | 0.40 (0.29–0.61) | 0.41 (0.29–0.59) | <0.001 |
| 25(OH)D3, ng/mL | 20.9 (16.7–25.4) | 14.0 (11.9–15.6) | 18.9 (17.8–19.9) | 23.1 (22.0–24.2) | 28.9 (26.9–31.7) | <0.001 |
| Inflection Point (ng/mL) | Concentrations (ng/mL) | N | Model I | Model II | |||
|---|---|---|---|---|---|---|---|
| β (95% CI), µmol/L | p-Value | β (95% CI), µmol/L | p-Value | ||||
| Serum 25(OH)D a | 24.31 | <24.31 | 3159 | 12.89 (4.76, 21.01) | 0.002 | 9.15 (1.48, 16.83) | 0.019 |
| ≥24.31 | 1618 | −4.85 (−14.09, 4.39) | 0.303 | −4.41 (−13.38, 4.56) | 0.335 | ||
| Log-Serum 25(OH)D2 b | −0.389 c | <−0.389 | 2497 | 18.43 (−2.21, 39.07) | 0.08 | 13.17 (−6.37, 32.72) | 0.187 |
| ≥−0.389 | 2280 | −22.23 (−35.12, −9.34) | <0.001 | −16.80 (−29.22, −4.39) | 0.008 | ||
| Serum 25(OH)D3 a | 23.79 | <23.79 | 3181 | 1.59 (0.79, 2.40) | <0.001 | 12.15 (4.56, 19.74) | 0.002 |
| ≥23.79 | 1596 | −0.45 (−1.38, 0.47) | 0.301 | −3.60 (−12.58, 5.38) | 0.432 | ||
| Inflection Point (ng/mL) | Concentrations (ng/mL) | N | Model I | Model II | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||||
| Serum 25(OH)D a | 21.29 | <21.29 | 2349 | 1.52 (1.00–2.35) | 0.055 | 1.45 (0.92–2.31) | 0.11 |
| ≥21.29 | 2428 | 0.45 (0.30–0.67) | <0.001 | 0.52 (0.34–0.78) | 0.002 | ||
| Log-Serum 25(OH)D2 b | −0.356 c | <−0.356 | 2797 | 2.00 (0.92–4.52) | 0.086 | 2.14 (0.93–5.10) | 0.081 |
| ≥−0.356 | 1980 | 0.54 (0.27–1.03) | 0.071 | 0.71 (0.34–1.39) | 0.300 | ||
| Serum 25(OH)D3 a | 20.92 | <20.92 | 2397 | 1.86 (1.22–2.86) | 0.004 | 1.68 (1.07–2.66) | 0.025 |
| ≥20.92 | 2380 | 0.50 (0.33–0.73) | <0.001 | 0.55 (0.36–0.84) | 0.006 | ||
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
|---|---|---|---|---|---|
| Serum 25(OH)D | |||||
| N | 412 | 411 | 408 | 410 | |
| Model I | 0.00 (reference) | −2.97 (−8.89, 2.96) | −7.50 (−13.48, −1.51) | −5.29 (−11.32, 0.74) | 0.0519 |
| Model II | 0.00 (reference) | −3.91 (−9.84, 2.02) | −9.27 (−15.30, −3.24) | −6.35 (−12.40, −0.30) | 0.0225 |
| Serum 25(OH)D2 | |||||
| N | 412 | 419 | 400 | 410 | |
| Model I | 0.00 (reference) | −4.82 (−10.69, 1.05) | −2.48 (−8.43, 3.47) | −6.73 (−12.65, −0.80) | 0.0529 |
| Model II | 0.00 (reference) | −4.34 (−10.19, 1.51) | −1.96 (−7.90, 3.98) | −5.91 (−11.83, 0) | 0.0929 |
| Serum 25(OH)D3 | |||||
| N | 412 | 409 | 410 | 410 | |
| Model I | 0.00 (reference) | −4.34 (−10.27, 1.59) | −7.47 (−13.44, −1.50) | −5.97 (−12.00, 0.06) | 0.0431 |
| Model II | 0.00 (reference) | −4.80 (−10.74, 1.13) | −9.00 (−15.03, −2.99) | −6.77 (−12.83, −0.71) | 0.0212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Xiong, T.; Li, Y.; Kong, B.; Lu, W.; Zhang, Z.; Chen, L.; Tang, Y.; Yao, P.; Xiong, J.; et al. The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis. Nutrients 2024, 16, 1492. https://doi.org/10.3390/nu16101492
Ma Z, Xiong T, Li Y, Kong B, Lu W, Zhang Z, Chen L, Tang Y, Yao P, Xiong J, et al. The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis. Nutrients. 2024; 16(10):1492. https://doi.org/10.3390/nu16101492
Chicago/Turabian StyleMa, Zhuang, Ting Xiong, Yan Li, Binxuan Kong, Wenlong Lu, Ziyang Zhang, Liangkai Chen, Yuhan Tang, Ping Yao, Jingfan Xiong, and et al. 2024. "The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis" Nutrients 16, no. 10: 1492. https://doi.org/10.3390/nu16101492
APA StyleMa, Z., Xiong, T., Li, Y., Kong, B., Lu, W., Zhang, Z., Chen, L., Tang, Y., Yao, P., Xiong, J., Li, Y., & Wu, Y. (2024). The Inverted U-Shaped Association between Serum Vitamin D and Serum Uric Acid Status in Children and Adolescents: A Large Cross-Sectional and Longitudinal Analysis. Nutrients, 16(10), 1492. https://doi.org/10.3390/nu16101492






