Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development
Abstract
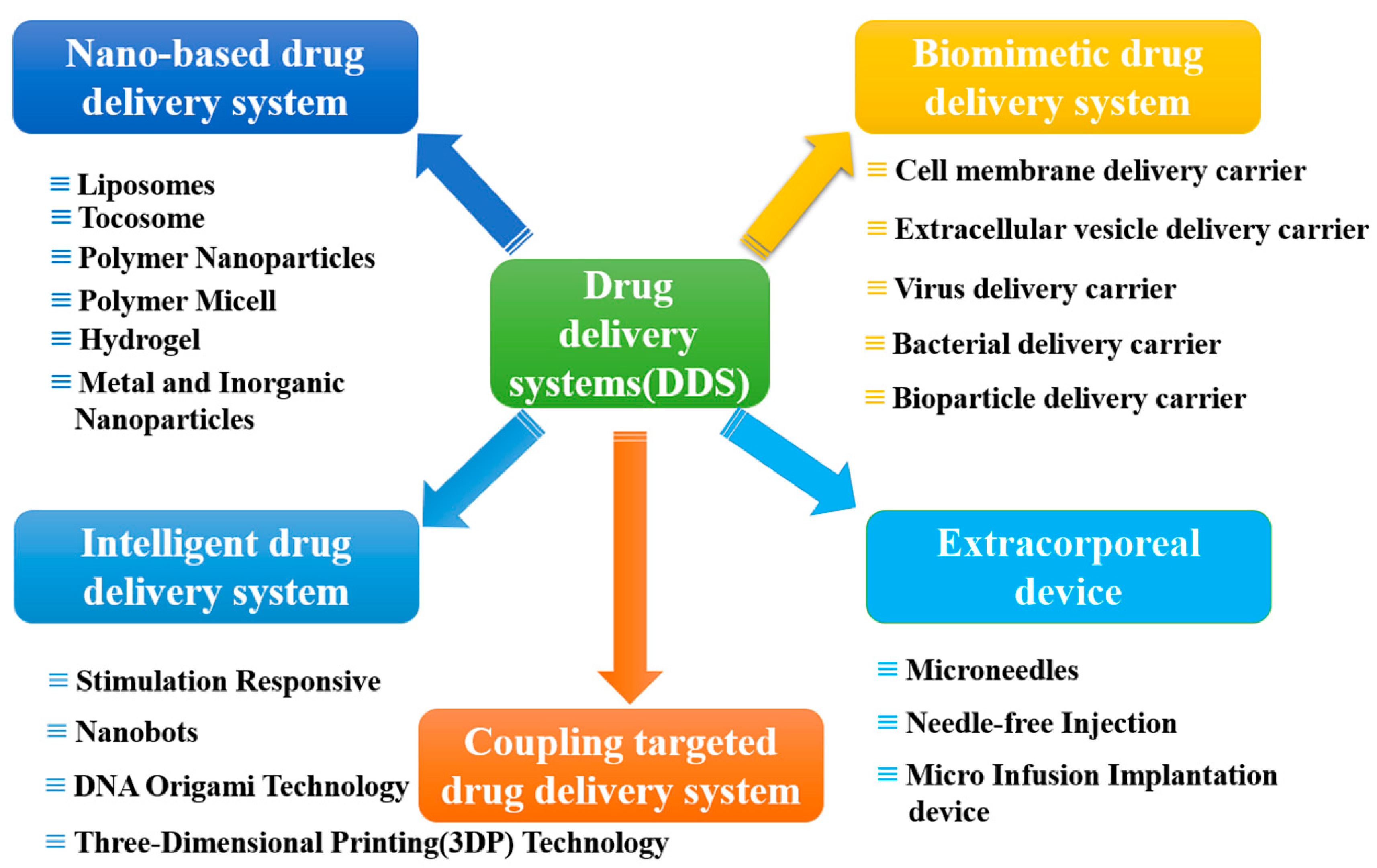
:1. Introduction
2. Carrier-Based Drug Delivery Systems
2.1. Nano-Based Drug Delivery Systems (NDDSs)
2.1.1. Liposomes
2.1.2. Tocosome
2.1.3. Polymer Nanoparticles
2.1.4. Polymer Micelle
2.1.5. Hydrogel
2.1.6. Metal and Inorganic Nanoparticles
2.2. Biomimetic Drug Delivery Systems
2.2.1. Cell Membrane Delivery Carrier
2.2.2. Extracellular Vesicle Delivery Carrier
2.2.3. Virus Delivery Carrier
2.2.4. Bacterial Delivery Carrier
2.2.5. Bioparticle Delivery Carrier
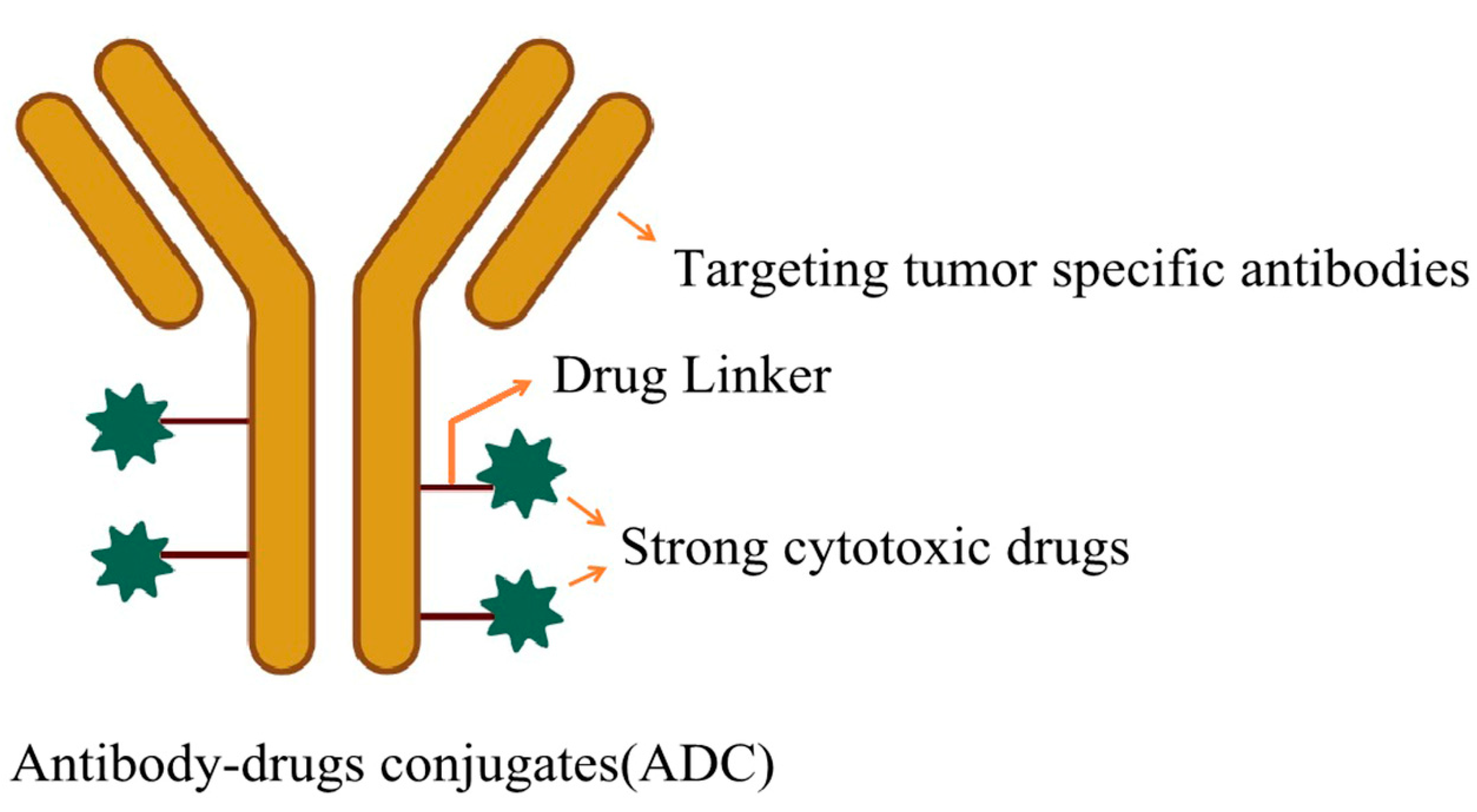
3. Coupling Targeted Drug Delivery Systems
4. Intelligent Drug Delivery System
4.1. Stimulation Responsive
4.2. Nanobots
4.3. DNA Origami Technology
4.4. Three-Dimensional Printing (3DP) Technology
5. Extracorporeal Device
5.1. Microneedles
5.2. Needle Free Injection
5.3. Micro Infusion Implantation Device
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Enrique, N.; Alberto, O.; Antonio, J.; Bravo, I.; Alonso-Moreno, C. Polyester polymeric nanoparticles as platforms in the development of novel nanomedicines for cancer treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, X.; Liang, X.; Yang, J.; Zhang, C.N.; Kong, D.L.; Wang, W.W. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta. Biomater. 2019, 85, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cao, Y.; Liu, Y.; Ouyang, B.S.; Ning, B.; Wang, Y.; Guo, H.S.; Pang, Z.Q.; Shen, S. Localized disruption of redox homeostasis boosting ferroptosis of tumor by hydrogel delivery system. Mater. Today. Bio. 2021, 12, 100154. [Google Scholar] [CrossRef] [PubMed]
- Abolfazl, A.; Rogaie, R.; Soodabeh, D.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Kisak, E.T.; Coldren, B.; Evans, C.A.; Boyer, C.; Zasadzinski, J.A. The vesosome-A multicompartment drug delivery vehicle. Curr. Med. Chem. 2004, 11, 199–219. [Google Scholar] [CrossRef]
- Lian, T.; Ho, J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef]
- Peyman, A.; Ahmad, M.; Nahid, S.; Abastabar, M.; Akhtari, J. Nanoliposome-loaded anti-fungal drugs for dermal administration: A review. Curr. Med. Mycol. 2021, 7, 71–78. [Google Scholar]
- Francian, A.; Widmer, A.; Olsson, T.; Ramirez, M.; Heald, D.; Rasic, K.; Adams, L.; Martinson, H.; Kullberg, M. Delivery of toll-like receptor agonists by complement C3-targeted liposomes activates immune cells and reduces tumour growth. J. Drug Target. 2021, 29, 754–760. [Google Scholar] [CrossRef]
- Park, S.J. Protein-nanoparticle interaction: Corona formation and conformational changes in proteins on nanoparticles. Int. J. Nanomed. 2020, 15, 5783–5802. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Goel, S.; Ni, D.L.; Ellison, P.A.; Siamof, C.M.; Jiang, D.W.; Cheng, L.; Kang, L.; Yu, F.Q.; Liu, Z.; et al. Reassembly of 89Zr-labeled cancer cell membranes into multicompartment membrane derived liposomes for PET-trackable tumor-targeted theranostics. Adv. Mater. 2018, 30, 1704–1734. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, X.X.; Chai, J.S.; Zhang, Z.Z.; Xue, X.; Huang, F.; Liu, J.F.; Shi, L.Q.; Liu, Y. Stapled liposomes enhance cross-priming of radio-immunotherapy. Adv. Mater. 2022, 34, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Gong, Y.C.; Li, Z.L.; Li, Y.P.; Xiong, X.Y. Application of nanoparticle-based co-delivery strategies for cancer therapy. Mater. Rep. 2020, 34, 516–522. [Google Scholar]
- Bo, R.N.; Dai, X.R.; Huang, J.; Wei, S.M.; Liu, M.J.; Li, J.G. Evaluation of optimum conditions for decoquinate nanoliposomes and their anticoccidial efficacy against diclazuril-resistant Eimeria tenella infections in broilers. Vet. Parasitol. 2020, 283, 109186. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Rodrigues, B.D.; Sun, C.W.; Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 2019, 307, 247–260. [Google Scholar] [CrossRef]
- Sanjeet, B. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar]
- Ferraz-Carvalho, R.S.; Pereira, M.A.; Linhares, L.A.; Lira-Nogueira, M.C.B.; Cavalcanti, I.M.F.; Santos-Magalhaes, N.S.; Montenegro, L.M.L. Effects of the encapsulation of usnic acid into liposomes and interactions with antituberculous agents against multidrug-resistant tuberculosis clinical isolates. Memórias Inst. Oswaldo Cruz 2016, 111, 330–334. [Google Scholar] [CrossRef]
- Ambati, S.; Pham, T.; Lewis, Z.A.; Lin, X.; Meagher, R.B. DC-SIGN targets amphotericin B-loaded liposomes to diverse pathogenic fungi. Fungal. Biol. Biotechnol. 2021, 8, 22. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (sort) nanoparticles for tissue-specific mrna delivery and crispr-cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Sun, Y.H.; Yu, X.L.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.Y.; Robinson, J.; Zhang, D.; et al. Preparation of selective organ-targeting (sort) lipid nanoparticles (lnps) using multiple technical methods for tissue-specific mrna delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.L.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Tang, Y.; Chen, J.J.; Muriph, R.; Ye, Z.F.; Huang, C.F.; Evans, J.; Henske, E.P.; Xu, Q.B. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Safford, H.C.; Geisler, H.C.; Hamilton, A.G.; Thatte, A.S.; Billingsley, M.M.; Joseph, R.A.; Mrksich, K.; Padilla, M.S.; Ghalsasi, A.A.; et al. Ionizable lipid nanoparticles for in vivo mrna delivery to the placenta during pregnancy. J. Am. Chem. Soc. 2023, 145, 4691–4706. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.H.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Fernández, P.O.M.; Shewale, S.V.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; Mui, B.L.; et al. Car T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Xu, F.; Zhang, Y. Research progress of mRNA vaccine delivery system. Chin. J. New Drugs 2022, 31, 2109–2113. [Google Scholar]
- Saunders, N.R.M.; Paolini, M.S.; Fenton, O.S.; Poul, L.; Devalliere, J.; Mpambani, F.; Darmon, A.; Bergère, M.; Jibault, O.; Germain, M.; et al. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020, 20, 4264–4269. [Google Scholar] [CrossRef]
- Cao, Z.P.; Wang, X.Y.; Pang, Y.; Cheng, S.S.; Liu, J.Y. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 2019, 10, 5783. [Google Scholar] [CrossRef]
- Cao, Y.F.; Dong, X.Y.; Chen, X.P. Polymer-modified liposomes for drug delivery: From fundamentals to applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Gao, B.; Wang, X.Y.; Li, W.Z.; Feng, Y.K. Enzyme-responsive strategy as a prospective cue to construct intelligent biomaterials for disease diagnosis and therapy. Biomater. Sci. 2022, 10, 1883–1903. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Javanmard, R.; Raji, M. Tocosome: Novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, A.; Alipoor, A.; Abadi, M.; Khorasani, S.; Mohammadabadi, M.R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Esra, O.; Roksan, L.; Robert, G. Modulation of cell proliferation and gene expression by alpha-tocopheryl phosphates: Relevance to atherosclerosis and inflammation. Ann. N. Y. Acad. Sci. 2004, 1031, 405–411. [Google Scholar]
- Roksan, L.; Sonja, T.; Aileen, H. Effect of tocopheryl phosphate on key biomarkers of inflammation: Implication in the reduction of atherosclerosis progression in a hypercholesterolaemic rabbit model. Clin. Exp. Pharmacol. Physiol. 2010, 37, 587–592. [Google Scholar]
- Yasukazu, S.; Atsushi, Y.; Nobuhiko, M. Alpha-tocopheryl phosphate suppresses tumor invasion concurrently with dynamic morphological changes and delocalization of cortactin from invadopodia. Int. J. Oncol. 2009, 35, 1277–1288. [Google Scholar]
- Fariba, R.; Gholamhossein, S. Investigation of temperature-responsive tocosomal nanocarriers as the efficient and robust drug delivery system for Sunitinib malate anti-cancer drug: Effects of MW and chain length of PNIPAAm on LCST and dissolution rate. J. Pharm. Sci. 2021, 111, 1937–1951. [Google Scholar]
- Fariba, R.; Gholamhossein, S. Evaluation of a temperature-responsive magnetotocosome as a magnetic targeting drug delivery system for sorafenib tosylate anticancer drug. Heliyon 2023, 9, e21794. [Google Scholar]
- Fitzpatrick, F.A.; Wheeler, R. The immunopharmacology of paclitaxel (Taxol), docetaxel (Taxotere), and related agents. Int. Immunopharmacol. 2003, 13, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.W.; Shi, J.; Song, Y.Z.; Deng, Y.H. A novel micellar carrier to reverse multidrug resistance of tumours: TPGS derivatives with end-grafted cholesterol. J. Drug Target. 2023, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, M. Precision medicine and therapies of the future. Epilepsia 2021, 62, 90–105. [Google Scholar] [CrossRef]
- Sartaj, A.; Qamar, Z.; Qizilbash, F.F.; Md, S.; Alhakamy, N.A.; Baboota, S.; Ali, J. Polymeric nanoparticles: Exploring the current drug development and therapeutic insight of breast cancer treatment and recommendations. Polymers 2021, 13, 4400. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Y.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: Current status, challenges and opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Geng, S.A.; Guo, M.Q.; Zhan, G.T.; Shi, D.W.; Shi, L.Y.; Gan, L.; Zhao, Y.B.; Yang, X.L. NIR-triggered ligandpresenting nanocarriers for enhancing synergistic photothermal-chemotherapy. J. Control. Release 2022, 353, 229–240. [Google Scholar] [CrossRef]
- Van de Ven, H.; Paulussen, C.; Feijens, P.B.; Matheeussen, A.; Rombaut, P.; Kayaert, P.; Van den Mooter, G.; Weyenberg, W.; Cos, P.; Maes, L.; et al. PLGA nanoparticles and nanosuspensions with amphotericin B: Potent in vitro and in vivo alternatives to Fungizone and AmBisome. J. Control. Release 2012, 161, 795–803. [Google Scholar] [CrossRef]
- Mishra, M.K.; Beaty, C.A.; Lesniak, W.G.; Kambhampati, S.R.; Zhang, F.; Wilson, M.A.; Blue, M.E.; Troncoso, J.C.; Kannan, S.; Johnston, M.V.; et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 2014, 8, 2134. [Google Scholar] [CrossRef]
- Zhang, F.; Magruder, J.T.; Lin, Y.A.; Crawford, T.C.; Grimm, J.C.; Sciortino, C.M.; Wilson, M.A.; Blue, M.E.; Kannan, S.; Johnston, M.V.; et al. Generation-6 hydroxyl PAMAM dendrimers improve CNS penetration from intravenous administration in a large animal brain injury model. J. Control. Release 2017, 249, 173. [Google Scholar] [CrossRef]
- Srinageshwar, B.; Peruzzaro, S.; Andrews, M.; Johnson, K.; Hietpas, A.; Clark, B.; McGuire, C.; Petersen, E.; Kippe, J.; Stewart, A.; et al. PAMAM Dendrimers Cross the Blood-Brain Barrier When Administered through the Carotid Artery in C57BL/6J Mice. Int. J. Mol. Sci. 2017, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.X.; Wu, S.; Lin, J.Q.; Cheng, L.T.; Zhou, J.; Xie, J.; Huang, K.X.; Wang, X.Y.; Yu, Y.; Chen, Z.B.; et al. Nanoparticles targeted against cryptococcal pneumonia by interactions between chitosan and its peptide ligand. Nano Lett. 2018, 18, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cui, X.; Cai, S.; Zou, X.; Zheng, S.; Li, Y.; Zhang, Z. Multifunctional phytochemical na noplatform for comprehensive treatment of all-stage MRSA biofilm associated infection and its accompanying inflammation. Chem. Eng. J. 2024, 480, 147951. [Google Scholar] [CrossRef]
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.K.; Islam, T.; Boyer, J.A.; Boué, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-selectin-targeted nanocarriers induce active crossing of the blood-brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023, 22, 391–399. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, L.; Bai, S.; Yang, H.; Cui, Z.; Zhang, X.; Li, Y. Advances of molecularly imprinted polymers (MIP) and the application in drug delivery. Eur. Polym. J. 2021, 143, 110179. [Google Scholar] [CrossRef]
- Hemmati, K.; Masoumi, A.; Ghaemy, M. Tragacanth gum-based nanogel as a superparamagnetic molecularly imprinted polymer for quercetin recognition and controlled release. Carbohydr. Polym. 2016, 136, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Suzuki, K.; Cho, H.; Youn, Y.S.; Bae, Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano 2018, 12, 8893–8900. [Google Scholar] [CrossRef]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K.; et al. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, D.; Kabanov, V. Polymeric micelles for the delivery of poorly soluble drugs: From nano-formulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Owens, E.; Peppasn, A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Albayaty, Y.N.; Thomas, N.; Ramirez-Garcia, P.D.; Davis, T.P.; Quinn, J.F.; Whittaker, M.R.; Prestidge, C.A. pH-Responsive copolymer micelles to enhance itraconazole efficacy against Candida albicans biofilms. J. Mater. Chem. 2020, 8, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Ronghui, Q. Polymer micelles for the protection and delivery of specialized proresolving mediators. Eur. J. Pharm. Biopharma. 2023, 184, 159–169. [Google Scholar]
- Zhang, X.; Xu, X.Y.; Wang, X.Y.; Lin, Y.J.; Zheng, Y.L.; Xu, W.; Liu, J.; Xu, W. Hepatoma-targeting and reactive oxygen species-responsive chitosan-based polymeric micelles for delivery of celastrol. Carbohydr. Polym. 2023, 303, 120439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Gao, X.L.; Gu, G.Z.; Rang, T.; Tu, Y.F.; Liu, Z.Y.; Song, Q.X.; Yao, L.; Pang, Z.Q.; Jiang, X.G.; et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG-PLA nanoparticles loaded with paclitaxel. Biomaterials 2013, 34, 5640–5650. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.J.; Zou, L.L.; Yu, H.J.; He, X.Y.; Cao, H.Q.; Zhang, Z.W.; Yin, Q.; Zhang, P.C.; Gu, W.W.; Chen, L.L.; et al. Treatment of malignant brain tumor by tumor-triggered programmed wormlike micelles with precise targeting and deep penetration. Adv. Funct. Mater. 2016, 26, 4201–4212. [Google Scholar] [CrossRef]
- Yu, L.L.; Bao, H.C. Research on the application of micelles in traditional Chinese medicine preparations. Shandong Chem. Ind. 2018, 47, 58–60. [Google Scholar]
- Gao, W.W.; Zhang, Y.; Zhang, Q.Z.; Zhang, L.F. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, W.; Wang, Z.; Zhu, Y.; Zhao, H.X.; Wu, K.; Wu, J.; Zhang, W.H.; Zhang, Q.; Guo, H.Q.; et al. NIR-II light powered asymmetric hydrogel nanomotors for enhanced immunochemotherapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202212866. [Google Scholar] [CrossRef]
- Li, S.S.; Li, X.Y.; Xu, Y.D.; Fan, C.R.; Li, Z.A.; Zheng, L.; Luo, B.C.; Li, Z.P.; Lin, B.F.; Zha, Z.G.; et al. Collagen fibril-like injectable hydrogels from self-assembled nanoparticles for promoting wound healing. Bioact. Mater. 2024, 32, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Shen, F.Y.; Tian, L.L.; Tao, H.Q.; Xiong, Z.J.; Xu, J.; Liu, Z. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, H.; Peng, L.; Gao, X.; Jiang, S. Development of PLGA-PEG-PLGA hydrogel delivery system for enhanced immunoreaction and efficacy of newcastle disease virus DNA vaccine. Molecules 2020, 25, 2505. [Google Scholar] [CrossRef]
- Shang, L.; Liu, J.; Wu, Y.T.; Wang, M.; Fei, C.Z.; Liu, Y.C.; Xue, F.Q.; Zhang, L.F.; Gu, F. Peptide supramolecular hydrogels with sustained Release Ability for Combating Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2023, 15, 26273–26284. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.; Al-Mahmood, S.M.A.; Chatterjee, B.; Sulaiman, W.M.A.W.; Elsayed, T.M.; Doolaanea, A. Encapsulation of black seed oil in alginate beads as a ph-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Li, j.; Liu, W.; Wang, J.; Li, J.; Liu, M.; Bo, R. Application prospect of new drug delivery system for oral administration. Prog. Vet. Med. 2023, 44, 121–126. [Google Scholar]
- Adapun, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ding, H.T.; Tan, P.; Fu, S.Q.; Tian, X.H.; Zhang, H.; Ma, X.L.; Gu, Z.W.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Akhter, S.; Mahdi, W.; Kazi, M.; Ahmad, J. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: Preclinical promise and translational challenges. Pharmaceutics 2020, 13, 24. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zheng, R.R.; Liu, L.S.; Chen, X.Y.; Guan, R.; Yang, N.; Chen, A.; Yu, X.Y.; Cheng, H.; Li, S.Y. Self-delivery oxidative stress amplifier for chemotherapy sensitized immunotherapy. Biomaterials 2021, 275, 120970. [Google Scholar] [CrossRef]
- Gong, X.J.; Zhang, Q.Y.; Gao, Y.F.; Shuang, S.M.; Choi, M.M.F.; Dong, C. Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging. ACS Appl. Mater. Interfaces 2016, 8, 11288–11297. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, L.L.; Wang, C.H.; Han, Y.; Lu, Y.L.; Liu, J.; Hu, X.C.; Yao, T.M.; Lin, Y.; Liang, S.J.; et al. Tumor-targeted drug and CpG delivery system for phototherapy and docetaxel-enhanced immunotherapy with polarization toward M1-type macrophages on triple negative breast cancers. Adv. Mater. 2019, 31, e1904997. [Google Scholar] [CrossRef] [PubMed]
- Raposo, L.R.; Roma-Rodrigues, C.; Jesus, J.; Martins, L.M.D.R.S.; Pombeiro, A.J.; Baptista, P.V.; Fernandes, A.R. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet. Comp. Oncol. 2017, 15, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.J.; Zhang, X.J.; Li, C.X.; Huang, Y.; Ding, Q.Q.; Pang, X.F. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings onTiO2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Mansourpanah, Y. Construction of chitosan-carboxymethyl β-cyclodextrin silver nanocomposite hydrogel to improve antibacterial activity. Plast. Rubber Compos. 2018, 47, 273–281. [Google Scholar] [CrossRef]
- Liu, B.L.; Liu, D.Y.; Chen, T.B.; Wang, X.H.; Xiang, H.; Wang, G.; Cai, R.J. ITRAQ-based quantitative proteomic analysis of the antibacterial mechanism of silver nanoparticles against multidrug-resistant Streptococcus suis. Front. Microbiol. 2023, 14, 1293363. [Google Scholar] [CrossRef] [PubMed]
- Kojouri, G.; Arbabi, F.; Mohebbi, A. The effects of selenium nanoparticles (SeNPs) on oxidant and antioxidant activities and neonatal lamb weight gain pattern. Comp. Clin. Pathol. 2020, 29, 369–374. [Google Scholar] [CrossRef]
- Kalinska, A.; Jaworski, S.; Wierzbicki, M.; Golebiewski, M. Silver and copper nanoparticles-an alternative in future mastitis treatment and prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef]
- Rodrigues, B.D.; Arora, S.; Kanekiyo, T.; Singh, J. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res. 2020, 1734, 146738. [Google Scholar] [CrossRef]
- Wang, K.; Shang, F.; Chen, D.; Cao, T.L.; Wang, X.W.; Jiao, J.P.; He, S.L.; Liang, X.F. Protein liposomes-mediated targeted acetylcholinesterase gene delivery for effective liver cancer therapy. J. Nanobiotechnol. 2021, 19, 31. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.Y.; Lin, Y.L.; Pacardo, D.B.; Wang, C.; Sun, W.J.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Ibrahim, M.F.; Alanazi, F.; Shazly, G.A. Engineering erythrocytes as a novel carrier for the targeted delivery of the anticancer drug paclitaxel. Saudi Pharm. J. 2014, 22, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.R.; He, C.S.; Hao, Y.Y.; Wang, L.L.; Li, L.; Zhu, G.Q. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Gu, Z. Bioinspired and biomimetic nanomedicines. Acc. Chem. Res. 2019, 52, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, X.; Li, C. Advances in anti-invasive fungal drug delivery systems. J. Zhejiang Univ. Med. Sci. 2023, 52, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.F.; Wang, M.; Chen, M.; Chen, Z.; Peng, X.; Zhou, F.F.; Song, J.; Qu, J.L. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 2020, 254, 120142. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.Y.; Jiang, X.C.; Gao, J.Q. Cell membrane-coated nanoparticles: A novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Tan, S.W.; Wu, T.T.; Zhang, D.; Zhang, Z.P. Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef]
- Tian, X.; Shi, A.; Wu, J. Construction of Biomimetic-Responsive Nanocar-riers and their Applications in Tumor Targeting. Anti-Cancer Agents Med. Chem. 2022, 22, 2255–2273. [Google Scholar]
- Guo, M.; Wang, L.; Qi, X. Advances in Red Blood Cells as Drug Delivery Systems. Chin. J. Pharm. 2023, 54, 496–503. [Google Scholar]
- Cao, Z.P.; Cheng, S.S.; Wang, X.Y.; Pang, Y.; Liu, J.Y. Camouflaging Bacteria by Wrapping with Cell Membranes. Nat. Commun. 2019, 10, 3452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, W.J.; Ye, Y.Q.; Hu, Q.Y.; Bomba, H.N.; Gu, Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 0011. [Google Scholar] [CrossRef]
- Wei, X.L.; Zhang, G.; Ran, D.N.; Krishnan, N.; Fang, R.H.; Gao, W.W.; Spector, S.A.; Zhang, L.F. T-cell-mimicking nanoparticles can neutralize HIV infectivity. Adv. Mater. 2018, 30, e1802233. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Zhu, D.M.; Li, J.H.; Chen, X.; Xie, W.; Jiang, X.; Wu, L.; Wang, G.G.; Xiao, Y.S.; Liu, Z.S.; et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 2020, 10, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, P.; Ogunnaike, E.A.; Ahn, S.; Froehlich, K.A.; Jansson, A.; Ligler, F.S.; Dotti, G.; Brudno, Y. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T Cells. Nat. Biotechnol. 2022, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.J.; Lee, J.P.; Bourne, C.M.; Wijewarnasuriya, D.; Kinarivala, N.; Kurtz, K.G.; Corless, B.C.; Dacek, M.M.; Chang, A.Y.; Mo, G.; et al. Engineering CAR-T cells to activate small-molecule drugs in situ. Nat. Chem. Biol. 2021, 18, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, J.; Hu, Y.; Miao, W.; Huang, H. Wrapping collagen-based nanoparticle with macrophage membrane for treating multidrug-resistant bacterial infection. J. Leather Sci. Eng. 2022, 4, 31. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Fan, Y.; Wang, Z.Q.; Li, G.M.; Zhan, M.S.; Gong, J.L.; Majoral, J.P.; Shi, X.Y.; Shen, M.W. Chemotherapy mediated by biomimetic polymeric nanoparticles potentiates enhanced tumor immunotherapy via amplification of endoplasmic reticulum stress and mitochondrial dysfunction. Adv. Mater. 2022, 34, e2206861. [Google Scholar] [CrossRef]
- Harris, C.; Scully, A.; Day, S. Cancer cell membrane-coated nanoparticles for cancer management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Wang, L.; Zhang, Z.; Zhang, W. Biomimetic cell membrane-coated nanocarriers for targeted siRNA delivery in cancer therapy. Drug Discov. Today 2023, 28, 103514. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cai, L.B.; Guo, Y.H.; Chen, J.Y.; Gao, Q.L.; Yang, J.X.; Li, Y.F. Cancer cell membrane-decorated zeolitic-imidazolate frameworks delivering cisplatin and oleanolic acid induce apoptosis and reversed multidrug resistance on bladder carcinoma cells. ACS Omega 2020, 5, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.; Stevens, M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.L.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.; Batrakova, E. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC)therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.M.; Lopes, D.; Lopes, J.; Yousefiasl, S.; Macário-Soares, A.; Peixoto, D.; Ferreira-Faria, I.; Veiga, F.; Conde, J.; Huang, Y.; et al. Review exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter 2023, 6, 761–799. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Y.; Li, X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J. Drug Target. 2020, 28, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.B.; Tang, H.L.; Li, Q.J.; Chen, G.P.; Li, D. Exosomes and their roles in the chemoresistance of pancreatic cancer. Cancer Med. 2022, 11, 4979–4988. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Rajadas, J.; Seifalian, A. Exosomes as nano-theragnostic delivery platforms for gene therapy. Adv. Drug Deliv. Rev. 2013, 65, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Depalo, N.; Tutino, V.; De Nunzio, V.; Ingrosso, C.; Rizzi, F.; Notarnicola, M.; Curri, M.L.; Giannelli, G. Exosomes for diagnosis and therapy in gastrointestinal cancers. Int. J. Mol. Sci. 2020, 21, 367. [Google Scholar] [CrossRef]
- Wu, Y.F.; Zhang, F.; Wang, K.; Luo, P.C.; Wei, Y.Q.; Liu, S.Q. Activatable fluorescence imaging and targeted drug delivery via extracellular vesicle-like porous coordination polymer nanoparticles. Anal. Chem. 2019, 91, 14036–14062. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Liu, T.Q.; Zhou, M.J. Immune-cell-derived exosomes for cancer therapy. Mol. Pharm. 2022, 19, 3042–3056. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.J.; Li, H.; Xu, G.; Hu, Y.X.; Zhang, W.G.; Tian, K. Current knowledge and future perspectives of exosomes as nanocarriers in diagnosis and treatment of diseases. Int. J. Nanomed. 2023, 18, 4751–4778. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119952. [Google Scholar] [CrossRef] [PubMed]
- Lucio, B. Extracellular vesicles as bridges between host immune cells and graft organ during cellular rejection. JACC Basic Transl. Sci. 2023, 8, 457–459. [Google Scholar]
- Godbole, N.; Quinn, A.; Carrion, F.; Pelosi, E.; Salomon, C. Extracellular vesicles as a potential delivery platform for CRISPR-Cas based therapy in epithelial ovarian cancer. Semin. Cancer Biol. 2023, 96, 64–81. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, C. Exosomes: Cell created drug delivery systems. Mol. Cell. Biochem. 2019, 495, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Z.Y.; Wei, X.W.; Gao, G.P.; Wei, Y.Q. Challenges in CRISPR/CAS9 delivery: Potential roles of nonviral vectors. Hum. Gene Ther. 2015, 26, 452–462. [Google Scholar] [CrossRef]
- Blenke, E.O.; Evers, M.J.W.; Mastrobattista, E.; van der Oost, J. CRISPR-Cas9 gene editing: Delivery aspects and therapeutic potential. J. Control. Release 2016, 244, 139–148. [Google Scholar] [CrossRef]
- Cheng, R.R.; Peng, J.; Yan, Y.H.; Cao, P.L.; Wang, J.W.; Qiu, C.; Tang, L.C.; Liu, D.; Tang, L.; Jin, J.P.; et al. Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9. FEBS Lett. 2014, 588, 3954–3958. [Google Scholar] [CrossRef]
- Yin, H.; Song, C.Q.; Dorkin, J.R.; Zhu, L.H.J.; Li, Y.X.; Wu, Q.Q.; Park, A.; Yang, J.; Suresh, S.; Bizhanova, A.; et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 2016, 34, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an aav capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.N.; Schubert, R.; Simic, B.; Brücher, D.; Schmid, M.; Kirk, N.; Freitag, P.C.; Gradinaru, V.; Plückthun, A. The shread gene therapy platform for paracrine delivery improves tumor localization and intratumoral effects of a clinical antibody. Proc. Natl. Acad. Sci. USA 2021, 118, e2017925118. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.P.; van Gogh, M.; Freitag, P.C.; Kast, F.; Nagy-Davidescu, G.; Borsig, L.; Plückthun, A. FAP-retargeted Ad5 enables in vivo gene delivery to stromal cells in the tumor microenvironment. Mol. Ther. 2023, 31, 2914–2928. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Huang, H.W.; Sun, M.C.; Zhang, R.Z.; Wang, J.X.; Zheng, H.Q.; Zhu, C.J.; Yang, S.H.; Shen, X.Y.; Shi, J.Q.; et al. Inhibition of tumor metastasis by liquid-nitrogen-shocked tumor cells with oncolytic viruses infection. Adv. Mater. 2023, 35, 2212210. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Y.; Lu, Z.; Zhang, T.; Zhang, X. Research status and trends of CRISPR delivery systems. Chin. J. Pharm. 2018, 49, 1041–1052. [Google Scholar]
- Raman, V.; Van Dessel, N.; Hall, C.L.; Wetherby, V.E.; Whitney, S.A.; Kolewe, E.L.; Bloom, S.M.K.; Sharma, A.; Hardy, J.A.; Bollen, M.; et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat. Commun. 2021, 12, 6116. [Google Scholar] [CrossRef] [PubMed]
- Geriatric, R.; Arabian, N.; Danino, T. Engineering bacteria as interactive cancer therapies. Science 2022, 378, 858–864. [Google Scholar]
- Li, Z.T.; Wang, Y.X.; Liu, J.; Rawding, P.; Bu, J.Y.; Hong, S.P.; Hu, Q.Y. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy. Adv. Mater. 2021, 33, e2102580. [Google Scholar] [CrossRef]
- Luo, C.H.; Huang, C.T.; Su, C.H.; Yeh, C.S. Bacteria mediated hypoxia-specific delivery of nanoparticles for tumors imaging and therapy. Nano Lett. 2016, 16, 3493–3499. [Google Scholar] [CrossRef]
- Kowalski, C.H.; Morelli, K.A.; Schultz, D.; Nadell, C.D.; Cramer, R.A. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 22473–22483. [Google Scholar] [CrossRef] [PubMed]
- Grahl, N.; Puttikamonkul, S.; Macdonald, J.M.; Gamcsik, M.P.; Ngo, L.Y.; Hohl, T.M.; Cramer, R.A. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011, 7, e1002145. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Y.; Hogan, A.; Melonakos, E. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Lala, H.C.; Shepherd, M.G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 1990, 58, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; McNab, R.; Jenkinson, H.F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 1996, 64, 4680–4685. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.; Gopal, K.; Jenkinson, F. Adherence of Candida albicans to a cell surface polysaccharide receptor on Streptococcus gorgoniid. Infect. Immun. 1995, 63, 1827–1834. [Google Scholar] [CrossRef]
- Ocana, S.; Nader, M.E. Vaginal lactobacilli: Self- and co-aggregating ability. Br. J. Biomed. Sci. 2002, 59, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Desai, J.; Rosenthal, M.; McArthur, G.A.; Pattison, S.T.; Pattison, S.L.; MacDiarmid, J.; Brahmbhatt, H.; Scott, A.M. A first-time-in-human phase I clinical trial of bispecific antibody-targeted, paclitaxel-packaged bacterial minicells. PLoS ONE 2015, 10, e0144559. [Google Scholar] [CrossRef]
- Gao, W.W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.M.; Angsantikul, P.; Zhang, Q.Z.; Hu, C.M.J.; Zhang, L.F. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cao, Z.P.; Zhang, M.M.; Meng, L.; Ming, Z.Z.; Liu, J.Y. Bioinspired oral delivery of gut microbiota by self-coating with biofilms. Sci. Adv. 2020, 6, eabb1952. [Google Scholar] [CrossRef]
- Segel, M.; Lash, B.; Song, J.W.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein peg10 packages its own mrna and can be pseudotyped for mrna delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Friedrich, M.J.; Guru, A.; Lash, B.; Saito, M.; Macrae, R.K.; Zhang, F. Programmable protein delivery with a bacterial contractile injection system. Nature 2023, 616, 357–364. [Google Scholar] [CrossRef]
- Angsantikul, P.; Thamphiwatana, S.; Gao, W.W.; Zhang, L.F. Cell membrane-coated nanoparticles as an emerging antibacterial vaccine platform. Vaccines 2015, 3, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Wu, M.; Chen, J.L.; Liu, Y.F.; Chen, Y.R.; Fan, G.L.; Liu, Y.Y.; Cheng, J.; Wang, Z.H.; Wang, S.X.; et al. Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Zhang, M.H.; Cheng, S.S.; Jin, Y.; Zhang, N.; Wang, Y. Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics. Clin. Transl. Med. 2021, 11, e292. [Google Scholar] [CrossRef]
- Kesireddy, M.; Kothapalli, S.R.; Gundepalli, S.G.; Asif, S. A review of the current FDA-approved antibody-drug conjugates: Landmark cclinical trials and indications. Pharm. Med. 2024, 38, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lee, J.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Edupuganti, V.V.S.R.; Hsu, K.S.; Dower, C.; Yu, G.; et al. Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability. Cell Rep. 2023, 42, 113503. [Google Scholar] [CrossRef]
- Xu, C.L.; Zhu, M.; Wang, Q.; Cui, J.J.; Huang, Y.P.; Huang, X.T.; Huang, J.; Gai, J.W.; Li, G.H.; Qiao, P.; et al. TROP2-directed nanobody-drug conjugate elicited potent antitumor effect in pancreatic cancer. J. Nanobiotechnology 2023, 21, 410. [Google Scholar] [CrossRef]
- Brown, K.M.; Nair, J.K.; Janas, M.M.; Anglero-Rodriguez, Y.I.; Dang, L.T.H.; Peng, H.Y.; Theile, C.S.; Castellanos-Rizaldos, E.; Brown, C.; Foster, D.; et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 2022, 40, 1500–1508. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.B.; Cai, K.M.; He, H.; Liu, Y.; Yen, J.; Wang, Z.Y.; Xu, M.; Sun, Y.W.; Zhou, X.; et al. Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat. Chem. Biol. 2017, 13, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Peplow, M. ‘Clicked’ drugs: Researchers prove the remarkable chemistry in humans. Nat. Biotechnol. 2023, 41, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.M.; Zhao, H.M.; Liu, Y.H.; Wu, H.; Liu, C.; Chang, S.Y.; Chen, L.Q.; Jin, M.J.; Wang, Q.M.; Gao, Z.G.; et al. Research advances in peptide–drug conjugates. Acta Pharm. Sin. B 2023, 13, 3659–3677. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, J.J.; Ruokoranta, T.; Ikonen, V.; Huppunen, M.E.; Acs, K.; Lehmann, F.; Heckman, C.A. The novel peptide drug conjugate OPDC3 is highly active in different hematological malignancies. Blood 2022, 140, 10697–10698. [Google Scholar] [CrossRef]
- Gao, L.P.; Wang, H.; Nan, L.J.; Peng, T.; Sun, L.; Zhou, I.G.; Xiao, Y.; Wang, J.; Sun, J.H.; Lu, W.Y.; et al. Erythrocyte membrane-wrapped pH sensitive polymeric nanoparticles for non-small cell lung cancer therapy. Bioconjugate Chem. 2017, 28, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.N.; Bains, A.; Hu, J.; Gong, Q.Y.; Luo, K.; Gu, Z.W. Enzyme-sensitive and amphiphilic PEGylated dendrimer-paclitaxel prodrug-based-nanoparticles for enhanced stability and anticancer efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.R.; Chen, Y.Y.; Cheng, Z.Y.; Deng, K.R.; Ma, P.A.; Hou, Z.Y.; Liu, B.; Huang, S.S.; Jin, D.Y.; Lin, J. Rational design of a comprehensive cancer therapy platform using temperature-sensitive polymer grafted hollow gold nanospheres: Simultaneous chemo/photothermal/photodynamic therapy triggered by a 650 nm laser with enhanced anti-tumor efficacy. Nanoscale 2016, 8, 6837–6850. [Google Scholar] [CrossRef]
- Feng, P.; Cao, Z.; Wang, X.; Li, J.; Liu, J. On-demand bacterial reactivation by restraining within a triggerable nanocoating. Adv. Mater. 2020, 32, 2002406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, S.J.; Pei, W.J.; Huang, B.Y.; Niu, C.C. Magnetically targeted erythrocyte membrane coated nanosystem for synergistic photothermal/chemotherapy of cancer. J. Mater. Chem. B 2020, 8, 4132–4142. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Hao, J.; Lee, L.A.; Biewer, M.C.; Wang, Q.; Stefan, M.C. Thermally controlled release of anticancer drug from self-assembled γ-substituted amphiphilic poly (ε-caprolactone) micellar nanoparticles. Biomacromolecules 2012, 13, 2163–2173. [Google Scholar] [CrossRef]
- Huang, J.; Shu, Q.; Wang, L.Y.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.X.; Cheng, D.; Jiang, G.Y.; Xing, W.Q.; Liu, P.W.; Wang, B.; Zhu, W.P.; Sun, H.T.; Sun, Z.R.; Xu, Y.F.; et al. Engineering naphthalimide-cyanine integrated near-infrared dye into ROS-responsive nanohybrids for tumor PDT/PTT/chemotherapy. Bioact. Mater. 2022, 14, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, X.X.; Song, L.N.; Cui, H.T.; Myers, J.N.; Bai, T.T.; Zhou, Y.; Chen, Z.; Gu, N. Controlled drug release and hydrolysis mechanism of polymer-magnetic nanoparticle composite. ACS Appl. Mater. Interfaces 2015, 7, 9410–9419. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Sun, J.F.; Guo, Z.B.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magneto thermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Panahifar, A.; Adeli, M.; Amiri, H.; Lascialfari, A.; Orsini, F.; Doschak, M.R.; Mahmoudi, M. Synthesis of pseudopolyrotaxanes-coated superparamagnetic iron oxide nanoparticles as new MRI contrast agent. Colloids Surf. B 2013, 103, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Guo, J.; He, C.; Geng, H.; Yu, G.; Li, J.; Zheng, H.; Ji, X.; Yan, F. Ultrasound triggered image-guided drug delivery to inhibit vascular reconstruction via paclitaxel-loaded microbubbles. Sci. Rep. 2016, 6, 21683. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, L.; Mou, L.; Dong, K.; Jiang, J.; Xue, S.; Xu, Y.; Wang, X.; Lu, Y.; Ye, H. A green tea-triggered genetic control system for treating diabetes in mice and monkeys. Sci. Transl. Med. 2019, 11, eaav8826. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, N.; Zhou, M.; Zhang, T.; Tian, T.; Li, S.; Tang, Z.; Lin, Y.; Cai, X. DNA nanorobot delivers antisense oligonucleotides silencing c-Met gene expression for cancer therapy. J. Biomed. Nanotechnol. 2019, 15, 1948–1959. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Xu, X.; Xu, K.; Zhang, M.; Huang, K.; Kang, H.; Lin, H.C.; Yang, Y.; Han, D. An intelligent DNA nanorobot for autonomous anticoagulation. Angew. Chem. Int. Ed. Engl. 2020, 59, 17697–17704. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Peng, R.; Peng, Y.; Deng, Z.; Xu, F.; Su, Y.; Wang, P.; Li, L.; Wang, X.Q.; Ke, Y.; et al. Hierarchical fabrication of DNA wireframe nanoarchitectures for efficient cancer imaging and targeted therapy. ACS Nano 2020, 14, 17365–17375. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.M.; Patel, G.C.; Patel, R.B.; Patel, J.K.; Patel, M. Nanorobot: A versatile tool in nanomedicine. J. Drug Target. 2006, 14, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A dual-targeted organic photothermal agent for enhanced photothermal therapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Han, X.; Hu, Y.; Luo, Y.; Chen, C.H.; Chen, Z.; Shi, P. A remotely controlled transformable soft robot based on engineered cardiac tissue construct. Small 2019, 15, e1900006. [Google Scholar] [CrossRef] [PubMed]
- Yasa, O.; Erkoc, P.; Alapan, Y.; Sitti, M. Microalga-powered microswimmers toward active cargo delivery. Adv. Mater. 2018, 30, e1804130. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.S.; Alshareef, A.; Hwang, A.V.; Kang, Z.L.; Kuosmanen, J.; Ishida, K.; Jenkins, J.; Liu, S.; Madani, W.A.M.; Lennerz, J.; et al. RoboCap: Robotic mucus-clearing capsule for enhanced drug delivery in the gastrointestinal tract. Sci. Robot. 2022, 7, eabp9066. [Google Scholar] [CrossRef] [PubMed]
- Holley, M.T.; Nagarajan, N.; Danielson, C.; Zorlutuna, P.; Park, K. Development and characterization of muscle-based actuators for self-stabilizing swimming biorobots. Lab Chip 2016, 16, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/nanorobots at work in active drug delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Jing, X.; Pan, M.; Liu, P.; Li, W.; Zhu, B.; Li, J.; Chen, H.; Wang, L.; et al. Complex silica composite nanomaterials templated with DNA origami. Nature 2018, 559, 593–598. [Google Scholar] [CrossRef]
- Agarwal, N.P.; Matthies, M.; Gür, F.N.; Osada, K.; Schmidt, T.L. Block Copolymer Micellization as a Protection Strategy for DNA Origami. Angew. Chem. Int. Ed. Engl. 2017, 56, 5460–5464. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Gray, M.A.; Xuan, S.; Lin, Y.; Byrnes, J.; Nguyen, A.I.; Todorova, N.; Stevens, M.M.; Bertozzi, C.R.; Zuckermann, R.N.; et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc. Natl. Acad. Sci. USA 2020, 117, 6339–6348. [Google Scholar] [CrossRef] [PubMed]
- Bastings, M.M.C.; Anastassacos, F.M.; Ponnuswamy, N.; Leifer, F.G.; Cuneo, G.; Lin, C.; Ingber, D.E.; Ryu, J.H.; Shih, W.M. Modulation of the cellular uptake of DNA origami through control over mass and shape. Nano Lett. 2018, 18, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ge, Z.; Im, H.J.; England, C.G.; Ni, D.; Hou, J.; Zhang, L.; Kutyreff, C.J.; Yan, Y.; Liu, Y.; et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat. Biomed. Eng. 2018, 2, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Komal, A.; Noreen, M.; El-Kott, A.F. TLR3 agonists: RGC100, ARNAX, and poly-IC: A comparative review. Immunol. Res. 2021, 69, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, F.; Kick, B.; Behler, K.L.; Honemann, M.N.; Weuster-Botz, D.; Dietz, H. Biotechnological mass production of DNA origami. Nature 2017, 552, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Cima, L.G.; Cima, M.J. Preparation of medical devices by solid free-form fabrication methods. Robot. Comput.-Integr. Manuf. 1996, 12, 371. [Google Scholar]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free-form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Wojtyłko, M.; Lamprou, D.A.; Froelich, A.; Kuczko, W.; Wichniarek, R.; Osmałek, T. 3D-printed solid oral dosage forms for mental and neurological disorders: Recent advances and future perspectives. Expert Opin. Drug Deliv. 2023, 11, 1–19. [Google Scholar] [CrossRef]
- Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D printed punctal plugs for controlled ocular drug delivery. Pharmaceutics 2021, 13, 1421. [Google Scholar] [CrossRef]
- Adhami, M.; Picco, C.J.; Detamornrat, U.; Anjani, Q.K.; Cornelius, V.A.; Robles-Martinez, P.; Margariti, A.; Donnelly, R.F.; Domínguez-Robles, J.; Larrañeta, E. Clopidogrel-loaded vascular grafts prepared using digital light processing 3D printing. Drug Deliv. Transl. Res. 2023, 14, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Hussain, C.M. 3D-printed hydrogel for diverse applications: A review. Gels 2023, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hu, Y.; Feng, H. Investigation of 3D-printed PNIPAM-based constructs for tissue engineering applications: A review. J. Mater. Sci. 2023, 58, 17727–17750. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Ostapchuk, G.; Catalano, P.N.; Hardy, J.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. 4D printing: The development of responsive materials using 3D-printing technology. Pharmaceutics 2023, 15, 2743. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, H.; Sun, T.; Wang, H. Responsive microneedles as a new platform for precision immunotherapy. Pharmaceutics 2023, 15, 1407. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Nguyen, C.N. Microneedle-mediated transdermal delivery of biopharmaceuticals. Pharmaceutics 2023, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Fitaihi, R.; Abukhamees, S.; Orlu, M.; Craig, D.Q.M. Transscleral Delivery of Dexamethasone-Loaded Microparticles Using a Dissolving Microneedle Array. Pharmaceutics 2023, 15, 1622. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Wang, Z.Z.; Aikelamu, K.; Bai, J.Y.; Shen, Q.; Gao, X.L.; Wang, M. Preparation and in vitro characterization of microneedles containing inclusion complexes loaded with progesterone. Pharmaceutics 2023, 15, 1765. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Ye, Z.; Lin, Z.; Ma, X.; He, Q. Biomedical micro-/nanomotors: From overcoming biological barriers to In vivo imaging. Adv. Mater. 2021, 33, e2000512. [Google Scholar] [CrossRef]
- Casula, L.; Pireddu, R.; Cardia, M.C.; Pini, E.; Valenti, D.; Schlich, M.; Sinico, C.; Marceddu, S.; Dragicevic, N.; Fadda, A.M. Nanosuspension-based dissolvable microneedle arrays to enhance diclofenac skin delivery. Pharmaceutics 2023, 15, 2308. [Google Scholar] [CrossRef]
- Yim, S.G.; Seong, K.Y.; Thamarappalli, A.; Lee, H.; Lee, S.; Lee, S.; Kim, S.; Yang, S.Y. Fast-embeddable grooved microneedles by shear actuation for accurate transdermal drug delivery. Pharmaceutics 2023, 15, 1966. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, T.; Lai, R.R.; Liu, Z.Y.; Luo, W.X.; Wang, Y.X.; Fu, S.J.; Chai, G.H.; Jia, J.J.; Xu, Y.H. Co-delivery of loxoprofen and tofacitinib by photothermal microneedles for rheumatoid arthritis treatment. Pharmaceutics 2023, 15, 1500. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.M.; Priya, S.; Gorantla, S.; Singhvi, G. Revolutionizing therapeutic delivery with microneedle technology for tumor treatment. Pharmaceutics 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Taberner, A.; Hogan, C.; Hunter, I.W. Needle-free jet injection using real-time controlled linear lorentz-force actuators. Med. Eng. Phys. 2012, 34, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Mizoguchi, I.; Sonoda, J.; Sakamoto, E.; Katahira, Y.; Hasegawa, H.; Watanabe, A.; Furusaka, Y.; Xu, M.; Yoneto, T.; et al. Induction of potent antitumor immunity by intradermal DNA injection using a novel needle-free pyro-drive jet injector. Cancer Sci. 2023, 114, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.L.; Fernandes, A.; Pelletier, M.; Takami, E.A.; Emery, C.; Dyer, B.; Jacoski, M.V.; Lozko, V.; Burgess, B.; Smith, R.H. Advances in large volume subcutaneous injections: A pilot tolerability study of an innovative needle-free injection platform. PDA J. Pharm. Sci. Technol. 2022, 76, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.; Murphy, M.; Bulluss, K.; D’Souza, W.; Plummer, C.; Priest, E.; Williams, C.; Sharan, A.; Fisher, R.; Pincus, S.; et al. Anti-seizure therapy with a long-term, implanted intra-cerebroventricular delivery system for drug-resistant epilepsy: A first-in-man study. Eclinicalmedicine 2020, 22, 100326. [Google Scholar] [CrossRef]
- Abramson, A.; Frederiksen, M.R.; Vegge, A.; Jensen, B.; Poulsen, M.; Mouridsen, B.; Jespersen, M.O.; Kirk, R.K.; Windum, J.; Hubálek, F.; et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol. 2022, 40, 103–109. [Google Scholar] [CrossRef]
- Zhang, D.X.; Zhong, D.N.; Ouyang, J.; He, J.; Qi, Y.C.; Chen, W.; Zhang, X.C.; Tao, W.; Zhou, M. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat. Commun. 2022, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.N.; Zhang, D.X.; Chen, W.; He, J.; Ren, C.J.; Zhang, X.C.; Kong, N.; Tao, W.; Zhou, M. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci. Adv. 2021, 7, eabi9265. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, B.; Mao, K. Analysis of R&D structure of new drug delivery systems. Chin. Biotechnol. 2024, 43, 1693–1707. [Google Scholar]
- Zhang, Z.X.; Yan, H.; Li, H.Q.; Wang, J.Z. Research progress in novel drug delivery systems for veterinary pharmaceuticals. Chin. J. Veter. Drug 2023, 57, 59–72. [Google Scholar]



| Drug Molecular | Challenges | Solutions |
|---|---|---|
| Protac (Proteolysis Targeting Chimeras) | High molecular weight, poor bioavailability, poor stability | Antibody drug conjugates (ADC) Three-dimensional printing(3DP) Transdermal preparation Implanted catheter |
| Peptides and Proteins | Immunogenicity, short half-life, | Polymeric nanoparticles (PNPs) Peptide drug conjugate (PDC) Implanted catheter |
| Anti-body | Toxicity, Immunogenicity, | Cell drug delivery systems Antibody drug conjugates (ADC) Implanted catheter |
| Nucleic acid | Extrahepatic delivery, Immunogenicity, Enzyme degradation, | Liposomal drug delivery systems Viral drug delivery systems Bioparticle drug delivery systems Coupling targeted drug delivery systems |
| Cell | Unstable drug characteristics, Poor tissue permeability | Liposomal drug delivery systems Polymeric nanoparticles (PNPs) |
| Generic Name | Trade Name | Target | Payload/Payload Class | Payload Action | Approval Year |
|---|---|---|---|---|---|
| Mirvetuximab soravtansine | ELAHERE | FRα | Maytansinoid DM4 | Folate receptor alpha | 2022 |
| Tisotumab vedotin-tftv | Tivdak | Tissue factor | MMAE/auristatin | microtubule inhibitor | 2021 |
| Loncastuximab tesirine-lpyl | Zynlonta | CD19 | SG3199/PBD dimer | DNA cleavage | 2021 |
| Belantamab mafodotin-blmf | Blenrep | BCMA | MMAF/auristatin | microtubule inhibitor | 2020, withdrawn in 2022 |
| Sacituzumab govitecan | Trodelvy | TROP2 | SN-38/camptothecin | TOP1 inhibitor | 2020 |
| Trastuzumab deruxtecan | Enhertu | HER2 | DXd/camptothecin | TOP1 inhibitor | 2019 |
| Enfortumab vedotin | Padcev | Nectin4 | MMAE/auristatin | microtubule inhibitor | 2019 |
| Polatuzumab vedotin-piiq | Polivy | CD79 | MMAE/auristatin | microtubule inhibitor | 2019 |
| Moxetumomab pasudotox | Lumoxiti | CD22 | PE38 (Pseudotox) | / | 2018 |
| Inotuzumab ozogamicin | Besponsa | CD22 | ozogamicin/calicheamicin | DNA cleavage | 2017 |
| Trastuzumab emtansine | Kadcyla | HER2 | DM1/maytansinoid | microtubule inhibitor | 2013 |
| Brentuximab vedotin | Adcetris | CD30 | MMAE/auristatin | microtubule inhibitor | 2011 |
| Gemtuzumab ozogamicin | Mylotarg | CD33 | ozogamicin/calicheamicin | DNA cleavage | 2017; 2000 |
| DDS | Drug | Advantages | Disadvantages |
|---|---|---|---|
| Liposomal DDS | protac Peptides and proteins nucleic acid cell | low toxicity biocompatibility non-immunogenicity | low drug loading poor stability high production costs potential toxic side effects |
| Nanoparticles-based DDS | protac Peptides and proteins cell | biodegradability biocompatibility low toxicity safety and efficacy | potential toxicity unclear mechanism and polymer stability |
| Polymer Micelle DDS | insoluble protac Chinese herbal monomer | stability Solubilization low toxicity | long-term safety limitations in clinical application |
| Hydrogel DDS | protac Peptides and proteins | biocompatibility Biodegradability low toxic side effects | heavily depends on the internal microenvironment |
| Inorganic Nanoparticles DDS | protac Peptides and proteins nucleic acid | bioavailability low toxic side effects tolerance | unclear toxicity biological distribution, and clearance methods |
| cell DDS | anti-body protac Peptides and proteins nucleic acid Bacteria and viruses | biocompatibility low toxicity biological functions targeting low immunogenicity | poor release control limited loading capacity |
| Extracellular Vesicle DDS | Proteins, lipids, nucleic acids, sugars, and other macromolecules | cycling stability biocompatibility biological barrier permeability | immature technology unclear side effects |
| Viral DDS | nucleic acid | high infection rate, targeting, and mature technology | one time delivery high immunogenicity safety issues limited loading capacity |
| Bacterial DDS | protac Peptides and proteins | targeting good transport ability | weak survivability imprecise colonization safety issues |
| Bioparticle DDS | nucleic acid | targeting carry mRNA | unknown half-life and pharmacokinetics potential immunogenicity |
| Coupling Targeted DDS | protac anti-body nucleic acid Peptides and proteins | targeting extrahepatic delivery biodegradable low immunogenicity solubilization organizational permeability | enzymatic degradation chemical instability poor cycle stability immunogenicity high production cost |
| Intelligent DDS | protac Peptides and proteins nucleic acid cell | precise control targeting penetrate tissues structural designability programmability biocompatibility | unclear harmacokinetics, intracellular metabolic pathways, in vivo distribution, and clearance mechanisms |
| Extracorporeal Device | protac Peptides and proteins anti-body nucleic acid cell | Targeting improve drug delivery efficiency and patient compliance | developing towards high-capacity drug delivery |
| DDS | Trade Name | Significance | Year |
|---|---|---|---|
| the spansule technology | Spansule® | the first 12-h release technology | 1952 |
| Contac® | delivering phenylpropanolamine hydrochloride and chlorpheniramine maleate | 1974 | |
| Dexedrine® | delivering dextroamphetamine sulfate | 1982 | |
| polymer | Lupron Depot® | the first long-acting injectable PLGA polymer formulation | 1989 |
| Abraxane® | drug-polymer composite nanoparticles | 2005 | |
| liposomes | Doxil® | the first PEGylated liposome | 1995 |
| Onpattro® | lipid-based nanoparticles used for the delivery of siRNA | 2018 | |
| Comiranty® | the first lipid-based nanoparticles used in COVID-19 vaccine | 2021 | |
| nanomedicine | Rapamune® | the first nanocrystal formulation | 2000 |
| Onpattro® | lipid-based nanoparticles used for the delivery of siRNA | 2018 | |
| ADC | Mylotarg® | the first antibody–drug conjugate approved for clinical use | 2009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. https://doi.org/10.3390/pharmaceutics16050674
Chen Q, Yang Z, Liu H, Man J, Oladejo AO, Ibrahim S, Wang S, Hao B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics. 2024; 16(5):674. https://doi.org/10.3390/pharmaceutics16050674
Chicago/Turabian StyleChen, Qian, Zhen Yang, Haoyu Liu, Jingyuan Man, Ayodele Olaolu Oladejo, Sally Ibrahim, Shengyi Wang, and Baocheng Hao. 2024. "Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development" Pharmaceutics 16, no. 5: 674. https://doi.org/10.3390/pharmaceutics16050674






