A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years
Abstract
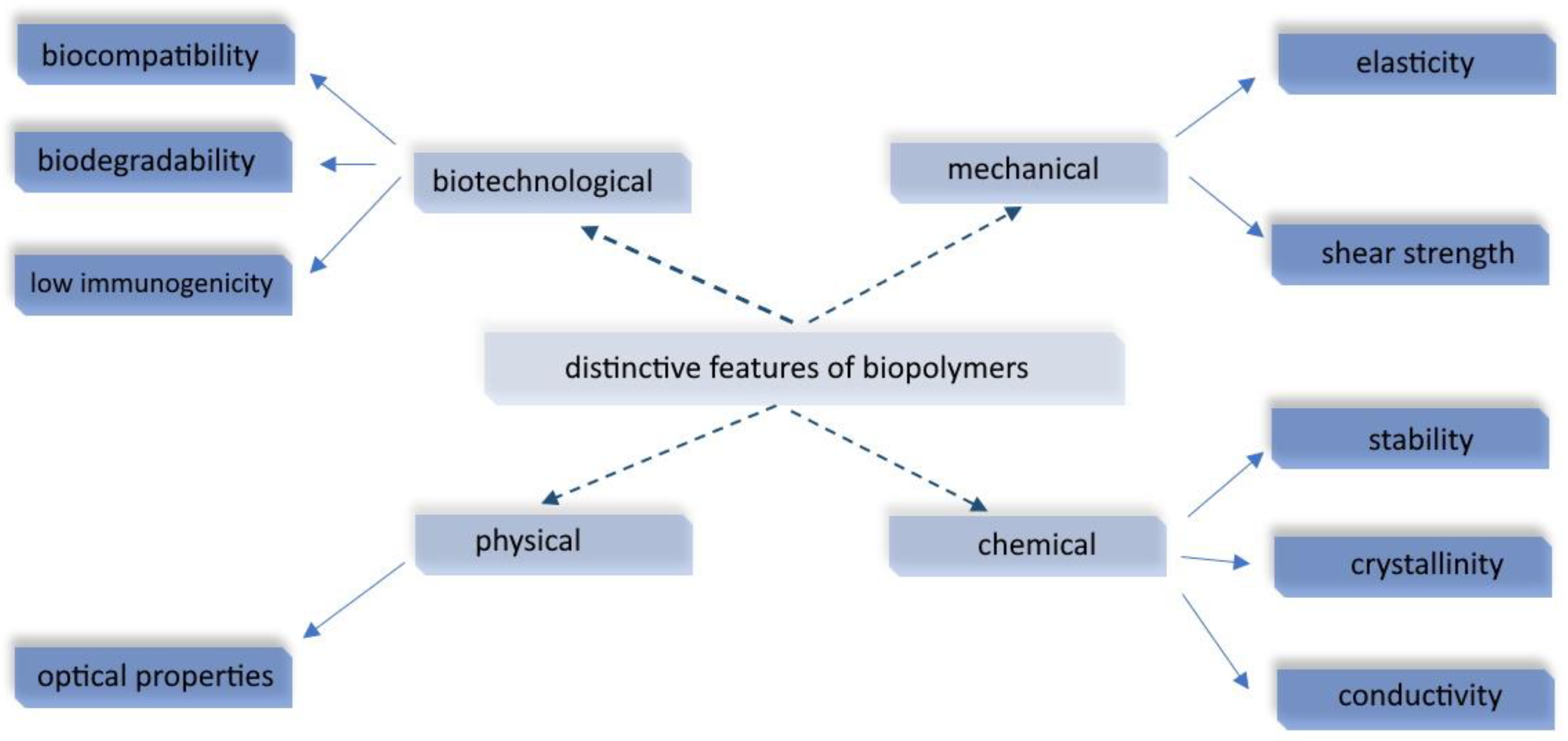
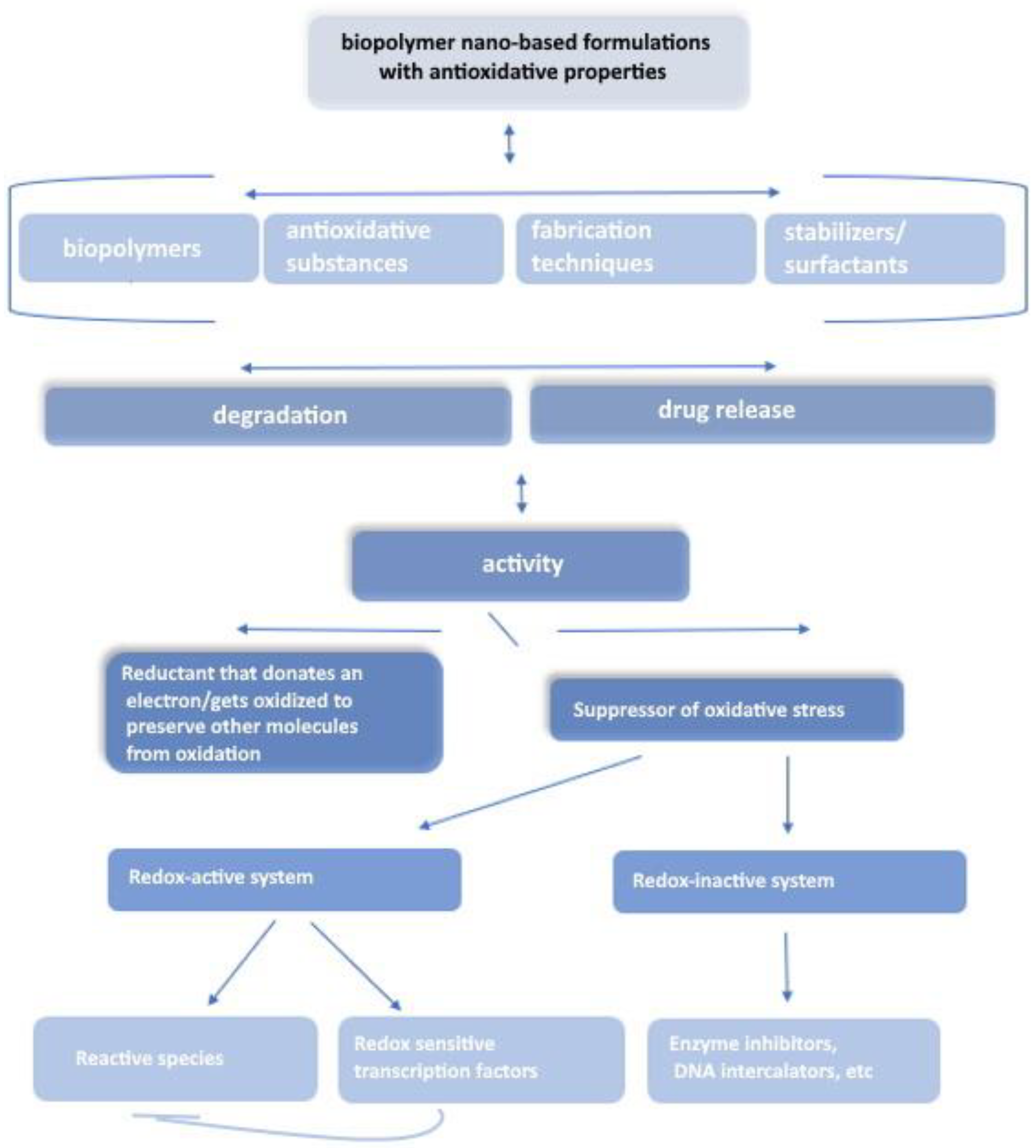
:1. Introduction
2. Polysaccharide-Based Nanoformulations
2.1. Polysaccharides
2.2. Different Formulations
2.2.1. Nanoparticles
2.2.2. Nanogels
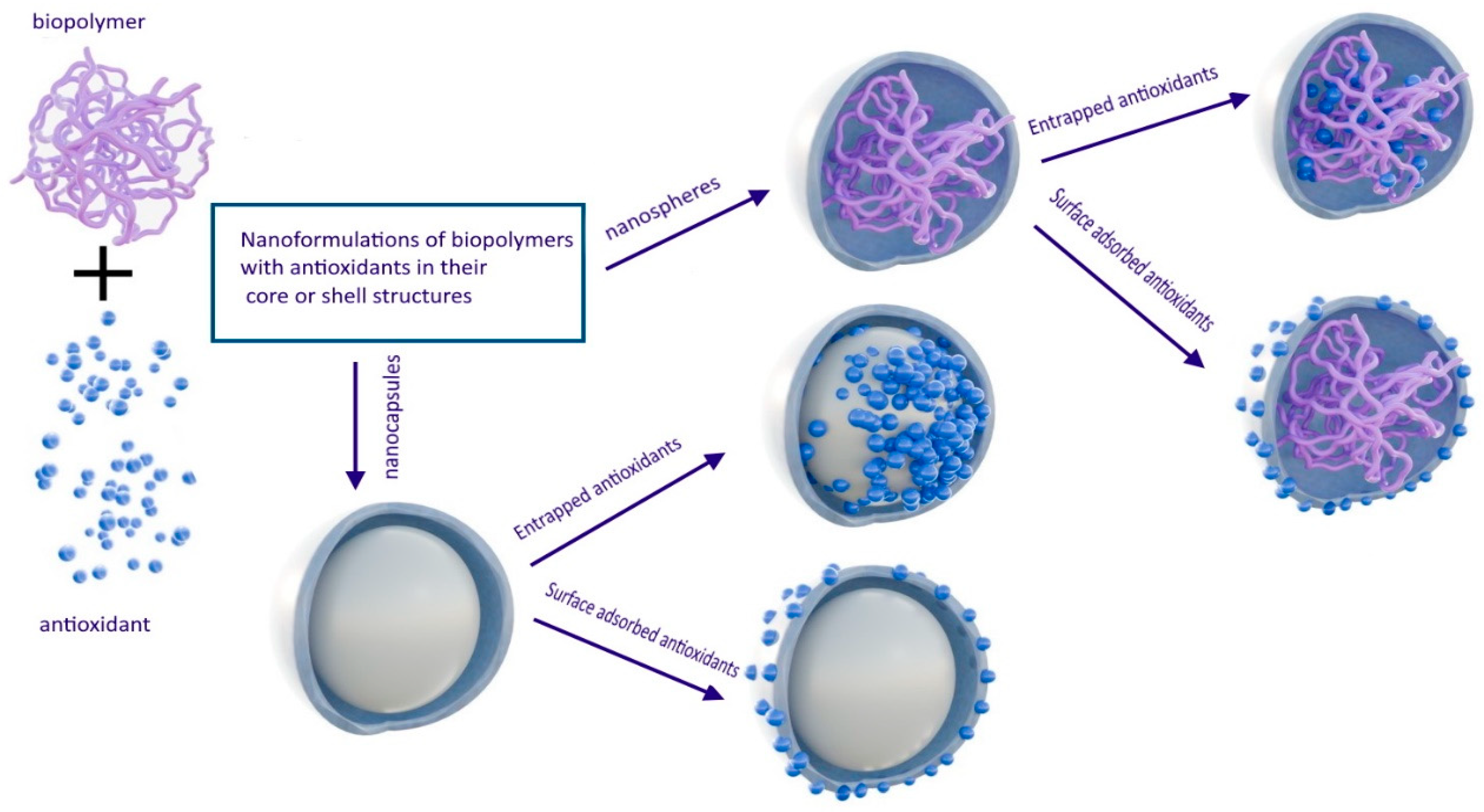
2.2.3. Nanospheres and Nanocapsules
2.2.4. Nanoemulsions
2.3. Surface Modification
2.4. Factors Influencing Degradation and Drug Release Kinetics
3. Polynucleotide-Based Nanoformulations
4. Protein-Based Nanoformulations
4.1. Nanoparticle-Based Systems
4.2. Hydrogel-Based (Nano)Formulations
4.3. Other Formulations
5. Polyester-Based Nanoformulations
| Active Agent | Form of DDS | Reported Results/Remarks | Reference | |
|---|---|---|---|---|
| Nanoparticles | tanshinone IIA | PLGA-b-PEG-OH NPs; size 92 nm | In vitro—reduction in SOD activity. In vivo (ischemic stroke pigs)—reduced cerebral swelling, lesion volume, and improved White Matter Integrity | [250] |
| epigallocatechin-3-gallate (EGCG) | PEGylated PLGA NPs, diameter 167 nm | Sustained-release profile up to 8 days. In vivo (subarachnoid hemorrhage SAH models)—superior antioxidative activity compared with unloaded EGCG. Combined with nimodipine = suppressed oxidative stress, Ca2+ overloading, mitochondrial dysfunction, and autophagy after SAH. | [251] | |
| phytol | PLGA NPs stabilized with PVA; average size 177 nm | In vitro—reducing ROS and RNS levels by activating the antioxidative defense system (superoxide dismutase and catalase) and restoring glutathione-metabolizing enzyme systems. In vivo (scopolamine-induced memory dysfunction in Wistar rats)—enhanced biodistribution and sustained release profile of phytol in the brain and plasma. | [252] | |
| rutin and the (S)-N-(2-oxo-3-oxetanyl)biphenyl-4-carboxamide derivative | PLGA NPs stabilized with poloxamer 188; average size 200 nm | In vitro—the multidrug formulation exhibited a dose-dependent enhancement of protective effect against H2O2-induced oxidative stress when compared to nanosystems containing the active compounds individually. | [253] | |
| naringin | PLGA NPs stabilized with PVA; Diameter 137 nm | Burst release in the initial 24 h followed by sustained release lasting for 10 days. DNA-binding activity was maintained after nanoencapsulation | [254] | |
| caffeic acid—covalently bonded | PHB–diethanolamine (PHB-DEA) nanoparticles; Diameter 232 nm | In vitro—powerful antioxidant capacity and bacterial inhibition. | [255] | |
| quercetin | nano-silica and PLGA nanocomposite; NPs Size 100–200 nm | In vitro and in vivo testing—potential application for cardiovascular diseases. | [256] | |
| Nanoporous formulations (membranes, films) hydrogels | niobium carbide nanosheets | Hydrogel: PLGA–PEG–PLGA triblock copolymer | In vitro and in vivo confirmation of ROS—scavenging activity/strategy for the treatment of diabetic wounds. | [257] |
| AuNPs and antimicrobial peptides (Os) | Polydopamine-modified PLGA membrane | In vitro—bactericidal and antioxidant effects. In vivo (full-thickness skin defect model in rats)—combined with electrical stimulation—acceleration of vascularization, collagen deposition, and promotion of wound healing. | [258] | |
| oregano essential oil | PLGA/gelatin-based nanofibrous membrane with spinnable bioactive glass particles | In vitro—rapid hemostasis, improved chemotactic response, antibacterial and anti-inflammatory response. In vivo (rat tail amputation model, an ear artery injury model, and a liver trauma model in rabbits)—rapid hemostasis and significant tissue regeneration. | [259] | |
| curcumin | PHB–chitosan film | In vitro drug release—sustained pattern: 32% after 12 h, 48% after 20 h, and around 69% after 36 h. | [260] | |
| ammonium derivatives of tannic acid | network mcl-PHAs containing sulfonate groups | In vitro DPPH assay—high radical scavenging activity of 82%, stable for 5 months in a buffered physiological environment. | [261] | |
| ascorbic acid | P(3HB-co-3HV) copolymer | In vitro DPPH assay—increased scavenging effect. | [262] | |
| Fibers/filaments | lignin | PLA fibers for 3D printing | In vitro DPPH assay—high radical scavenging activity of 80%. Meshes with curcumin for wound dressing. | [263] |
| mango leaf extract | PLA filament for 3D printing | In vitro DPPH assay—reduction of 88% | [264] | |
| lignin | PLA fibers, electrospinning; Diameters 314–587 nm | In vitro, DDPH and ABTS—ultrafine fibers containing 2.5% lignin exhibited the highest antioxidant activity, with around 70%. | [265] |
6. Looking Ahead
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghorbani, A. Demand for Health and Healthcare. In Healthcare Access; IntechOpen: London, UK, 2022. [Google Scholar]
- Kiriiri, G.K.; Njogu, P.M.; Mwangi, A.N. Exploring Different Approaches to Improve the Success of Drug Discovery and Development Projects: A Review. Futur. J. Pharm. Sci. 2020, 6, 27. [Google Scholar] [CrossRef]
- Kong, M.; D’Atri, D.; Bilotta, M.T.; Johnson, B.; Updegrove, T.B.; Gallardo, D.L.; Machinandiarena, F.; Wu, I.-L.; Constantino, M.A.; Hewitt, S.M.; et al. Cell-Specific Cargo Delivery Using Synthetic Bacterial Spores. Cell Rep. 2023, 42, 111955. [Google Scholar] [CrossRef]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming Barriers to Patient Adherence: The Case for Developing Innovative Drug Delivery Systems. Nat. Rev. Drug Discov. 2023, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, P.C.; Ezeako, E.C.; Okpara, J.O.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Rana, A.; Adhikary, M.; Singh, P.K.; Das, B.C.; Bhatnagar, S. “Smart” Drug Delivery: A Window to Future of Translational Medicine. Front. Chem. 2023, 10, 1095598. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, M. Polymeric Micro- and Nanoparticles for Controlled and Targeted Drug Delivery. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 355–378. [Google Scholar]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. Int. J. Nanomed. 2023, 18, 509. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The Future of Drug Delivery. Chem. Mater. 2023, 35, 359. [Google Scholar] [CrossRef]
- He, B.; Sui, X.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent Advances in Drug Delivery Systems for Enhancing Drug Penetration into Tumors. Drug Deliv. 2020, 27, 1474. [Google Scholar] [CrossRef]
- de La Torre, L.G.; Sipoli, C.C.; Oliveira, A.F.; Eş, I.; Pessoa, A.C.S.N.; Vitor, M.T.; Vit, F.F.; Naves, T.F. Biopolymers for Gene Delivery Applications. In Biopolymer-Based Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 289–323. [Google Scholar]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Kumar, V.; Parveen, R.; Ahmad, S. Pharmacokinetics of Nanomedicine. In Nanotechnology Principles in Drug Targeting and Diagnosis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 127–142. [Google Scholar]
- Stevanović, M.M.; Škapin, S.D.; Bračko, I.; Milenković, M.; Petković, J.; Filipič, M.; Uskoković, D.P. Poly(Lactide-Co-Glycolide)/Silver Nanoparticles: Synthesis, Characterization, Antimicrobial Activity, Cytotoxicity Assessment and ROS-Inducing Potential. Polymer 2012, 53, 2818. [Google Scholar] [CrossRef]
- Stater, E.P.; Sonay, A.Y.; Hart, C.; Grimm, J. The Ancillary Effects of Nanoparticles and Their Implications for Nanomedicine. Nat. Nanotechnol. 2021, 16, 1180. [Google Scholar] [CrossRef]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Gaikwad, V.L.; Choudhari, P.B.; Bhatia, N.M.; Bhatia, M.S. Characterization of Pharmaceutical Nanocarriers: In Vitro and in Vivo Studies. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–58. [Google Scholar]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Stevanović, M.; Uskoković, V.; Filipović, M.; Škapin, S.D.; Uskoković, D. Composite PLGA/AgNpPGA/AscH Nanospheres with Combined Osteoinductive, Antioxidative, and Antimicrobial Activities. ACS Appl. Mater. Interfaces 2013, 5, 9034. [Google Scholar] [CrossRef]
- Filipović, N.; Veselinović, L.; Ražić, S.; Jeremić, S.; Filipič, M.; Žegura, B.; Tomić, S.; Čolić, M.; Stevanović, M. Poly (ε-Caprolactone) Microspheres for Prolonged Release of Selenium Nanoparticles. Mater. Sci. Eng. C 2019, 96, 776. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681. [Google Scholar] [CrossRef]
- Bakar, N.F.A.; Tan, H.L.; Lim, Y.P.; Adrus, N.; Abdullah, J. Environmental Impact of Quantum Dots. In Graphene, Nanotubes and Quantum Dots-Based Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 837–867. [Google Scholar]
- Filipović, N.; Stevanović, M.; Nunić, J.; Cundrič, S.; Filipič, M.; Uskoković, D. Synthesis of Poly(ɛ-Caprolactone) Nanospheres in the Presence of the Protective Agent Poly(Glutamic Acid) and Their Cytotoxicity, Genotoxicity and Ability to Induce Oxidative Stress in HepG2 Cells. Colloids Surf. B Biointerfaces 2014, 117, 414. [Google Scholar] [CrossRef] [PubMed]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.-L.; Soran, A. An Overview of Biopolymers for Drug Delivery Applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Wu, J.; Shaidani, S.; Theodossiou, S.K.; Hartzell, E.J.; Kaplan, D.L. Localized, on-Demand, Sustained Drug Delivery from Biopolymer-Based Materials. Expert Opin. Drug Deliv. 2022, 19, 1317. [Google Scholar] [CrossRef]
- Tekade, R.K.; Maheshwari, R.; Tekade, M. Biopolymer-Based Nanocomposites for Transdermal Drug Delivery. In Biopolymer-Based Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 81–106. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Stevanović, M.; Savić, J.; Jordović, B.; Uskoković, D. Fabrication, in Vitro Degradation and the Release Behaviours of Poly(Dl-Lactide-Co-Glycolide) Nanospheres Containing Ascorbic Acid. Colloids Surf. B Biointerfaces 2007, 59, 215. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of Biodegradable Materials in Food Packaging: A Review. Alex. Eng. J. 2024, 91, 70. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer Based Nanomaterials in Drug Delivery Systems: A Review. Mater. Today Chem. 2018, 9, 43. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohydr. Polym. 2019, 221, 94. [Google Scholar] [CrossRef]
- Liu, Y.; Kishimura, A.; Katayama, Y.; Mori, T. Development of Polynucleotide-Loaded Nanoparticles for the Regulation of Intracellular Nucleotide Levels. Chem. Lett. 2022, 51, 1037. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, M.; Guo, F.; Yan, Y.; Tian, H.; Zhang, Q.; Ren, S.; Yang, L. Biodegradable Polyester-Based Nano Drug Delivery System in Cancer Chemotherapy: A Review of Recent Progress (2021–2023). Front. Bioeng. Biotechnol. 2023, 11, 1295323. [Google Scholar] [CrossRef]
- Kreua-ongarjnukool, N.; Soomherun, N.; Niyomthai, S.T.; Chumnanvej, S. Aliphatic Polyester Nanoparticles for Drug Delivery Systems. In Smart Drug Delivery; IntechOpen: London, UK, 2022. [Google Scholar]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Stevanović, M.M.; Filipović, N.; Kuzmanović, M.; Tomić, N.; Ušjak, D.; Milenković, M.; Zheng, K.; Stampfl, J.; Boccaccini, A.R. Synthesis and Characterization of a Collagen-Based Composite Material Containing Selenium Nanoparticles. J. Biomater. Appl. 2022, 36, 1800. [Google Scholar] [CrossRef]
- Numata, K. How to Define and Study Structural Proteins as Biopolymer Materials. Polym. J. 2020, 52, 1043. [Google Scholar] [CrossRef]
- Niaz, K.; Khan, F.; Shah, M.A. Analysis of Carbohydrates (Monosaccharides, Polysaccharides). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 621–633. [Google Scholar]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef]
- Stevanović, M.; Bračko, I.; Milenković, M.; Filipović, N.; Nunić, J.; Filipič, M.; Uskoković, D.P. Multifunctional PLGA Particles Containing Poly(l-Glutamic Acid)-Capped Silver Nanoparticles and Ascorbic Acid with Simultaneous Antioxidative and Prolonged Antimicrobial Activity. Acta Biomater. 2014, 10, 151. [Google Scholar] [CrossRef]
- Dutta, R.; Mohapatra, S.S.; Mohapatra, S. Biopolymeric Systems for the Delivery of Nucleic Acids. In Tailor-Made and Functionalized Biopolymer Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 635–661. [Google Scholar]
- Zehiroglu, C.; Sarikaya, S.B.O. The Importance of Antioxidants and Place in Today’s Scientific and Technological Studies. J. Food Sci. Technol. 2019, 56, 4757. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Hermund, D.B. Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–221. [Google Scholar]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, S.A.; Bhagwat, D.B. USDA Database for the Oxygen Radical Capacity (ORAC) of Selected Foods, Release 2; USDA National Nutrient Database for Standard: Washington, DC, USA, 2010.
- The National Center for Complementary and Integrative Health (NCCIH). Antioxidant Supplements: What You Need To Know. 2023. Available online: https://www.nccih.nih.gov/health/antioxidant-supplements-what-you-need-to-know (accessed on 16 January 2024).
- Chan, T.H. The Nutrition Source. Available online: https://www.hsph.harvard.edu/nutritionsource/what-should-you-eat/whole-grains/ (accessed on 16 January 2024).
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Prevention of Mortality in Healthy Participants and Patients with Various Diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar]
- Tomić, N.; Stevanović, M.M.; Filipović, N.; Ganić, T.; Nikolić, B.; Gajić, I.; Ćulafić, D.M. Resveratrol/Selenium Nanocomposite with Antioxidative and Antibacterial Properties. Nanomaterials 2024, 14, 368. [Google Scholar] [CrossRef]
- Tomić, N.; Matić, T.; Filipović, N.; Ćulafić, D.M.; Boccacccini, A.R.; Stevanović, M.M. Synthesis and Characterization of Innovative Resveratrol Nanobelt-like Particles and Assessment of Their Bioactivity, Antioxidative and Antibacterial Properties. J. Biomater. Appl. 2023, 38, 122. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements Used Worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Herdiana, Y.; Husni, P.; Nurhasanah, S.; Shamsuddin, S.; Wathoni, N. Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy. Polymers 2023, 15, 2953. [Google Scholar] [CrossRef]
- Bashir, S.M.; Rather, G.A.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.U.H.; Singh, H.; Alam Khan, A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Quester, K.; Rodríguez-González, S.; González-Dávalos, L.; Lozano-Flores, C.; González-Gallardo, A.; Zapiain-Merino, S.J.; Shimada, A.; Mora, O.; Vazquez-Duhalt, R. Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement. Animals 2022, 12, 417. [Google Scholar] [CrossRef]
- Kongkaoroptham, P.; Piroonpan, T.; Pasanphan, W. Chitosan Nanoparticles Based on Their Derivatives as Antioxidant and Antibacterial Additives for Active Bioplastic Packaging. Carbohydr. Polym. 2021, 257, 117610. [Google Scholar] [CrossRef]
- Mo, E.; Ebedy, Y.A.; Ibrahim, M.A.; Farroh, K.Y.; Hassanen, E.I. Newly Synthesized Chitosan-Nanoparticles Attenuate Carbendazim Hepatorenal Toxicity in Rats via Activation of Nrf2/HO1 Signalling Pathway. Sci. Rep. 2022, 12, 9986. [Google Scholar] [CrossRef]
- Hussein, A.A.; Aldujaili, N.H. Antimicrobial, Antibiofilm, and Antioxidant Activity of Chitosan Nanoparticles Synthesized by E. coli. J. Phys. Conf. Ser. 2020, 1664, 012118. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Ardakani, M.T.; Verdom, B.H.; Bagheri, A.; Mohammad-Beigi, H.; Aliakbari, F.; Salehpour, Z.; Alipour, M.; Afrouz, S.; Bardania, H. Chitosan Nanoparticles Containing Physalis Alkekengi-L Extract: Preparation, Optimization and Their Antioxidant Activity. Bull. Mater. Sci. 2019, 42, 131. [Google Scholar] [CrossRef]
- Hajizadeh, H.S.; Dadashzadeh, R.; Azizi, S.; Mahdavinia, G.R.; Kaya, O. Effect of Chitosan Nanoparticles on Quality Indices, Metabolites, and Vase Life of Rosa Hybrida Cv. Black Magic. Chem. Biol. Technol. Agric. 2023, 10, 12. [Google Scholar] [CrossRef]
- Othman, N.; Jamil, S.N.A.M.; Masarudin, M.J.; Jusoh, R.A.B.M.; Alamassi, M.N. Increased Radical Scavenging Activity of Thymoquinone and L-Ascorbic Acid Dual Encapsulated in Palmitoyl-Chitosan Nanoparticles in a Human Normal Lung Fibroblast, MRC-5 Due to Synergistic Antioxidative Effects. RSC Adv. 2023, 13, 27965. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, Antioxidant and Antibacterial Activities of Chitosan Nanoparticles Loaded with Nettle Essential Oil. J. Food Meas. Charact. 2021, 15, 1395. [Google Scholar] [CrossRef]
- Al-Baqami, N.; Hamza, R. Synergistic Antioxidant Capacities of Vanillin and Chitosan Nanoparticles against Reactive Oxygen Species, Hepatotoxicity, and Genotoxicity Induced by Aging in Male Wistar Rats. Hum. Exp. Toxicol. 2021, 40, 183. [Google Scholar] [CrossRef]
- Maheo, A.R.; Vithiya, B.S.M.; Prasad, T.A.A.; Mangesh, V.L.; Perumal, T.; Al-Qahtani, W.H.; Govindasamy, M. Cytotoxic, Antidiabetic, and Antioxidant Study of Biogenically Improvised Elsholtzia Blanda and Chitosan-Assisted Zinc Oxide Nanoparticles. ACS Omega 2023, 8, 10954. [Google Scholar] [CrossRef]
- Araujo, J.M.; Fortes-Silva, R.; Pola, C.C.; Yamamoto, F.Y.; Gatlin, D.M.; Gomes, C.L. Delivery of Selenium Using Chitosan Nanoparticles: Synthesis, Characterization, and Antioxidant and Growth Effects in Nile Tilapia (Orechromis Niloticus). PLoS ONE 2021, 16, e0251786. [Google Scholar] [CrossRef] [PubMed]
- Zaid, O.A.; Elsonbaty, S.; Moawad, F.; Abdelghaffar, M. Antioxidants and Hepatoprotective Effects of Chitosan Nanoparticles against Hepatotoxicity Induced in Rats. Benha Vet. Med. J. 2019, 36, 252. [Google Scholar] [CrossRef]
- El-Denshary, E.S.; Aljawish, A.; El-Nekeety, A.A.; Hassan, N.S.; Saleh, R.H.; Rihn, B.H.; Abdel-Wahhab, M.A. Possible Synergistic Effect and Antioxidant Properties of Chitosan Nanoparticles and Quercetin against Carbon Tetrachloride-Induce Hepatotoxicity in Rats. Soft Nanosci. Lett. 2015, 5, 36. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Alian, D.M.E.; Haroun, M.; Nounou, M.I.; Patel, A.; El-Khordagui, L. Chitosan Nanoparticles Alleviated the Adverse Effects of Sildenafil on the Oxidative Stress Markers and Antioxidant Enzyme Activities in Rats. Oxid. Med. Cell. Longev. 2023, 2023, 9944985. [Google Scholar] [CrossRef]
- Sankar, N.A.A.; Ramesh, S.; Rajeshkumar, S. Antioxidant Activity of Chitosan Nanoparticles with Chlorhexidine—An In Vitro Study. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 41–48. [Google Scholar]
- Paul, P.; Nandi, G.; Abosheasha, M.A.; Bera, H. Alginate-Based Systems for Protein and Peptide Delivery. In Tailor-Made and Functionalized Biopolymer Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–113. [Google Scholar]
- Carolin, C.F.; Kamalesh, T.; Kumar, P.S.; Hemavathy, R.V.; Rangasamy, G. A Critical Review on Sustainable Cellulose Materials and Its Multifaceted Applications. Ind. Crops Prod. 2023, 203, 117221. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893. [Google Scholar] [CrossRef]
- Chandel, N.; Jain, K.; Jain, A.; Raj, T.; Patel, A.K.; Yang, Y.-H.; Bhatia, S.K. The Versatile World of Cellulose-Based Materials in Healthcare: From Production to Applications. Ind. Crops Prod. 2023, 201, 116929. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, Y.; Liu, Q.; Jang, J.; Han, J. Preparation, Characterization, and Antioxidant Activities of Cellulose Nanocrystals/Genistein Nanocomposites. BioResources 2018, 14, 336. [Google Scholar] [CrossRef]
- El-Waseif, A.A.; Alshehrei, F.; Al-Ghamdi, S.B.; El-Ghwas, D.E. Antioxidant and Anticoagulant Activity of Microbial Nano Cellulose-ZnO-Ag Composite Components. Pakistan J. Biol. Sci. 2022, 25, 531. [Google Scholar] [CrossRef]
- Davoodbasha, M.A.; Saravanakumar, K.; Abdulkader, A.M.; Lee, S.-Y.; Kim, J.-W. Synthesis of Biocompatible Cellulose-Coated Nanoceria with PH-Dependent Antioxidant Property. ACS Appl. Bio Mater. 2019, 2, 1792. [Google Scholar] [CrossRef]
- Musino, D.; Devcic, J.; Lelong, C.; Luche, S.; Rivard, C.; Dalzon, B.; Landrot, G.; Rabilloud, T.; Capron, I. Impact of Physico-Chemical Properties of Cellulose Nanocrystal/Silver Nanoparticle Hybrid Suspensions on Their Biocidal and Toxicological Effects. Nanomaterials 2021, 11, 1862. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef] [PubMed]
- Medically Reviewed by Adrienne Seitz, MS, RD, LDN, Nutrition—By Zia Sherrell, MPH on January 19 2022. MedicalNEwsToday. What to Know About Starch. Available online: https://www.medicalnewstoday.com/articles/what-is-starch (accessed on 14 March 2024).
- Fatima, S.; Khan, M.R.; Ahmad, I.; Sadiq, M.B. Recent Advances in Modified Starch Based Biodegradable Food Packaging: A Review. Heliyon 2024, 10, e27453. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Sudha, P.N.; Rose, M.H. Beneficial Effects of Hyaluronic Acid. Adv Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [PubMed]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–569. [Google Scholar] [CrossRef]
- Wang, S.; Fontana, F.; Shahbazi, M.-A.; Santos, H.A. Acetalated Dextran Based Nano- and Microparticles: Synthesis, Fabrication, and Therapeutic Applications. Chem. Commun. 2021, 57, 4212. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Dextran of Diverse Molecular-Configurations Used as a Blood-Plasma Substitute, Drug-Delivery Vehicle and Food Additive Biosynthesized by Leuconostoc, Lactobacillus and Weissella. Appl. Sci. 2023, 13, 12526. [Google Scholar] [CrossRef]
- Paliya, B.S.; Sharma, V.K.; Sharma, M.; Diwan, D.; Nguyen, Q.D.; Aminabhavi, T.M.; Rajauria, G.; Singh, B.N.; Gupta, V.K. Protein-Polysaccharide Nanoconjugates: Potential Tools for Delivery of Plant-Derived Nutraceuticals. Food Chem. 2023, 428, 136709. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent Advances in Polysaccharides Based Biomaterials for Drug Delivery and Tissue Engineering Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Ahmad, M.; Seidi, F.; Qurtulen; Song, J.; Jin, Y.; Xiao, H. Polysaccharide-Based Nanoassemblies: From Synthesis Methodologies and Industrial Applications to Future Prospects. Adv. Colloid Interface Sci. 2023, 318, 102953. [Google Scholar] [CrossRef] [PubMed]
- Wardani, G.; Nugraha, J.; Mustafa, M.R.; Kurnijasanti, R.; Sudjarwo, S.A. Antioxidative Stress and Antiapoptosis Effect of Chitosan Nanoparticles to Protect Cardiac Cell Damage on Streptozotocin-Induced Diabetic Rat. Oxid. Med. Cell. Longev. 2022, 2022, 3081397. [Google Scholar] [CrossRef] [PubMed]
- Mizrahy, S.; Peer, D. Polysaccharides as Building Blocks for Nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623. [Google Scholar] [CrossRef] [PubMed]
- Hanna, D.M.F.; Youshia, J.; Fahmy, S.F.; George, M.Y. Nose to Brain Delivery of Naringin-Loaded Chitosan Nanoparticles for Potential Use in Oxaliplatin-Induced Chemobrain in Rats: Impact on Oxidative Stress, CGAS/STING and HMGB1/RAGE/TLR2/MYD88 Inflammatory Axes. Expert Opin. Drug Deliv. 2023, 20, 1859. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and Chitosan/Ethylene Oxide-Propylene Oxide Block Copolymer Nanoparticles as Novel Carriers for Proteins and Vaccines. Pharm. Res. 1997, 14, 1431. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125. [Google Scholar] [CrossRef]
- Hoang, N.H.; Le Thanh, T.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan Nanoparticles-Based Ionic Gelation Method: A Promising Candidate for Plant Disease Management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef]
- Kim, E.S.; Baek, Y.; Yoo, H.-J.; Lee, J.-S.; Lee, H.G. Chitosan-Tripolyphosphate Nanoparticles Prepared by Ionic Gelation Improve the Antioxidant Activities of Astaxanthin in the In Vitro and In Vivo Model. Antioxidants 2022, 11, 479. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Parize, A.L. In Vitro Cytotoxic and Antioxidant Evaluation of Quercetin Loaded in Ionic Cross-Linked Chitosan Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 74, 103561. [Google Scholar] [CrossRef]
- Canbolat, F.; Demir, N.; Yayıntas, O.T.; Pehlivan, M.; Eldem, A.; Ayna, T.K.; Senel, M. Chitosan Nanoparticles Loaded with Quercetin and Valproic Acid: A Novel Approach for Enhancing Antioxidant Activity against Oxidative Stress in the SH-SY5Y Human Neuroblastoma Cell Line. Biomedicines 2024, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-J.; Yu, Y.-G.; Yin, S.-W.; Tang, C.-H.; Yang, X.-Q. Cellular Uptake and Intracellular Antioxidant Activity of Zein/Chitosan Nanoparticles Incorporated with Quercetin. J. Agric. Food Chem. 2018, 66, 12783. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.S.; Dara, P.K.; Raman, S.P.; Vijayan, D.K.; Sadasivam, J.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Nanoencapsulation in Low-molecular-weight Chitosan Improves in Vivo Antioxidant Potential of Black Carrot Anthocyanin. J. Sci. Food Agric. 2021, 101, 5264. [Google Scholar] [CrossRef] [PubMed]
- Al-Eisa, R.A. Synergistic Antioxidant Capacity of Chitosan Nanoparticles and Lycopene against Aging Hepatotoxicity Induced by D-Galactose in Male Rats. Int. J. Pharmacol. 2018, 14, 811. [Google Scholar] [CrossRef]
- Dave, S.; Gajendran, P.H.; Arjunkumar, P.L.; Rajeshkumar, R. In Vitro Antioxidant Activity of Chitosan Nanoparticles and Its Incorporated Lycopene. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 45. [Google Scholar]
- de Silva, W.N.D.; Attanayake, A.P.; Arawwawala, L.D.A.M.; Karunaratne, D.N.; Pamunuwa, G.K. In Vitro Antioxidant Activity of Alginate Nanoparticles Encapsulating the Aqueous Extract of Coccinia grandis L. Turkish J. Chem. 2023, 47, 715. [Google Scholar] [CrossRef]
- Alves, M.J.D.S.; de Sousa, M.H.O.; de Moura, N.F.; Cesca, K.; Verruck, S.; Monteiro, A.R.; Valencia, G.A. Starch Nanoparticles Containing Phenolic Compounds from Green Propolis: Characterization and Evaluation of Antioxidant, Antimicrobial and Digestibility Properties. Int. J. Biol. Macromol. 2024, 255, 128079. [Google Scholar]
- Yan, X.; Ramos, R.A.N.S.; Alcouffe, P.; Munoz, L.E.; Bilyy, R.O.; Ganachaud, F.; Bernard, J. Programmable Hierarchical Construction of Mixed/Multilayered Polysaccharide Nanocapsules through Simultaneous/Sequential Nanoprecipitation Steps. Biomacromolecules 2019, 20, 3915. [Google Scholar] [CrossRef]
- Martínez-Muñoz, O.I.; Ospina-Giraldo, L.F.; Mora-Huertas, C.E. Nanoprecipitation: Applications for Entrapping Active Molecules of Interest in Pharmaceutics. In Nano- and Microencapsulation—Techniques and Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Mehra, M.; Sheorain, J.; Bakshi, J.; Grewal, S.; Dhingra, D.; Bernela, M.; Kumari, S. Sodium Alginate Polymer Nanoformulation as Promising Carrier for Berberine Delivery: Synthesis, Morphology and in-Vitro Evaluation. Carbohydr. Polym. Technol. Appl. 2024, 7, 100408. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide Nanoparticles: From Fabrication to Applications. J. Mater. Chem. B 2021, 9, 7030. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-Based Nanocomposites and Their Applications. Carbohydr. Res. 2015, 405, 23. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and Interactions in Covalently and Ionically Crosslinked Chitosan Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 19. [Google Scholar] [CrossRef]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide Colloidal Particles as Delivery Systems for Macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83. [Google Scholar] [CrossRef]
- Taib, M.N.A.M.; Yehye, W.A.; Julkapli, N.M. Influence of Crosslinking Density on Antioxidant Nanocellulose in Bio-Degradation and Mechanical Properties of Nitrile Rubber Composites. Fibers Polym. 2019, 20, 165. [Google Scholar] [CrossRef]
- Furlani, F.; Parisse, P.; Sacco, P. On the Formation and Stability of Chitosan/Hyaluronan-Based Complex Coacervates. Molecules 2020, 25, 1071. [Google Scholar] [CrossRef]
- Vuillemin, M.E.; Michaux, F.; Seiler, A.; Linder, M.; Muniglia, L.; Jasniewski, J. Polysaccharides Enzymatic Modification to Control the Coacervation or the Aggregation Behavior: A Thermodynamic Study. Food Hydrocoll. 2022, 122, 107092. [Google Scholar] [CrossRef]
- Gonçalves, M.M.; Maluf, D.F.; Pontarolo, R.; Saul, C.K.; Almouazen, E.; Chevalier, Y. Negatively Charged Chitosan Nanoparticles Prepared by Ionotropic Gelation for Encapsulation of Positively Charged Proteins. Int. J. Pharm. 2023, 642, 123164. [Google Scholar] [CrossRef]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by Layer Buildup of Polysaccharide Films: Physical Chemistry and Cellular Adhesion Aspects. Langmuir 2004, 20, 448. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.S.; Silva, N.H.; Sintra, T.E.; Santos, S.A.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.; Silvestre, A.J.; et al. Anti-Inflammatory and Antioxidant Nanostructured Cellulose Membranes Loaded with Phenolic-Based Ionic Liquids for Cutaneous Application. Carbohydr. Polym. 2019, 206, 187. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Wu, Y.; Dai, F.; Yuan, M.; Wang, F.; Bai, Y.; Deng, H. Natural Polysaccharides Based Self-Assembled Nanoparticles for Biomedical Applications—A Review. Int. J. Biol. Macromol. 2021, 192, 1240. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, B.B.; Schmid, I.; Wich, P.R. Amphiphilic Polysaccharide Block Copolymers for PH-Responsive Micellar Nanoparticles. Biomacromolecules 2017, 18, 2839. [Google Scholar]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Parasuraman, V.; Hsieh-Chih-Tsai; Arunagiri, V.; Gunaseelan, S.; Chou, H.-Y.; Anbazhagan, R.; Lai, J.-Y.; Prasad, N.R. Radioprotective Effect of Self-Assembled Low Molecular Weight Fucoidan–Chitosan Nanoparticles. Int. J. Pharm. 2020, 579, 119161. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Chitosan-Based Pickering Emulsion: A Comprehensive Review on Their Stabilizers, Bioavailability, Applications and Regulations. Carbohydr. Polym. 2023, 304, 120491. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as Nanoreactors for Synthesis of Biopolymer Nanoparticles. Trends Food Sci. Technol. 2019, 86, 118. [Google Scholar] [CrossRef]
- Fan, Y.; Luo, D.; Yi, J. Resveratrol-Loaded α-Lactalbumin-Chitosan Nanoparticle-Encapsulated High Internal Phase Pickering Emulsion for Curcumin Protection and Its in Vitro Digestion Profile. Food Chem. X 2022, 15, 100433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Liu, L.; Chen, Z. Emulsions Stabilized by Cellulose-Based Nanoparticles for Curcumin Encapsulations: In Vitro Antioxidant Properties. Front. Nutr. 2022, 9, 931581. [Google Scholar] [CrossRef]
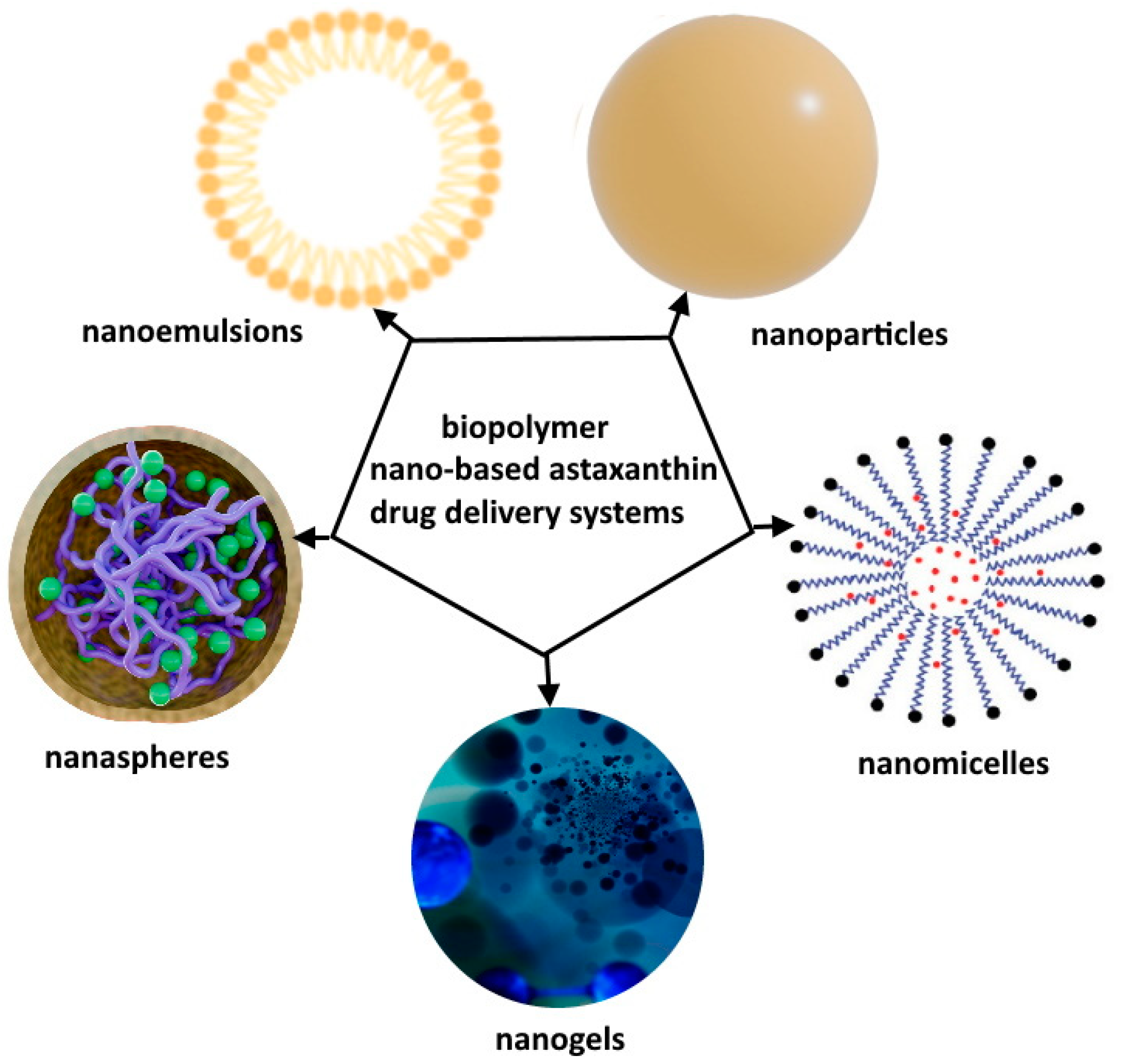
- Chen, S.; Wang, J.; Feng, J.; Xuan, R. Research Progress of Astaxanthin Nano-Based Drug Delivery System: Applications, Prospects and Challenges? Front. Pharmacol. 2023, 14, 931581. [Google Scholar] [CrossRef]
- Su, W.; Polyakov, N.E.; Xu, W.; Su, W. Preparation of Astaxanthin Micelles Self-Assembled by a Mechanochemical Method from Hydroxypropyl β-Cyclodextrin and Glyceryl Monostearate with Enhanced Antioxidant Activity. Int. J. Pharm. 2021, 605, 120799. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, Z.; Xue, C. Recent Research Advances in Astaxanthin Delivery Systems: Fabrication Technologies, Comparisons and Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 3497. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Lee, Y.W.; Kang, H.W. Cellulose Nanocrystals/Nanofibrils Loaded Astaxanthin Nanoemulsion for the Induction of Apoptosis via ROS-Dependent Mitochondrial Dysfunction in Cancer Cells under Photobiomodulation. Int. J. Biol. Macromol. 2020, 149, 165. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, E.; Özkan, B.; Güngör, S.; Özsoy, Y. Biopolymer-Based Nanogel Approach in Drug Delivery: Basic Concept and Current Developments. Pharmaceutics 2023, 15, 1644. [Google Scholar] [CrossRef]
- Pedrali, D.; Scarafoni, A.; Giorgi, A.; Lavelli, V. Binary Alginate-Whey Protein Hydrogels for Antioxidant Encapsulation. Antioxidants 2023, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chu, T.; Yu, T.; Li, N.; Wang, C.; Li, C.; Zhang, Y.; Meng, H.; Nie, G. Design of Diselenide-Bridged Hyaluronic Acid Nano-Antioxidant for Efficient ROS Scavenging to Relieve Colitis. ACS Nano 2022, 16, 13037. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Hopkins, A.K.; Espinosa, E.; Larrañeta, E.; Malinova, D.; McShane, A.N.; Domínguez-Robles, J.; Rodríguez, A. Antioxidant Cellulose Nanofibers/Lignin-Based Aerogels: A Potential Material for Biomedical Applications. Chem. Biol. Technol. Agric. 2023, 10, 72. [Google Scholar] [CrossRef]
- Sarma, S.; Agarwal, S.; Bhuyan, P.; Hazarika, J.; Ganguly, M. Resveratrol-Loaded Chitosan–Pectin Core–Shell Nanoparticles as Novel Drug Delivery Vehicle for Sustained Release and Improved Antioxidant Activities. R. Soc. Open Sci. 2022, 9, 210784. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-W.; Yue, X.-J.; Yuan, X.-F.; Zhao, B. Hemp Seed Globulin-Alginate Nanoparticles for Encapsulation of Cannabisin A with Enhanced Colloidal Stability and Antioxidant Activity. Int. J. Biol. Macromol. 2024, 256, 128380. [Google Scholar] [CrossRef]
- Ali, A.; Rehman, A.; Jafari, S.M.; Ranjha, M.M.A.N.; Shehzad, Q.; Shahbaz, H.M.; Khan, S.; Usman, M.; Kowalczewski, P.; Jarzębski, M.; et al. Effect of Co-Encapsulated Natural Antioxidants with Modified Starch on the Oxidative Stability of β-Carotene Loaded within Nanoemulsions. Appl. Sci. 2022, 12, 1070. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.; Bumajdad, A.; Al-Sagheer, F. To What Extent Do Polymeric Stabilizers Affect Nanoparticles Characteristics? Adv. Colloid Interface Sci. 2019, 270, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Peterson, T.; Fan, Z.; Wang, S. The Commonly Used Stabilizers for Phytochemical-Based Nanoparticles: Stabilization Effects, Mechanisms, and Applications. Nutrients 2023, 15, 3881. [Google Scholar] [CrossRef] [PubMed]
- Manojlović-Stojanoski, M.; Borković-Mitić, S.; Nestorović, N.; Ristić, N.; Trifunović, S.; Stevanović, M.; Filipović, N.; Stojsavljević, A.; Pavlović, S. The Effects of BSA-Stabilized Selenium Nanoparticles and Sodium Selenite Supplementation on the Structure, Oxidative Stress Parameters and Selenium Redox Biology in Rat Placenta. Int. J. Mol. Sci. 2022, 23, 13068. [Google Scholar] [CrossRef] [PubMed]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed]
- Tamang, N.; Shrestha, P.; Khadka, B.; Mondal, M.H.; Saha, B.; Bhattarai, A. A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers 2021, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Bustos, D.; Guzmán, L.; Valdés, O.; Muñoz-Vera, M.; Morales-Quintana, L.; Castro, R.I. Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel. Polymers 2023, 15, 3217. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Jangde, R.K. Current Updated Review on Preparation of Polymeric Nanoparticles for Drug Delivery and Biomedical Applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Altomare, L.; Bonetti, L.; Campiglio, C.E.; De Nardo, L.; Draghi, L.; Tana, F.; Farè, S. Biopolymer-Based Strategies in the Design of Smart Medical Devices and Artificial Organs. Int. J. Artif. Organs 2018, 41, 337. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural Biodegradable Polymers Based Nano-Formulations for Drug Delivery: A Review. Int. J. Pharm. 2019, 561, 244. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and Targeted Delivery of Hydrophilic Drug Compounds: A Review of Existing and Novel Technologies from Bench to Bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. DNA Binding Efficacy with Functionalized Folic Acid-PAMAM Nanoparticles. Chem. Biol. Interact. 2018, 290, 52. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Jahanban-Esfahlan, A.; Jaymand, M.; Alizadeh, E.; Majdi, H.; Najjar, R.; Javaheri, T.; Zare, P. Static DNA Nanostructures For Cancer Theranostics: Recent Progress In Design And Applications. Nanotechnol. Sci. Appl. 2019, 12, 25. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Prim. 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Subjakova, V.; Oravczova, V.; Hianik, T. Polymer Nanoparticles and Nanomotors Modified by DNA/RNA Aptamers and Antibodies in Targeted Therapy of Cancer. Polymers 2021, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, L.; Yang, L.; Pan, X.; Yazd, H.S.; Cui, C.; Li, J.; Moroz, L.; Sun, Y.; et al. Lipid–Oligonucleotide Conjugates for Bioapplications. Natl. Sci. Rev. 2020, 7, 1933. [Google Scholar] [CrossRef]
- Subramanian, P.V.; AlSalhi, M.S.; Devanesan, S.; Thomas, P.A. Evaluation of Antioxidant, Anticancer and DNA Binding Potentials of Noble Metal Nanoparticles Synthesized Using Aristolochia Indica and Indigofera Tinctoria. J. Clust. Sci. 2021, 32, 917. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Wang, Z.; Wang, H.; Liu, F.; Long, Q.; Jiang, S. Advanced Applications of DNA Nanostructures Dominated by DNA Origami in Antitumor Drug Delivery. Front. Mol. Biosci. 2023, 10, 1239952. [Google Scholar] [CrossRef]
- Chen, J.; Seeman, N.C. Synthesis from DNA of a Molecule with the Connectivity of a Cube. Nature 1991, 350, 631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeman, N.C. Construction of a DNA-Truncated Octahedron. J. Am. Chem. Soc. 1994, 116, 1661. [Google Scholar] [CrossRef]
- Mao, C.; Sun, W.; Seeman, N.C. Assembly of Borromean Rings from DNA. Nature 1997, 386, 137. [Google Scholar] [CrossRef]
- Wang, M. DNA Origami Scavenges ROS in the Kidney. Nat. Rev. Nephrol. 2019, 15, 61. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, Y. DNA Origami as Scaffolds for Self-Assembly of Lipids and Proteins. ChemBioChem 2019, 20, 2422. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Z.; Jia, B.; Shi, Y.; Dong, J.; Jiang, S.; Li, Z. DNA Origami as a Nanomedicine for Targeted Rheumatoid Arthritis Therapy through Reactive Oxygen Species and Nitric Oxide Scavenging. ACS Nano 2022, 16, 12520. [Google Scholar] [CrossRef]
- Lima, E.S.; dos Santos, D.; Souza, A.L.; Macedo, M.E.; Bandeira, M.E.; Junior, S.S.S.; Fiuza, B.S.D.; Rocha, V.P.C.; Fonseca, L.M.d.S.; Nunes, D.D.G.; et al. RNA Combined with Nanoformulation to Advance Therapeutic Technologies. Pharmaceuticals 2023, 16, 1634. [Google Scholar] [CrossRef]
- Sinitsyna, V.V.; Vetcher, A.A. Nucleic Acid Aptamers in Nanotechnology. Biomedicines 2022, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Riaz, M.; Zhang, X.; Lin, C.; Wong, K.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Ouyang, Y.; Fadeev, M.; Zhang, P.; Carmieli, R.; Li, J.; Sohn, Y.S.; Karmi, O.; Nechushtai, R.; Pikarsky, E.; Fan, C.; et al. Aptamer-Modified Au Nanoparticles: Functional Nanozyme Bioreactors for Cascaded Catalysis and Catalysts for Chemodynamic Treatment of Cancer Cells. ACS Nano 2022, 16, 18232. [Google Scholar] [CrossRef] [PubMed]
- Grechkin, Y.A.; Grechkina, S.L.; Zaripov, E.A.; Fedorenko, S.V.; Mustafina, A.R.; Berezovski, M.V. Aptamer-Conjugated Tb(III)-Doped Silica Nanoparticles for Luminescent Detection of Leukemia Cells. Biomedicines 2020, 8, 14. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Barar, J.; Eskandani, M.; Omidi, Y. Aptamer-Conjugated Mesoporous Silica Nanoparticles for Simultaneous Imaging and Therapy of Cancer. TrAC Trends Anal. Chem. 2020, 123, 115759. [Google Scholar] [CrossRef]
- An, H.; Deng, X.; Wang, F.; Xu, P.; Wang, N. Dendrimers as Nanocarriers for the Delivery of Drugs Obtained from Natural Products. Polymers 2023, 15, 2292. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-Based Nanomaterials: A New Tool for Targeted Drug Delivery. Ther. Deliv. 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Qiao, L.; Qiao, B.; Guo, B. Conductive Hydrogels for Tissue Repair. Chem. Sci. 2023, 14, 3091. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, L.; Chen, X.; Yin, W.; Ni, L.; Wang, M. Photothermal Nanohybrid Hydrogels for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 1066617. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-Crosslinked Dynamic Hydrogels for Biomedical Applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Chopra, H.; Mohanta, Y.K.; Mahanta, S.; Mohanta, T.K.; Singh, I.; Avula, S.K.; Mallick, S.P.; Rabaan, A.A.; AlSaihati, H.; Alsayyah, A.; et al. Recent Updates in Nanotechnological Advances for Wound Healing: A Narrative Review. Nanotechnol. Rev. 2023, 12, 1–34. [Google Scholar]
- Li, J.; Zhai, Y.-N.; Xu, J.-P.; Zhu, X.-Y.; Yang, H.-R.; Che, H.-J.; Liu, C.-K.; Qu, J.-B. An Injectable Collagen Peptide-Based Hydrogel with Desirable Antibacterial, Self-Healing and Wound-Healing Properties Based on Multiple-Dynamic Crosslinking. Int. J. Biol. Macromol. 2024, 259, 129006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022, 2022, 4656037. [Google Scholar] [CrossRef]
- Kianfar, E. Protein Nanoparticles in Drug Delivery: Animal Protein, Plant Proteins and Protein Cages, Albumin Nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral Administration of Protein Nanoparticles: An Emerging Route to Disease Treatment. Pharmacol. Res. 2020, 158, 104685. [Google Scholar] [CrossRef]
- Kaltbeitzel, J.; Wich, P.R. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chemie Int. Ed. 2023, 62, e202216097. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, M.L.E.; Fogli, S.; Colavita, P.E.; Scanlan, E.M. Aggregation of Protein Therapeutics Enhances Their Immunogenicity: Causes and Mitigation Strategies. RSC Chem. Biol. 2021, 2, 1004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant Peptides, the Guardian of Life from Oxidative Stress. Med. Res. Rev. 2024, 44, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, C.; Bordoni, L.; Petracci, I.; Wu, D.; Fang, L.; Wang, J.; Wang, X.; Gabbianelli, R.; Min, W. Advances on the Antioxidant Peptides from Nuts: A Narrow Review. Antioxidants 2022, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- César, A.P.C.; Lopes, F.E.S.; Azevedo, F.F.N.; Pinto, Y.O.; Andrade, C.R.; Mesquita, F.P.; Silva, G.O.; Freitas, C.D.T.; Souza, P.F.N. Antioxidant Peptides from Plants: A Review. Phytochem. Rev. 2024, 23, 95. [Google Scholar] [CrossRef]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant Peptides: Overview of Production, Properties, and Applications in Food Systems. Compr. Rev. Food Sci. Food Saf. 2023, 22, 46. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-Derived Antioxidant Peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Ram, T.B.; Tang, C.C.B.; Kiew, S.F.; Lau, S.Y.; Gobi, G.; Jaison, J.; Danquah, M.K. Nanoformulation of Peptides for Pharmaceutical Applications: In Vitro and In Vivo Perspectives. Appl. Sci. 2022, 12, 12777. [Google Scholar] [CrossRef]
- Ma, X.; Gong, H.; Liu, Y.; Liu, Y.; Ogino, K.; Xing, R.; Yan, X. Orally Administered Covalently-Assembled Antioxidative Peptide Nanoparticles for Inflammatory Bowel Disease Therapy. J. Colloid Interface Sci. 2022, 626, 156. [Google Scholar] [CrossRef]
- Xia, X.; Li, H.; Xu, X.; Wu, C.; Wang, Z.; Yi, J.; Zhao, G.; Du, M. LYC Loaded Ferritin Nanoparticles for Intracerebral Delivery and the Attenuation of Neurodegeneration in D-Gal-Induced Mice. Biomater. Adv. 2023, 151, 213419. [Google Scholar] [CrossRef] [PubMed]
- Younis, F.A.; Saleh, S.R.; El-Rahman, S.S.A.; Newairy, A.-S.A.; El-Demellawy, M.A.; Ghareeb, D.A. Preparation, Physicochemical Characterization, and Bioactivity Evaluation of Berberine-Entrapped Albumin Nanoparticles. Sci. Rep. 2022, 12, 17431. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Yang, E.; Park, E.J.; Lee, Y.J.; Safavi, M.S.; Song, K.; Na, D.H. Synthesis and Characterization of β-carotene-loaded Albumin Nanoparticles by high-speed Homogenizer. Bull. Korean Chem. Soc. 2022, 43, 677. [Google Scholar] [CrossRef]
- Fan, Y.; Zeng, X.; Yi, J.; Zhang, Y. Fabrication of Pea Protein Nanoparticles with Calcium-Induced Cross-Linking for the Stabilization and Delivery of Antioxidative Resveratrol. Int. J. Biol. Macromol. 2020, 152, 189. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, S.; Zhou, T.; Wu, K.; Qiao, Z.; Zhang, Y.; Xin, N.; Liu, X.; Wei, D.; Sun, J.; et al. Antioxidative and Conductive Nanoparticles-Embedded Cell Niche for Neural Differentiation and Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2021, 13, 52346. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; El Aziz, A.E.A.; Raouf, B.M.A.; Mohamed, S.A.; Nada, D. Antioxidant-Biocompatible and Stable Catalase-Based Gelatin–Alginate Hydrogel Scaffold with Thermal Wound Healing Capability: Immobilization and Delivery Approach. 3 Biotech 2022, 12, 73. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Pérez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis Sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Thongchai, K.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Characterization, Release, and Antioxidant Activity of Caffeic Acid-Loaded Collagen and Chitosan Hydrogel Composites. J. Mater. Res. Technol. 2020, 9, 6512. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Polkowska, I.; Małek, M.; Kluczyński, J.; Paździor-Czapula, K.; Wekwejt, M.; Michno, A.; Ronowska, A.; Pałubicka, A.; Nowicka, B.; et al. The Characterization of Collagen-Based Scaffolds Modified with Phenolic Acids for Tissue Engineering Application. Sci. Rep. 2023, 13, 9966. [Google Scholar] [CrossRef]
- Fiorentini, F.; Suarato, G.; Summa, M.; Miele, D.; Sandri, G.; Bertorelli, R.; Athanassiou, A. Plant-Based, Hydrogel-like Microfibers as an Antioxidant Platform for Skin Burn Healing. ACS Appl. Bio Mater. 2023, 6, 3103. [Google Scholar] [CrossRef]
- Hamdi, M.; Feki, A.; Bardaa, S.; Li, S.; Nagarajan, S.; Mellouli, M.; Boudawara, T.; Sahnoun, Z.; Nasri, M.; Nasri, R. A Novel Blue Crab Chitosan/Protein Composite Hydrogel Enriched with Carotenoids Endowed with Distinguished Wound Healing Capability: In Vitro Characterization and in Vivo Assessment. Mater. Sci. Eng. C 2020, 113, 110978. [Google Scholar] [CrossRef]
- Yu, S.H.; Kim, D.-Y.; Baek, Y.; Lee, H.G. Combination of Nanoparticles and Gelatin-Genipin Hydrogel Enhances the Antioxidant Activity, Stability, and Release Properties of Curcumin. J. Food Eng. 2024, 365, 111814. [Google Scholar] [CrossRef]
- Darban, Z.; Singh, H.; Singh, U.; Bhatia, D.; Gaur, R.; Kuddushi, M.; Dhanka, M.; Shahabuddin, S. β-Carotene Laden Antibacterial and Antioxidant Gelatin/Polyglyceryl Stearate Nano-Hydrogel System for Burn Wound Healing Application. Int. J. Biol. Macromol. 2024, 255, 128019. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.; Bártolo, R.; Pedro, S.N.; Valente, B.F.A.; Pinto, R.J.B.; Vilela, C.; Shahbazi, M.-A.; Santos, H.A.; Freire, C.S.R. Injectable Nanocomposite Hydrogels of Gelatin-Hyaluronic Acid Reinforced with Hybrid Lysozyme Nanofibrils-Gold Nanoparticles for the Regeneration of Damaged Myocardium. ACS Appl. Mater. Interfaces 2023, 15, 25860. [Google Scholar] [CrossRef]
- Ojeda-Piedra, S.A.; Quintanar-Guerrero, D.; Cornejo-Villegas, M.A.; Zambrano-Zaragoza, M.L. A Green Method for Nanoencapsulation of Thymol in Chitosan–Gelatin with Antioxidant Capacity. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- de Brito, V.P.; de Souza Ribeiro, M.M.; Viganó, J.; de Moraes, M.A.; Veggi, P.C. Silk Fibroin Hydrogels Incorporated with the Antioxidant Extract of Stryphnodendron Adstringens Bark. Polymers 2022, 14, 4806. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, H.; Chen, J.; Zhang, Y.; Mo, Q.; Zhang, P.; Wang, M.; Liu, H.; Bao, X.; Sun, Y.; et al. Silk-Based Hydrogel Incorporated with Metal-Organic Framework Nanozymes for Enhanced Osteochondral Regeneration. Bioact. Mater. 2023, 20, 221. [Google Scholar] [CrossRef]
- Yi, J.; He, Q.; Peng, G.; Fan, Y. Improved Water Solubility, Chemical Stability, Antioxidant and Anticancer Activity of Resveratrol via Nanoencapsulation with Pea Protein Nanofibrils. Food Chem. 2022, 377, 131942. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, R.; Zhou, J.; Yim, W.; Jokerst, J.V.; Zhang, Y.; Mansel, B.W.; Yang, N.; Zhang, Y.; Ma, J. Supramolecular Assembly of Multifunctional Collagen Nanocomposite Film via Polyphenol-Coordinated Clay Nanoplatelets. ACS Appl. Bio Mater. 2022, 5, 1319. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Socka, M.; Kost, B. Microfluidics for Producing Polylactide Nanoparticles and Microparticles and Their Drug Delivery Application. Polym. Int. 2019, 68, 997. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Moraveji, M.K. Microfluidic Assisted Synthesis of PLGA Drug Delivery Systems. RSC Adv. 2019, 9, 2055. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Farooqi, M.Q.U.; Bhowmik, S.; Zahra, Z.; Mahmud, M.M.C.; Assadpour, E.; Gan, R.Y.; Kharazmi, M.S.; Jafari, S.M. Application of Micro/Nano-Fluidics for Encapsulation of Food Bioactive Compounds—Principles, Applications, and Challenges. Trends Food Sci. Technol. 2023, 136, 64. [Google Scholar] [CrossRef]
- Liu, H.; Singh, R.P.; Zhang, Z.; Han, X.; Liu, Y.; Hu, L. Microfluidic Assembly: An Innovative Tool for the Encapsulation, Protection, and Controlled Release of Nutraceuticals. J. Agric. Food Chem. 2021, 69, 2936. [Google Scholar] [CrossRef]
- Vu, H.T.H.; Streck, S.; Hook, S.M.; McDowell, A. Utilization of Microfluidics for the Preparation of Polymeric Nanoparticles for the Antioxidant Rutin: A Comparison with Bulk Production. Pharm. Nanotechnol. 2019, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-Based Drug Delivery Systems for Remotely Triggered Cancer Therapeutic and Diagnostic Applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.R.; Nikam, A.; Chandak, P.; Mandale, V.; Naik, J.B.; Giram, P.S. Recent Advances in PLGA Based Nanocarriers for Drug Delivery System: A State of the Art Review. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 49. [Google Scholar] [CrossRef]
- Qiu, E.; Liu, F. PLGA-Based Drug Delivery Systems in Treating Bone Tumors. Front. Bioeng. Biotechnol. 2023, 11, 1199343. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-Based Nanoparticles for the Treatment of Cancer: Current Strategies and Perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Dziadek, M.; Dziadek, K.; Checinska, K.; Salagierski, S.; Choinska, E.; Szatkowski, P.; Wajda, A.; Kopec, A.; Cholewa-Kowalska, K. Polyphenolic Compounds Affect the Long-Term Degradation Behaviour of Polymer and Composite Materials Based on PCL, PLGA, and Bioactive Glass. Sustain. Mater. Technol. 2023, 35, e00568. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(Lactic-Co-Glycolic Acid) Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1759. [Google Scholar]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76. [Google Scholar] [CrossRef]
- Sahini, M.G. Polylactic Acid (PLA)-Based Materials: A Review on the Synthesis and Drug Delivery Applications. Emergent Mater. 2023, 6, 1461. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Clemente, M.; Grande, E.; Urciuoli, S.; Romani, A.; Torre, L.; Puglia, D.; Bernini, R.; Santi, L. Hydroxytyrosol and Oleuropein-Enriched Extracts Obtained from Olive Oil Wastes and By-Products as Active Antioxidant Ingredients for Poly (Vinyl Alcohol)-Based Films. Molecules 2021, 26, 2104. [Google Scholar] [CrossRef]
- Ortenzi, M.A.; Gazzotti, S.; Marcos, B.; Antenucci, S.; Camazzola, S.; Piergiovanni, L.; Farina, H.; Di Silvestro, G.; Verotta, L. Synthesis of Polylactic Acid Initiated through Biobased Antioxidants: Towards Intrinsically Active Food Packaging. Polymers 2020, 12, 1183. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Properties and Application of Bilayer Films Based on Poly (Lactic Acid) and Fish Gelatin Containing Epigallocatechin Gallate Fabricated by Thermo-Compression Molding. Food Hydrocoll. 2020, 105, 105792. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Fabrication of Quercetin-Loaded Biopolymer Films as Functional Packaging Materials. ACS Appl. Polym. Mater. 2021, 3, 2131. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(Lactic Acid)/Lignin Films with Enhanced Toughness and Anti-Oxidation Performance for Active Food Packaging. Int. J. Biol. Macromol. 2020, 144, 102. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Bioactive Functional Poly(Lactic Acid)/Curcumin Composite Film for Food Packaging Application. Int. J. Biol. Macromol. 2020, 162, 1780. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/Organoclay Bionanocomposites Impregnated with Thymol and Cinnamaldehyde by Supercritical Impregnation for Active and Sustainable Food Packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Waters, E.S.; Kaiser, E.E.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; Kumar, A.; Platt, S.R.; et al. Intracisternal Administration of Tanshinone IIA-Loaded Nanoparticles Leads to Reduced Tissue Injury and Functional Deficits in a Porcine Model of Ischemic Stroke. IBRO Neurosci. Rep. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Han, M.; Wang, X.; Wang, J.; Sun, X.; Zhang, C.; Yan, S.; Huang, L.; Chen, Y. Evaluation of the Synergistic Effects of Epigallocatechin-3-Gallate-Loaded PEGylated-PLGA Nanoparticles with Nimodipine against Neuronal Injury after Subarachnoid Hemorrhage. Front. Nutr. 2023, 9, 953326. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol Loaded PLGA Nanoparticles Ameliorate Scopolamine-Induced Cognitive Dysfunction by Attenuating Cholinesterase Activity, Oxidative Stress and Apoptosis in Wistar Rat. Nutr. Neurosci. 2022, 25, 485. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Voci, S.; Ambrosio, N.; Fresta, M.; Duranti, A.; Cosco, D. Characterization and Preliminary In Vitro Antioxidant Activity of a New Multidrug Formulation Based on the Co-Encapsulation of Rutin and the α-Acylamino-β-Lactone NAAA Inhibitor URB894 within PLGA Nanoparticles. Antioxidants 2023, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xue, T.; Niu, B.; Wei, L.; Wang, H. Preparation, Characterization and Antibacterial Property of Naringin Loaded PLGA Nanospheres. Prog. Nat. Sci. Mater. Int. 2022, 32, 498. [Google Scholar] [CrossRef]
- Abdelmalek, F.; Rofeal, M.; Pietrasik, J.; Steinbüchel, A. Novel Biodegradable Nanoparticulate Chain-End Functionalized Polyhydroxybutyrate–Caffeic Acid with Multifunctionalities for Active Food Coatings. ACS Sustain. Chem. Eng. 2023, 11, 7123. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, M.; Li, Y.; Du, Y.; Wang, H.; Chen, Y.; Li, L. Fabrication of Superparamagnetic Nano-Silica@ Quercetin-Encapsulated PLGA Nanocomposite: Potential Application for Cardiovascular Diseases. J. Photochem. Photobiol. B Biol. 2019, 196, 111508. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts ROS-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, e2201300. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Wang, J.; Sun, X.; Yang, X.; Liu, G. Mussel-Inspired Electroactive, Antibacterial and Antioxidative Composite Membranes with Incorporation of Gold Nanoparticles and Antibacterial Peptides for Enhancing Skin Wound Healing. J. Biol. Eng. 2024, 18, 3. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, L.; Jiang, S.; Shafiq, M.; Cai, Y.; Chen, Y.; Song, J.; Yu, X.; Ijima, H.; Xu, Y.; et al. Anti-Inflammatory, Antibacterial, and Antioxidative Bioactive Glass-Based Nanofibrous Dressing Enables Scarless Wound Healing. Smart Mater. Med. 2023, 4, 407. [Google Scholar] [CrossRef]
- Evangeline, S.; Sridharan, T.B. Biosynthesis and Statistical Optimization of Polyhydroxyalkanoate (PHA) Produced by Bacillus Cereus VIT-SSR1 and Fabrication of Biopolymer Films for Sustained Drug Release. Int. J. Biol. Macromol. 2019, 135, 945. [Google Scholar] [CrossRef]
- Brelle, L.; Renard, E.; Langlois, V. Antioxidant Network Based on Sulfonated Polyhydroxyalkanoate and Tannic Acid Derivative. Bioengineering 2021, 8, 9. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Wadhwa, P.; Hong, J.W.; Hong, Y.G.; Jeon, J.-M.; Lee, E.S.; Yang, Y.-H. Lipase Mediated Functionalization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Ascorbic Acid into an Antioxidant Active Biomaterial. Int. J. Biol. Macromol. 2019, 123, 117. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.; Fong, M.; Stewart, S.; Irwin, N.; Rial-Hermida, M.; Donnelly, R.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Fontes, M.R.V.; da Rosa, M.P.; Fonseca, L.M.; Beck, P.H.; da Rosa Zavareze, E.; Dias, A.R.G. Thermal Stability, Hydrophobicity and Antioxidant Potential of Ultrafine Poly (Lactic Acid)/Rice Husk Lignin Fibers. Braz. J. Chem. Eng. 2021, 38, 133. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.-Y.; Yang, H.; Chen, J.-N.; Lin, Y.; Han, S.-Y.; Cao, Q.; Zeng, H.-S.; Ye, J.-W. A Polyhydroxyalkanoates-Based Carrier Platform of Bioactive Substances for Therapeutic Applications. Front. Bioeng. Biotechnol. 2022, 9, 798724. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef]
- da Fonseca, M.S.; Mourão, M.M.; Xavier, L.P.; Santos, A.V. Recent Biotechnological Applications of Polyhydroxyalkanoates (PHA) in the Biomedical Sector—A Review. Polymers 2023, 15, 4405. [Google Scholar] [CrossRef]
- Cañadas, O.; García-García, A.; Prieto, M.; Pérez-Gil, J. Polyhydroxyalkanoate Nanoparticles for Pulmonary Drug Delivery: Interaction with Lung Surfactant. Nanomaterials 2021, 11, 1482. [Google Scholar] [CrossRef]
- Longé, L.F.; Michely, L.; Gallos, A.; De Anda, A.R.; Vahabi, H.; Renard, E.; Latroche, M.; Allais, F.; Langlois, V. Improved Processability and Antioxidant Behavior of Poly(3-Hydroxybutyrate) in Presence of Ferulic Acid-Based Additives. Bioengineering 2022, 9, 100. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Li, X.; Zhang, Y.; Mao, Z.; Wang, B.; Sui, X.; Feng, X. Fabrication of Lignin/Poly(3-Hydroxybutyrate) Nanocomposites with Enhanced Properties via a Pickering Emulsion Approach. Int. J. Biol. Macromol. 2020, 165, 3078. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Patanè, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of Polyhydroxyalkanoates (PHA)-Based Bioplastic with Phloretin for Active Food Packaging: Characterization of Its Mechanical, Antioxidant, and Antimicrobial Activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef]
- Geraili, A.; Xing, M.; Mequanint, K. Design and Fabrication of Drug-delivery Systems toward Adjustable Release Profiles for Personalized Treatment. View 2021, 2, 20200126. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs. PLGA-Based Nanoparticles Containing Rutin: A Comparative Investigation. Mater. Sci. Eng. C 2021, 111, 111538. [Google Scholar] [CrossRef]
- Nanotechnology Drug Delivery Market. Available online: https://www.precedenceresearch.com/nanotechnology-drug-delivery-market (accessed on 9 May 2024).
- Nanotechnology Drug Delivery Market Size & Share Analysis—Growth Trends & Forecasts (2024–2029). Available online: https://www.mordorintelligence.com/industry-reports/nanotechnology-drug-delivery-market (accessed on 9 May 2024).
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles–Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef]


| Polysaccharides | Chitosan | 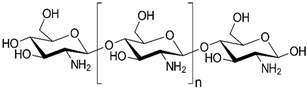 |
| Starch | 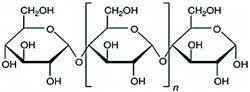 | |
| Alginates |  | |
| Cellulose |  | |
| Hyaluronic acid | 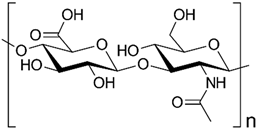 | |
| Polynucleotides | DNA | 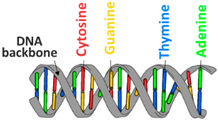 |
| RNA |  | |
| Proteins | Collagen |  |
| BSA |  | |
| Silk |  | |
| Zein |  | |
| Polyesters | PLGA | 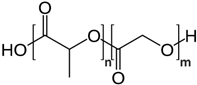 |
| PLA |  | |
| Polyhydroxy-alkanoates | 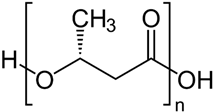 |
| Active Agent | Composition of Hydrogel | Reported Results/Remarks | Reference |
|---|---|---|---|
| Cannabis sativa oil extract and AgNPs | Collagen | At 150 µL of Cannabis sativa oil extract high scavenger activity of 80%. | [215] |
| Caffeic acid (CA) | Collagen–chitosan | CA increases the dimensional stability of hydrogel after immersing in medium. Gradual release profiles within 8 h. DPPH·, ABTH+, and FRAP assays confirmed good antioxidative potential. | [216] |
| Caffeic acid (CA), ferulic acid (FA), and gallic acid (GA) | Collagen | Polyphenolic compounds induce higher swelling rate and enzymatic stability of hydrogel compared to pure collagen. Radical scavenging activity in the range 85–91%, highest for FA. In vivo tests—only hydrogels with FA generate an inflammatory process. | [217] |
| Vit. C | Electrospinning—zein + pectin | In vitro—DCFH-DA assay on HaCaT cells confirmed ROS decrease of 50%. In vivo test on a skin UVB-burn mouse model—significant reduction of inflammatory cytokines expression. | [218] |
| Carotenoids | Chitosan + protein isolate from blue crab | Low pH promotes release. In vitro, DPPH assay—radical scavenging 93–100%. In vivo rat model, acceleration of wound healing and complete healing. | [219] |
| Curcumin NPs | Gelatin–genipin | In vitro—hydrogel exhibits slightly higher antioxidative activity. Stability of curcumin NPs increased by 174%. | [220] |
| β-Carotene | Gelatin/polyglyceryl stearate/graphene oxide (GO) | Increasing GO concentration showed sustained release of β-Carotene. GPGO-3 β hydrogel showed the highest antioxidant potency (57.75%). | [221] |
| Au nanoparticles with lysozyme nanofibrils AuNPs@LNFs | Gelatin–hyaluronic Acid | Improved rheological properties, mechanical resilience, antioxidant activity, and electrical conductivity. The swelling and bioresorbable ratios were favorable at lower pH. | [222] |
| Thymol NPs | Chitosan–gelatin composite, gallic acid as crosslinker. | Composition of chitosan–type A gelatin with ratio 1:4 exhibits the best properties. Antioxidant capacity evaluated by DPPH, ABTS—radical inhibition 87% and 88.5%. FRAP assay—1085 µM Trolox equivalents. | [223] |
| Barbatimão extracts | Silk fibroin | Extractions in propylene glycol were superior to ethanol and result in better physical–chemical and structural performance of hydrogel. High antioxidant activity (ORAC and FRAP). | [224] |
| metal–organic framework nanozymes (CuTA@SF) | silk-based | In vitro—CuTA@SF hydrogel accelerates cell proliferation, enhances cell viability, and antioxidant and antibacterial properties. In vivo (rabbit model)—successful in situ osteochondral defect regeneration. | [225] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevanović, M.; Filipović, N. A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years. Pharmaceutics 2024, 16, 670. https://doi.org/10.3390/pharmaceutics16050670
Stevanović M, Filipović N. A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years. Pharmaceutics. 2024; 16(5):670. https://doi.org/10.3390/pharmaceutics16050670
Chicago/Turabian StyleStevanović, Magdalena, and Nenad Filipović. 2024. "A Review of Recent Developments in Biopolymer Nano-Based Drug Delivery Systems with Antioxidative Properties: Insights into the Last Five Years" Pharmaceutics 16, no. 5: 670. https://doi.org/10.3390/pharmaceutics16050670





