In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Characterization of the Scaffolds
2.1.1. Production of the Scaffolds
2.1.2. Chemical Composition
2.1.3. Determination of Compressive Strength
2.1.4. Porosity
2.1.5. Energy Dispersive X-ray Analysis (EDX) and Scanning Electron Microscopy (SEM) before Implantation
2.2. Animal Model
2.2.1. Implantation of the Wedges
2.2.2. Plate Removal
2.3. Radiological Examination and Semi-Quantitative Evaluation
2.4. In Vivo µCT Examination
2.4.1. Semi-Quantitative Evaluation of the In Vivo µCT Examination
2.4.2. Quantitative Evaluation of the In Vivo µCT Examination
2.5. Histological Examination and Semi-Quantitative Evaluation
Histomorphometric Examination
2.6. Energy Dispersive X-ray Analysis (EDX) and Scanning Electron Microscopy (SEM) of the Implanted Wedges
2.7. Statistics
3. Results
3.1. Chemical and Mechanical Properties of the Scaffolds
3.2. SEM/EDX Analyses before Implantation
3.3. OP and Clinical Examination
3.4. X-ray Examinations
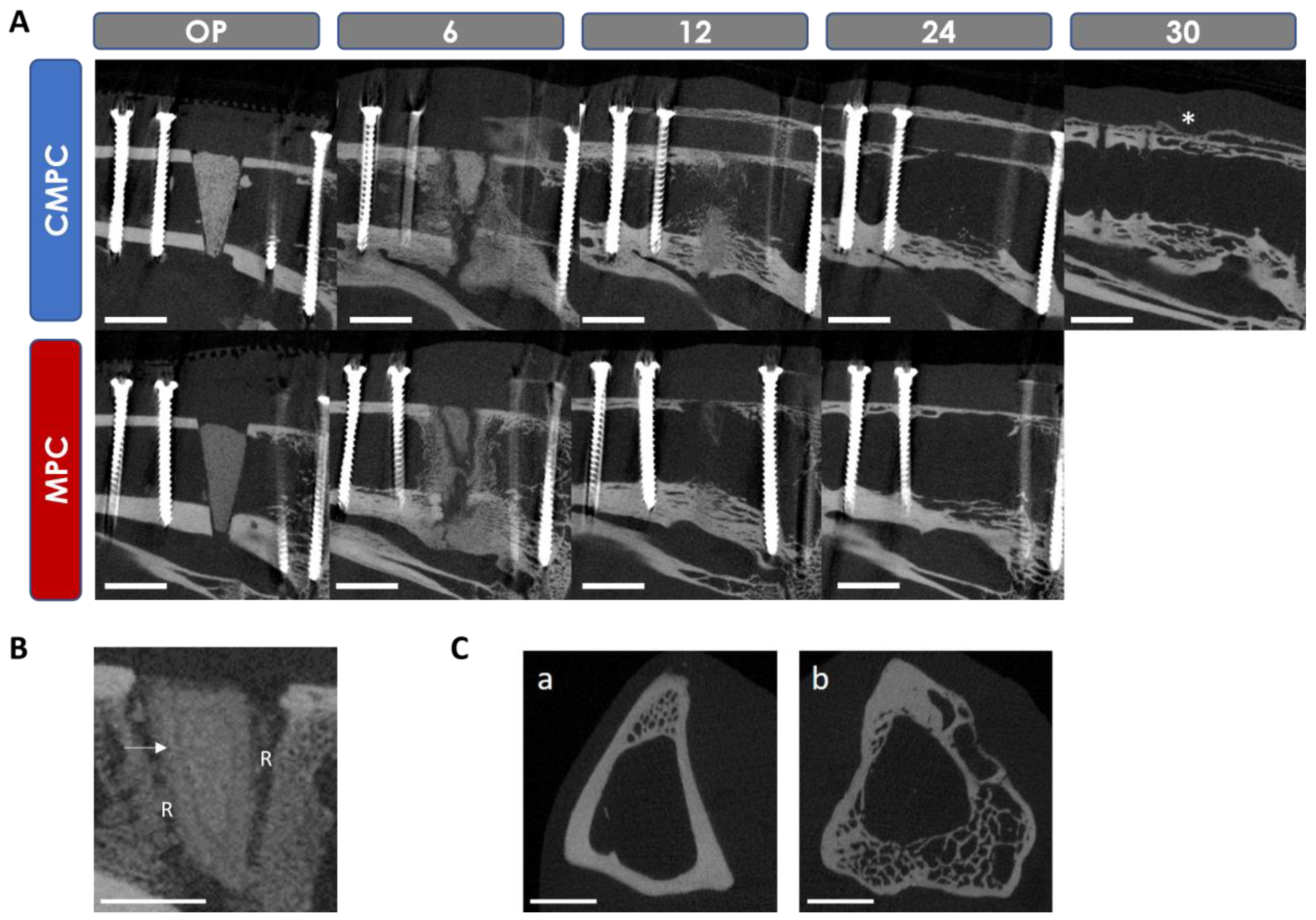
3.5. In Vivo µCT Examinations
3.5.1. Semi-Quantitative Evaluation of the Scans
3.5.2. Quantitative Evaluation of the Scans
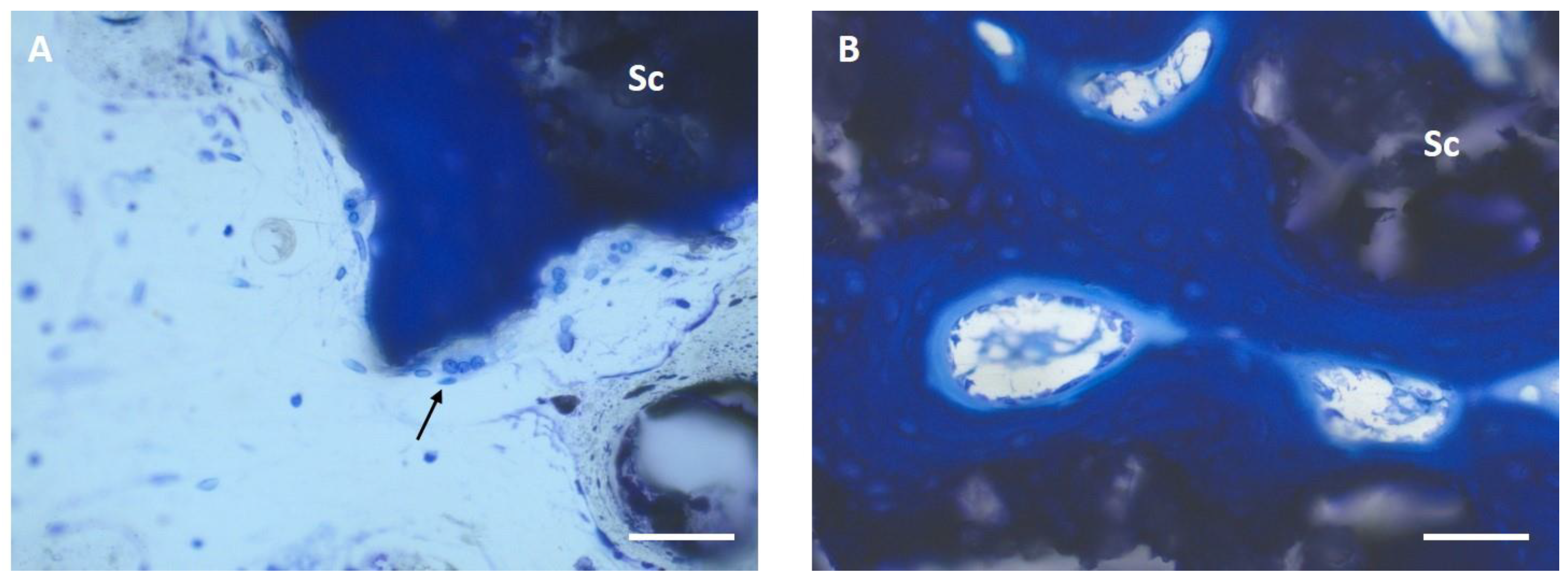
3.6. Histological Examination
3.6.1. Semi-Quantitative Assessment
3.6.2. Histomorphometric Examinations
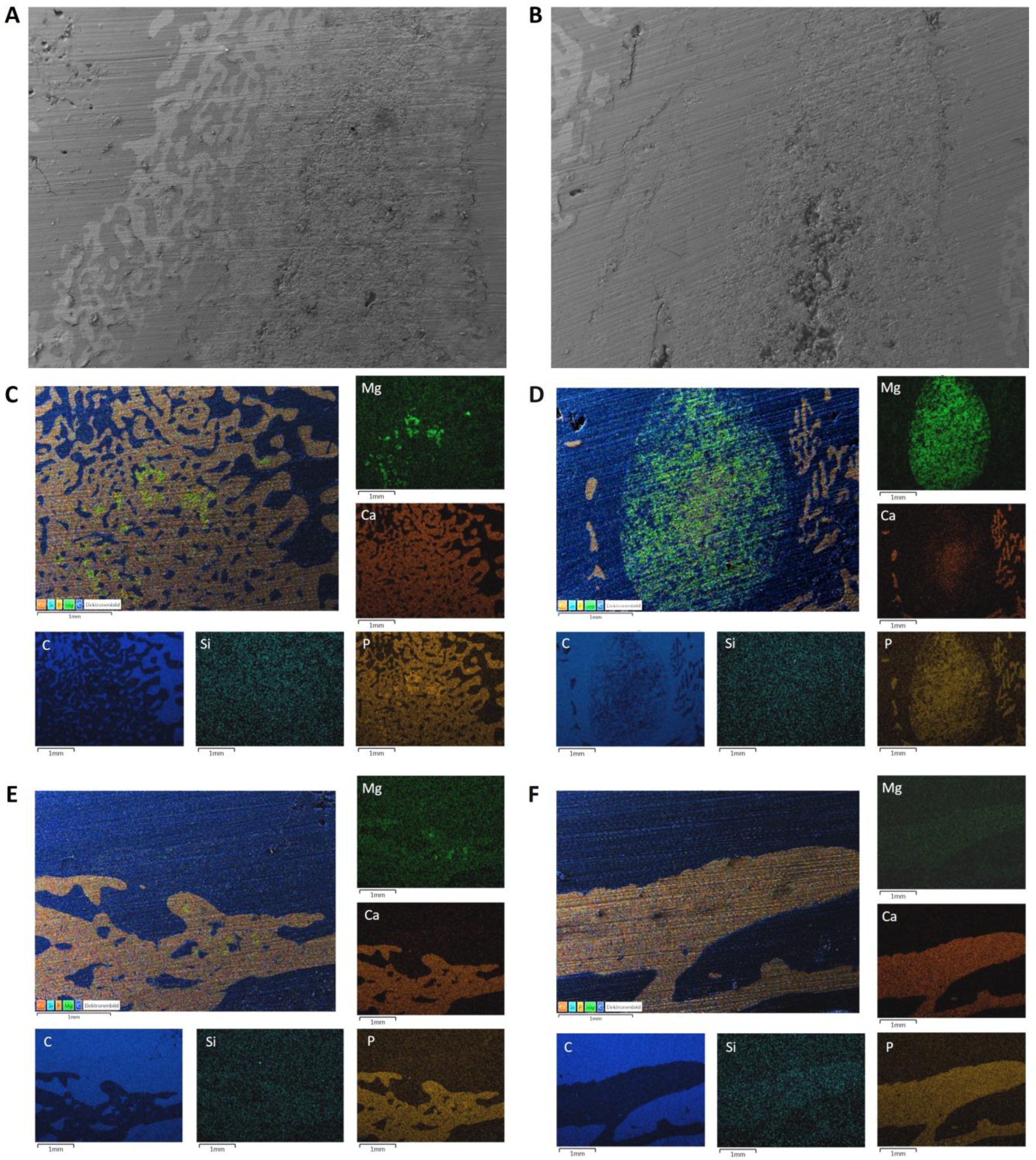
3.7. SEM/EDX Analyses after Implantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| µ-CT | µ-computed tomography |
| ATMP | Amorphous trimagnesium phosphate |
| Ca | Calcium |
| CMPC | Ca0.75Mg2.25(PO4)2 post-treated with phosphoric acid |
| CMPCs | Calcium magnesium phosphate cements |
| CPCs | Calcium phosphate cements |
| CTMP | Crystalline trimagnesium phosphate |
| DAHP | Diammonium hydrogen phosphate |
| EDX | Energy dispersive X-ray analysis |
| HA | Hydroxyapatite |
| Mg | Magnesium |
| MPC | Mg3(PO4)2 post-treated with diammonium hydrogen phosphate |
| MPCs | Magnesium phosphate cements |
| mRUST | Modified radiographic union scale for tibial fractures |
| P | Phosphate |
| PA | Phosphoric acid, H3PO4 |
| PEEK | Polyetheretherketone |
| PR | Plate removal |
| RT | Room temperature |
| SD | Scaffold density |
| SEM | Scanning electron microscopy |
| SV | Scaffold volume |
| TCP | Tricalcium phosphate |
| Th | Threshold |
References
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52 (Suppl. S2), S72–S77. [Google Scholar] [CrossRef]
- Kheirallah, M.; Almeshaly, H. Bone graft substitutes for bone defect regeneration. A collective review. Int. J. Dent. Oral. Sci. 2016, 3, 247–257. [Google Scholar]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical In Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef]
- Linhart, W.; Briem, D.; Peters, A.; Lehmann, W.; Windolf, J.; Rueger, J. Resorbierbare Kalziumphosphatzemente. Trauma Berufskrankh. 2004, 6, 277–284. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Simon, M.H.; Grünwald, L.; Schenke, M.; Dickschas, J.; Strecker, W. Corrective osteotomies of femur and tibia: Which factors influence bone healing? Arch. Orthop. Trauma. Surg. 2020, 140, 303–311. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium phosphate cement systems for hard tissue applications: A review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ferreira, J.M. Brushite-forming Mg-, Zn-and Sr-substituted bone cements for clinical applications. Materials 2010, 3, 519–535. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Palm, C.; Willbold, E. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. J. Biomed. Mater. Res. A 2007, 81, 757–765. [Google Scholar] [CrossRef]
- Fuchs, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Stahlhut, P.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; et al. Bone regeneration capacity of newly developed spherical magnesium phosphate cement granules. Clin. Oral. Investig. 2021, 26, 2619–2633. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bose, S. Osteoclastogenesis and osteoclastic resorption of tricalcium phosphate: Effect of strontium and magnesium doping. J. Biomed. Mater. Res. A 2012, 100, 2450–2461. [Google Scholar] [CrossRef]
- Kanter, B.; Vikman, A.; Bruckner, T.; Schamel, M.; Gbureck, U.; Ignatius, A. Bone regeneration capacity of magnesium phosphate cements in a large animal model. Acta Biomater. 2018, 69, 352–361. [Google Scholar] [CrossRef]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, H.; Wei, J.; Jiang, X.; Hua, H.; Chen, F.; Wei, S.; Shin, J.W.; Liu, C. Development of magnesium calcium phosphate biocement for bone regeneration. J. R. Soc. Interface 2010, 7, 1171–1180. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Vorndran, E.; Feichtner, F.; Waselau, A.C.; Brueckner, M.; Meyer-Lindenberg, A. In-Vivo Degradation Behavior and Osseointegration of 3D Powder-Printed Calcium Magnesium Phosphate Cement Scaffolds. Materials 2021, 14, 946. [Google Scholar] [CrossRef]
- Kowalewicz, K.; Waselau, A.C.; Feichtner, F.; Schmitt, A.M.; Brückner, M.; Vorndran, E.; Meyer-Lindenberg, A. Comparison of degradation behavior and osseointegration of 3D powder-printed calcium magnesium phosphate cement scaffolds with alkaline or acid post-treatment. Front. Bioeng. Biotechnol. 2022, 10, 998254. [Google Scholar] [CrossRef]
- Ewald, A.; Kreczy, D.; Bruckner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A. Development and Bone Regeneration Capacity of Premixed Magnesium Phosphate Cement Pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef]
- Vorndran, E.; Klarner, M.; Klammert, U.; Grover, L.M.; Patel, S.; Barralet, J.E.; Gbureck, U. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv. Eng. Mater. 2008, 10, B67–B71. [Google Scholar] [CrossRef]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Wunder, K.; Moseke, C.; Biermann, I.; Müller, F.A.; Zorn, K.; Gbureck, U. Hydraulic setting Mg3(PO4)2 powders for 3D printing technology. Adv. Appl. Ceram. 2011, 110, 476–481. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; Marz, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1145–1158. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Klammert, U.; Vorndran, E.; Reuther, T.; Müller, F.A.; Zorn, K.; Gbureck, U. Low temperature fabrication of magnesium phosphate cement scaffolds by 3D powder printing. J. Mater. Sci. Mater. Med. 2010, 21, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, C.; Schmitt, A.M.; Moseke, C.; Stahlhut, P.; Geroneit, I.; Brückner, M.; Meyer-Lindenberg, A.; Vorndran, E. Physicochemical degradation of calcium magnesium phosphate (stanfieldite) based bone replacement materials and the effect on their cytocompatibility. Biomed. Mater. 2022, 18, 015022. [Google Scholar] [CrossRef] [PubMed]
- ISO 13175-3:2012; Implants for Surgery—Calcium Phosphates—Part 3: Hydroxyapatite and Beta-Tricalcium Phosphate Bone Substitutes. ISO: Geneva, Switzerland, 2012.
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral. Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Waselau, A.C.; Feichtner, F.; Julmi, S.; Klose, C.; Maier, H.J.; Wriggers, P.; Meyer-Lindenberg, A. In vivo investigation of open-pored magnesium scaffolds LAE442 with different coatings in an open wedge defect. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221142679. [Google Scholar] [CrossRef] [PubMed]
- Brosset, T.; Pasquier, G.; Migaud, H.; Gougeon, F. Opening wedge high tibial osteotomy performed without filling the defect but with locking plate fixation (TomoFix™) and early weight-bearing: Prospective evaluation of bone union, precision and maintenance of correction in 51 cases. Orthop. Traumatol. Surg. Res. 2011, 97, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Whelan, D.B.; Bhandari, M.; Stephen, D.; Kreder, H.; McKee, M.D.; Zdero, R.; Schemitsch, E.H. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma 2010, 68, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Litrenta, J.; Tornetta, P., 3rd; Mehta, S.; Jones, C.; O’Toole, R.V.; Bhandari, M.; Kottmeier, S.; Ostrum, R.; Egol, K.; Ricci, W.; et al. Determination of Radiographic Healing: An Assessment of Consistency Using RUST and Modified RUST in Metadiaphyseal Fractures. J. Orthop. Trauma 2015, 29, 516–520. [Google Scholar] [CrossRef]
- Leow, J.M.; Clement, N.D.; Tawonsawatruk, T.; Simpson, C.J.; Simpson, A.H. The radiographic union scale in tibial (RUST) fractures: Reliability of the outcome measure at an independent centre. Bone Joint Res. 2016, 5, 116–121. [Google Scholar] [CrossRef]
- Stengele, A. Systematische Analyse der Abbindereaktion von Magnesiumphosphat mit Polyacrylsäure im Vergleich zu Klassischen Wässrigen Zementsystemen. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2017. [Google Scholar]
- Gefel, E. Zelluläre Resorption 3D-Gedruckter Knochenimplantate auf Basis von Calciummagnesiumphosphaten. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2023. [Google Scholar]
- Zeng, D.; Xia, L.; Zhang, W.; Huang, H.; Wei, B.; Huang, Q.; Wei, J.; Liu, C.; Jiang, X. Maxillary sinus floor elevation using a tissue-engineered bone with calcium-magnesium phosphate cement and bone marrow stromal cells in rabbits. Tissue Eng. Part A 2012, 18, 870–881. [Google Scholar] [CrossRef]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase composition, mechanical performance and in vitro biocompatibility of hydraulic setting calcium magnesium phosphate cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef]
- Bavya Devi, K.; Lalzawmliana, V.; Saidivya, M.; Kumar, V.; Roy, M.; Kumar Nandi, S. Magnesium Phosphate Bioceramics for Bone Tissue Engineering. Chem. Rec. 2022, 22, e202200136. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xun, S.; Haoye, M.; Baichuan, S.; Peng, C.; Xuejian, L.; Kaihong, Z.; Xuan, Y.; Jiang, P.; Shibi, L. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 2017, 5, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Rodrigues, J.; Vorndran, E.; Gbureck, U.; Quental, C.; Folgado, J.; Fernandes, P.R. Computational design and fabrication of a novel bioresorbable cage for tibial tuberosity advancement application. J. Mech. Behav. Biomed. Mater. 2017, 65, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Gefel, E.; Moseke, C.; Schmitt, A.M.; Dümmler, N.; Stahlhut, P.; Ewald, A.; Meyer-Lindenberg, A.; Vorndran, E. Degradation of 3D-printed magnesium phosphate ceramics in vitro and a prognosis on their bone regeneration potential. Bioact. Mater. 2023, 19, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Dias, M.; Vorndran, E.; Gbureck, U.; Fernandes, P.; Pires, I.; Gouveia, B.; Armés, H.; Pires, E.; Rodrigues, J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication 2014, 6, 025005. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Ewald, A.; Müller, F.A.; Zorn, K.; Kufner, A.; Gbureck, U. Formation and properties of magnesium-ammonium-phosphate hexahydrate biocements in the Ca-Mg-PO4 system. J. Mater. Sci. Mater. Med. 2011, 22, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, C.; Yu, S.; Wu, X.; Jie, Z.; Dai, H. Citric acid enhances the physical properties, cytocompatibility and osteogenesis of magnesium calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2019, 94, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamamoto, M.; Ioku, K.; Goto, S. Relationship between compressive strength and pore structure of hardened cement pastes. Adv. Cem. Res. 1997, 9, 25–30. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef]
- Fiset, S.; Godbout, C.; Crookshank, M.C.; Zdero, R.; Nauth, A.; Schemitsch, E.H. Experimental Validation of the Radiographic Union Score for Tibial Fractures (RUST) Using Micro-Computed Tomography Scanning and Biomechanical Testing in an in-Vivo Rat Model. J. Bone Joint Surg. Am. 2018, 100, 1871–1878. [Google Scholar] [CrossRef]
- Mısır, A.; Yıldız, K.İ.; Kızkapan, T.B.; Uzun, E.; Özçamdallı, M.; Oğuzkaya, S. Reliability of rust and modified rust scores for evaluation of union in pediatric and adult femoral shaft fractures. Acta Orthop. Traumatol. Turc. 2021, 55, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Féron, J.M.; Mauprivez, R. Fracture repair: General aspects and influence of osteoporosis and anti-osteoporosis treatment. Injury 2016, 47 (Suppl. S1), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B. Osseointegration Kalthärtender Knochenzemente im Schafmodell. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2014. [Google Scholar]
- Planzer, K. Variable Fixation Locking Screw (VFLS): Investigation of Bone healing with a Device Intended to Optimize Strain and Micromotion for Each Phase of Fracture Healing. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2019. [Google Scholar]
- Naghavi, S.A.; Tamaddon, M.; Garcia-Souto, P.; Moazen, M.; Taylor, S.; Hua, J.; Liu, C. A novel hybrid design and modelling of a customised graded Ti-6Al-4V porous hip implant to reduce stress-shielding: An experimental and numerical analysis. Front. Bioeng. Biotechnol. 2023, 11, 1092361. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Eckert-Hübner, K.; Augat, P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J. Orthop. Res. 2002, 20, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.; Fernandez, A.; Regazzoni, P. Understanding fracture healing biomechanics based on the “strain” concept and its clinical applications. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Mati, L.; Ridi, F.; Baglioni, P. Tuning the properties of magnesium phosphate-based bone cements: Effect of powder to liquid ratio and aqueous solution concentration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 248–255. [Google Scholar] [CrossRef]
- Nuss, K.M.; von Rechenberg, B. Biocompatibility issues with modern implants in bone—A review for clinical orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef]
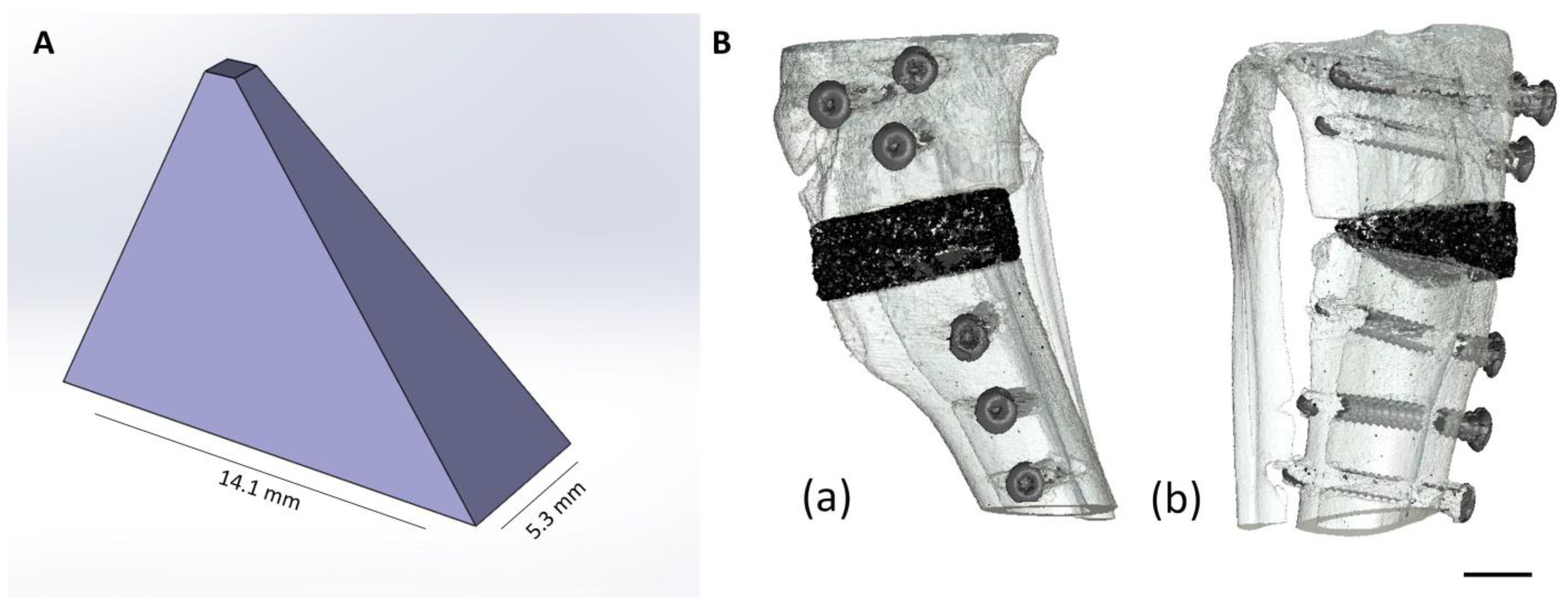
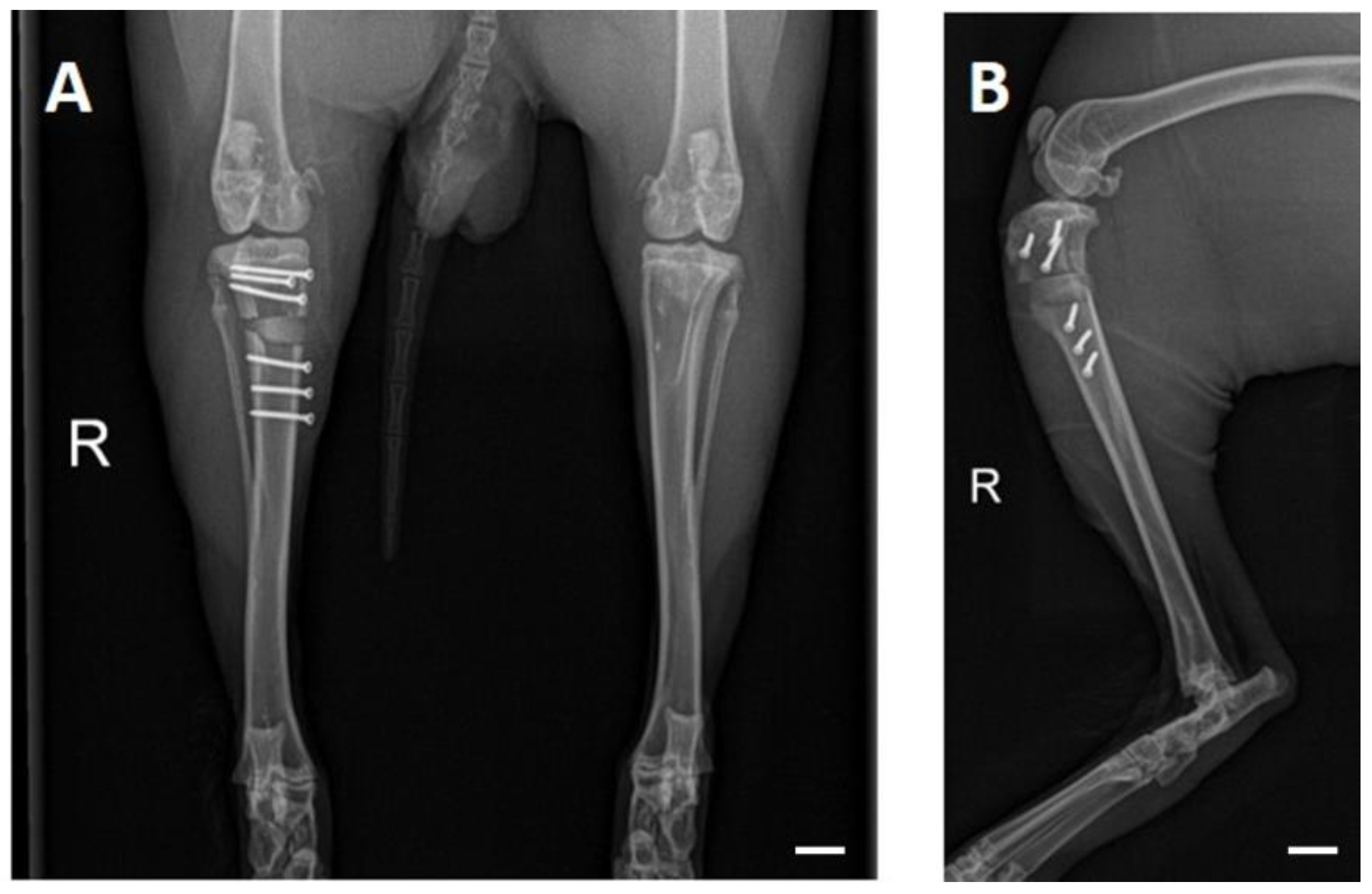

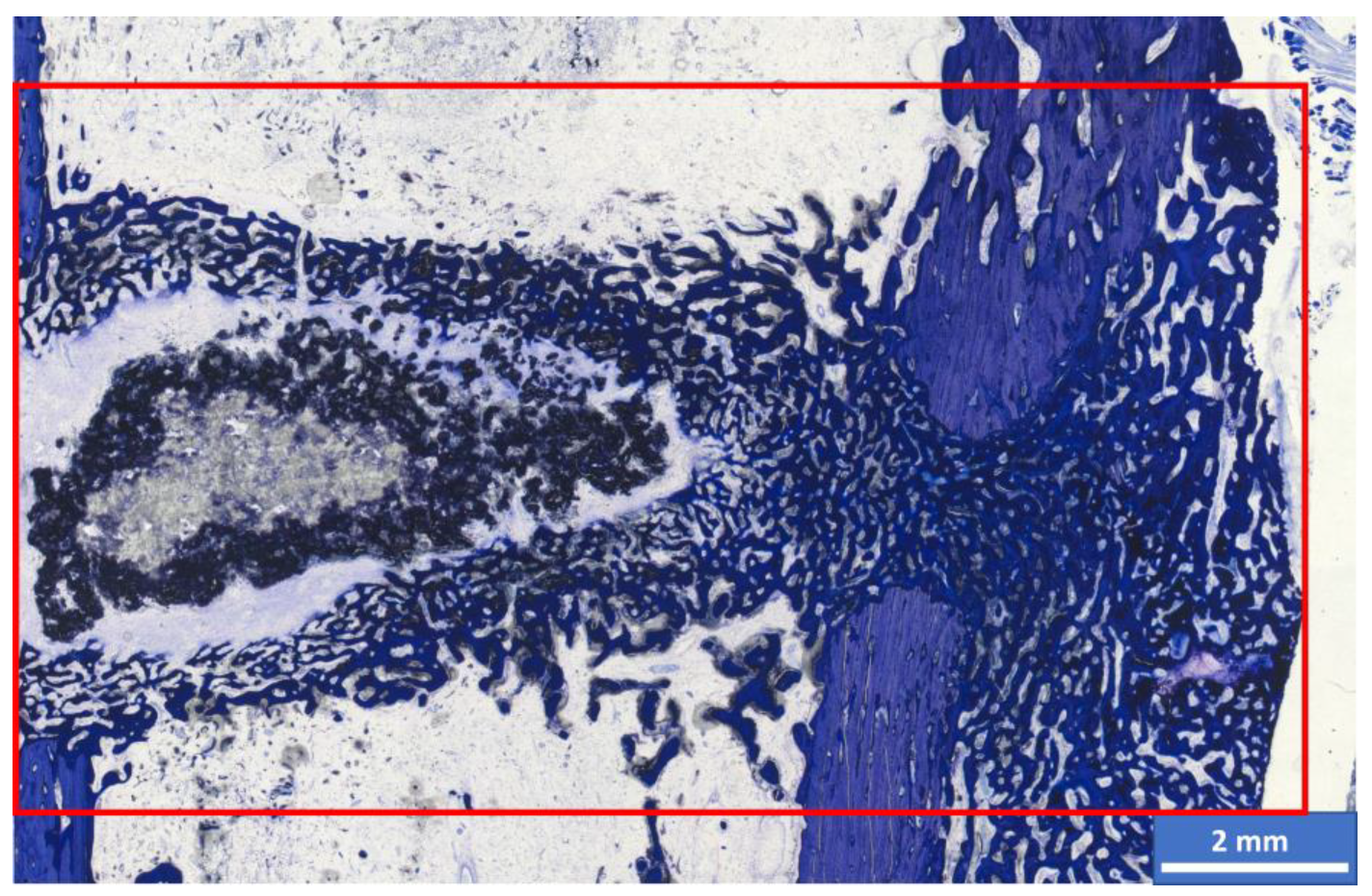
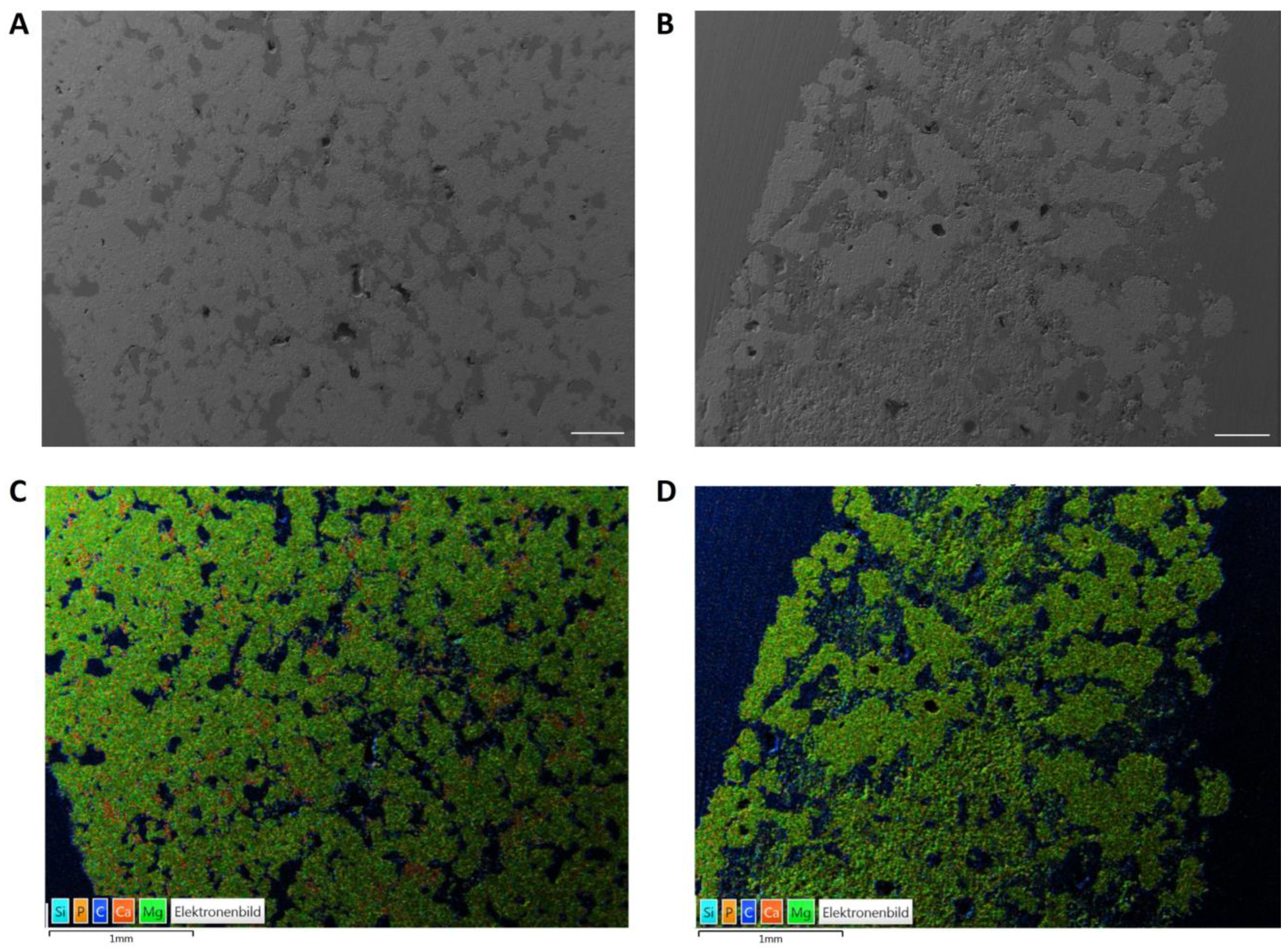


| CaxMg3−x(PO4)2 | CaHPO4 | CaCO3 | MgHPO4 3H2O | Mg(OH)2 |
|---|---|---|---|---|
| x = 0.00 | - | - | 2.00 | 1.00 |
| x = 0.75 | 0.50 | 0.25 | 1.50 | 0.75 |
| mRUST Score | Osteotomy Line | Callus |
|---|---|---|
| 1 | Visible | Missing |
| 2 | Visible | Available |
| 3 | Visible | Bridged |
| 4 | Not visible | Completely bridged or remodeled |
| Parameters | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Scaffold visibility | Not visible | Partially visible | Fully visible |
| Loss of shape | Wedge shape no longer recognizable | Wedge shape partially recognizable | Wedge shape clearly recognizable |
| Closure of osteotomy gap by callus tissue | Scaffold completely covered by callus | Partial callus formation | No callus formation |
| Scaffold integration | Continuous visible contact surface between scaffold and bone | Contact surface between scaffold and bone partially interrupted | No visible scaffold–bone contact |
| Resorption zone | No resorption zone | Scaffold partially surrounded by resorption zone | Scaffold completely surrounded by resorption zone |
| Delimitable zone within scaffold | No zone delimitable | Zone indistinctly delimitable | Zone clearly delimitable |
| Scaffold fit (on day of surgery) | Scaffold is attached to the cortices | Scaffold does not lie medially or laterally against cortical bone | Scaffold is neither medial nor lateral to cortical bone |
| Bridging of medial osteotomy gap (cis-cortex) | Complete bridging | Partial bridging | No bridging |
| Endosteal callus formation proximal to scaffold | None | Minor | Medium to high |
| Endosteal callus formation distal to scaffold | None | Minor | Medium to high |
| Periosteal callus formation (trans-cortex) | None | Minor | Medium to high |
| Remodeling trans-cortex | Even and narrow = physiological | Loosened and cancellous | Heavily curved |
| Parameters | Score 0 | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|---|
| Cis-cortex bridging | 76–100% | 51–75% | 26–50% | 1–25% | Not bridged |
| Trans-cortex bridging | 76–100% | 51–75% | 26–50% | 1–25% | Not bridged |
| Cis-cortex remodeling (bone maturation) | Mainly lamellar bone | Woven bone with no to little lamellar bone | Woven bone with cartilage | Cartilage tissue | No remodeling |
| Trans-cortex remodeling (bone maturation) | Mainly lamellar bone | Woven bone with no to little lamellar bone | Woven bone with cartilage | Cartilage tissue | No remodeling |
| Proximal endosteal callus | 76–100% | 51–75% | 26–50% | 1–25% | No callus |
| Distal endosteal callus | 76–100% | 51–75% | 26–50% | 1–25% | No callus |
| Scaffold degradation | 76–100% | 51–75% | 26–50% | 1–25% | No degradation |
| Scaffold integration | 76–100% | 51–75% | 26–50% | 1–25% | No integration |
| Resorption zone | Measured at level of scaffold center in mm | ||||
| Proportion of dark-colored material | Estimated percentage of scaffold | ||||
| Thickness of the trans-cortex in mm | Measured including periosteal callus | ||||
| Brushite | Stanfieldite | Farringtonite | Struvite | Newberyite | Periclase | |
|---|---|---|---|---|---|---|
| MPC | - | - | 81.0 ± 3.2 | 19.0 ± 3.2 | - | - |
| CMPC | 10.9 ± 2.4 | 20.1 ± 4.9 | 10.3 ± 1.4 | - | 58.1 ± 3.9 | 0.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmerlein, E.; Vorndran, E.; Schmitt, A.-M.; Feichtner, F.; Waselau, A.-C.; Meyer-Lindenberg, A. In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects. Materials 2024, 17, 2136. https://doi.org/10.3390/ma17092136
Hemmerlein E, Vorndran E, Schmitt A-M, Feichtner F, Waselau A-C, Meyer-Lindenberg A. In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects. Materials. 2024; 17(9):2136. https://doi.org/10.3390/ma17092136
Chicago/Turabian StyleHemmerlein, Elke, Elke Vorndran, Anna-Maria Schmitt, Franziska Feichtner, Anja-Christina Waselau, and Andrea Meyer-Lindenberg. 2024. "In Vivo Investigation of 3D-Printed Calcium Magnesium Phosphate Wedges in Partial Load Defects" Materials 17, no. 9: 2136. https://doi.org/10.3390/ma17092136





