Enhanced Long-Term Corrosion Resistance of 316L Stainless Steel by Multilayer Amorphous Carbon Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
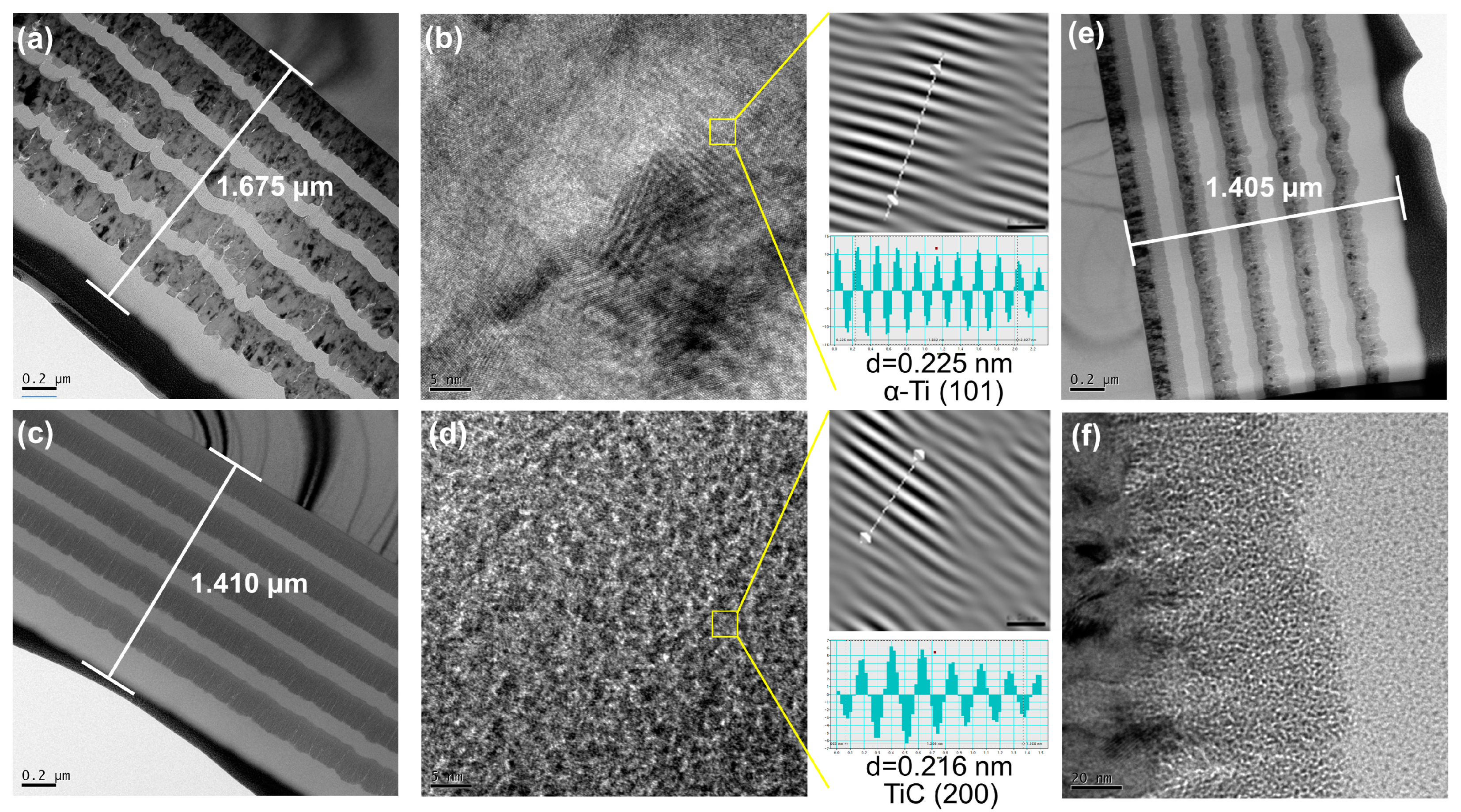
3.1. Microstructure Characterization
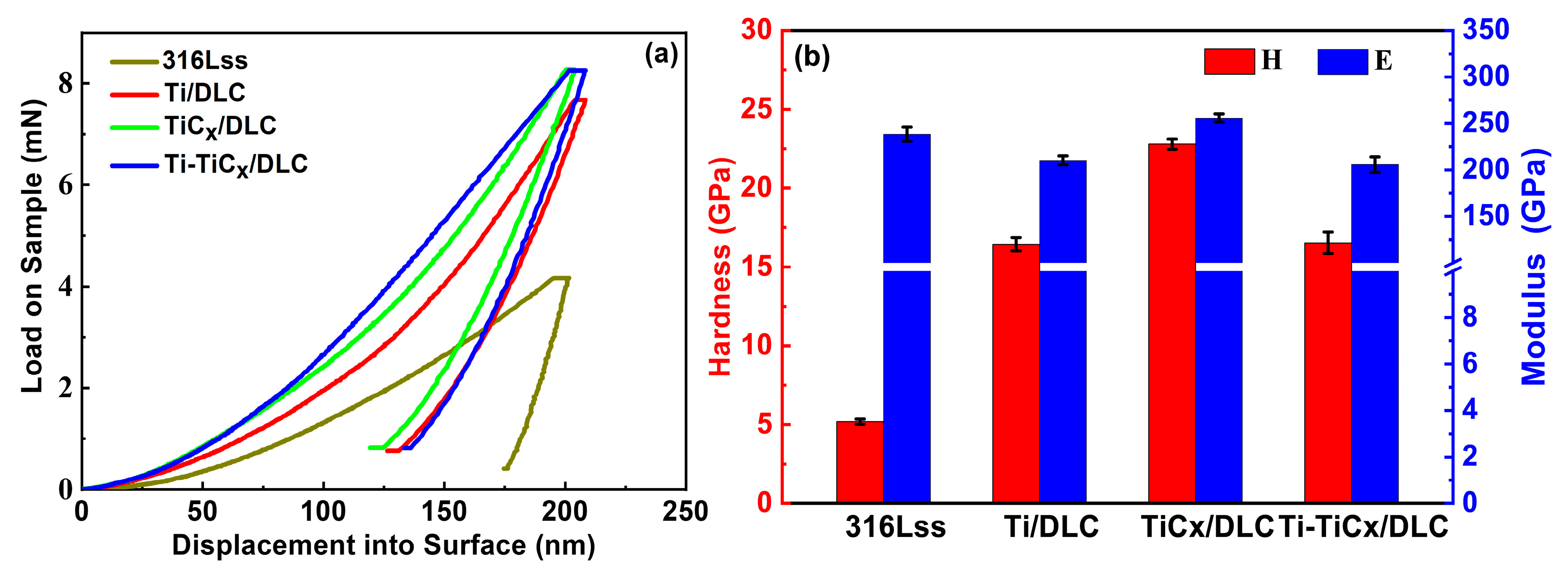
3.2. Mechanical Properties
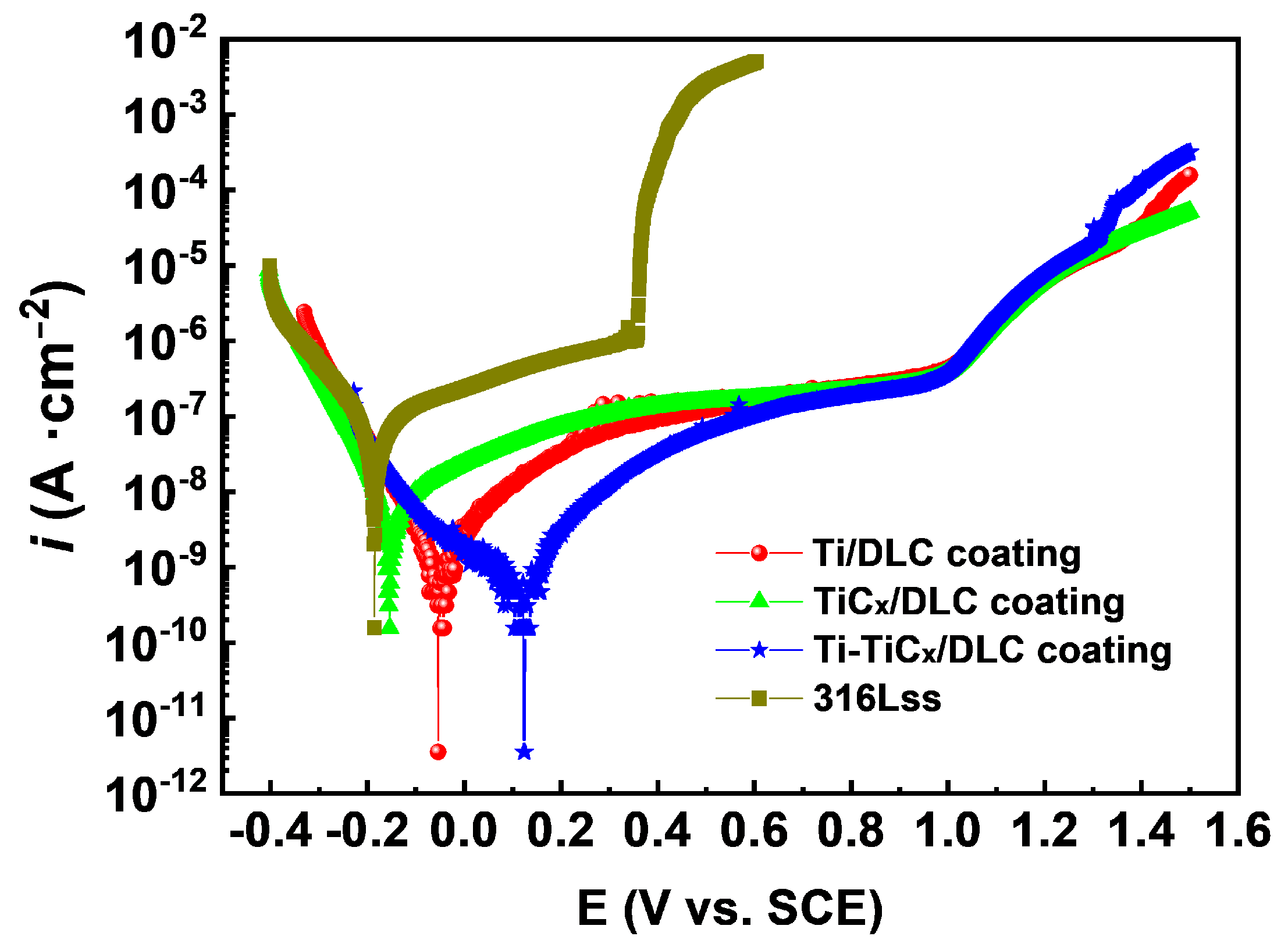
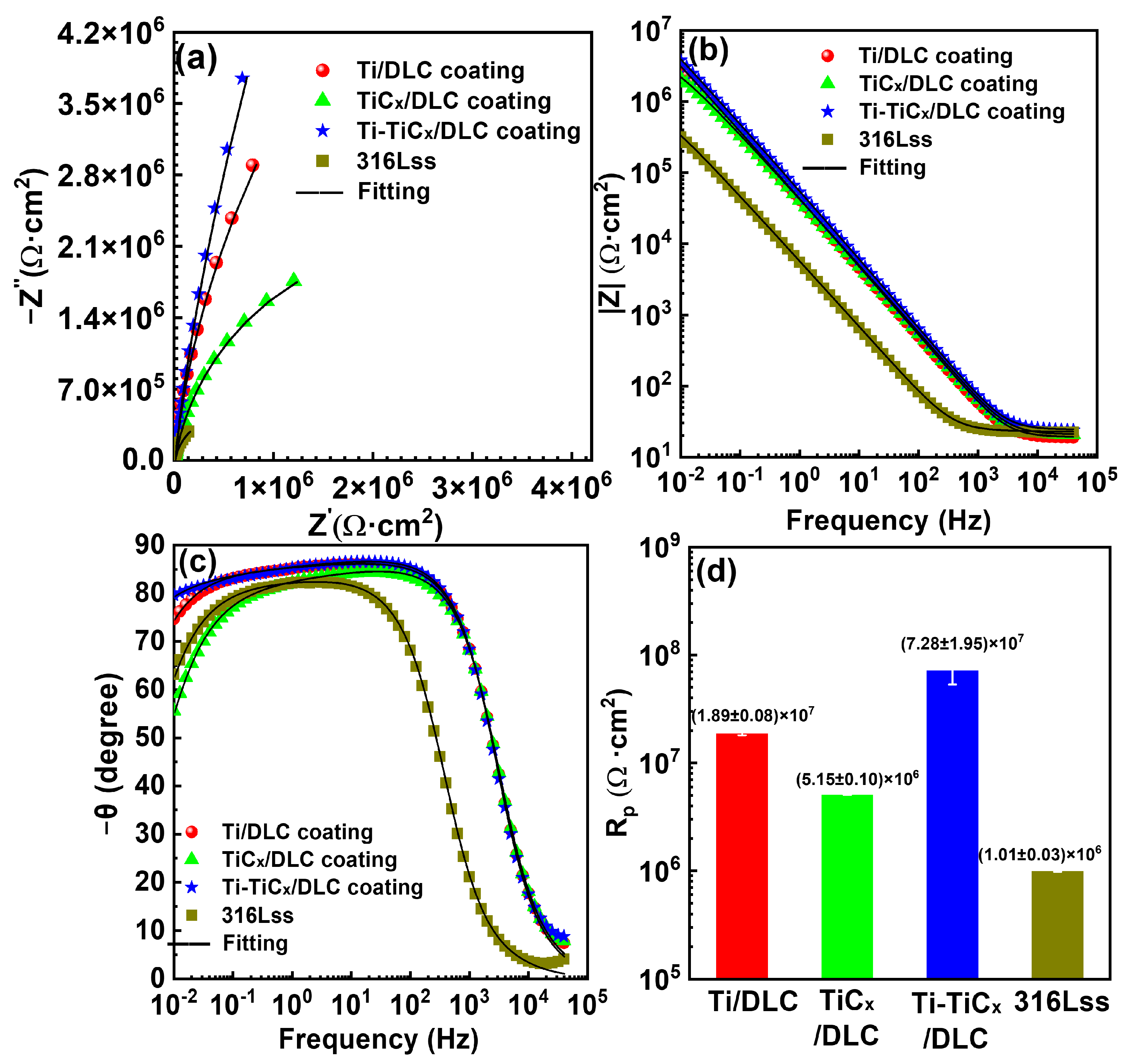
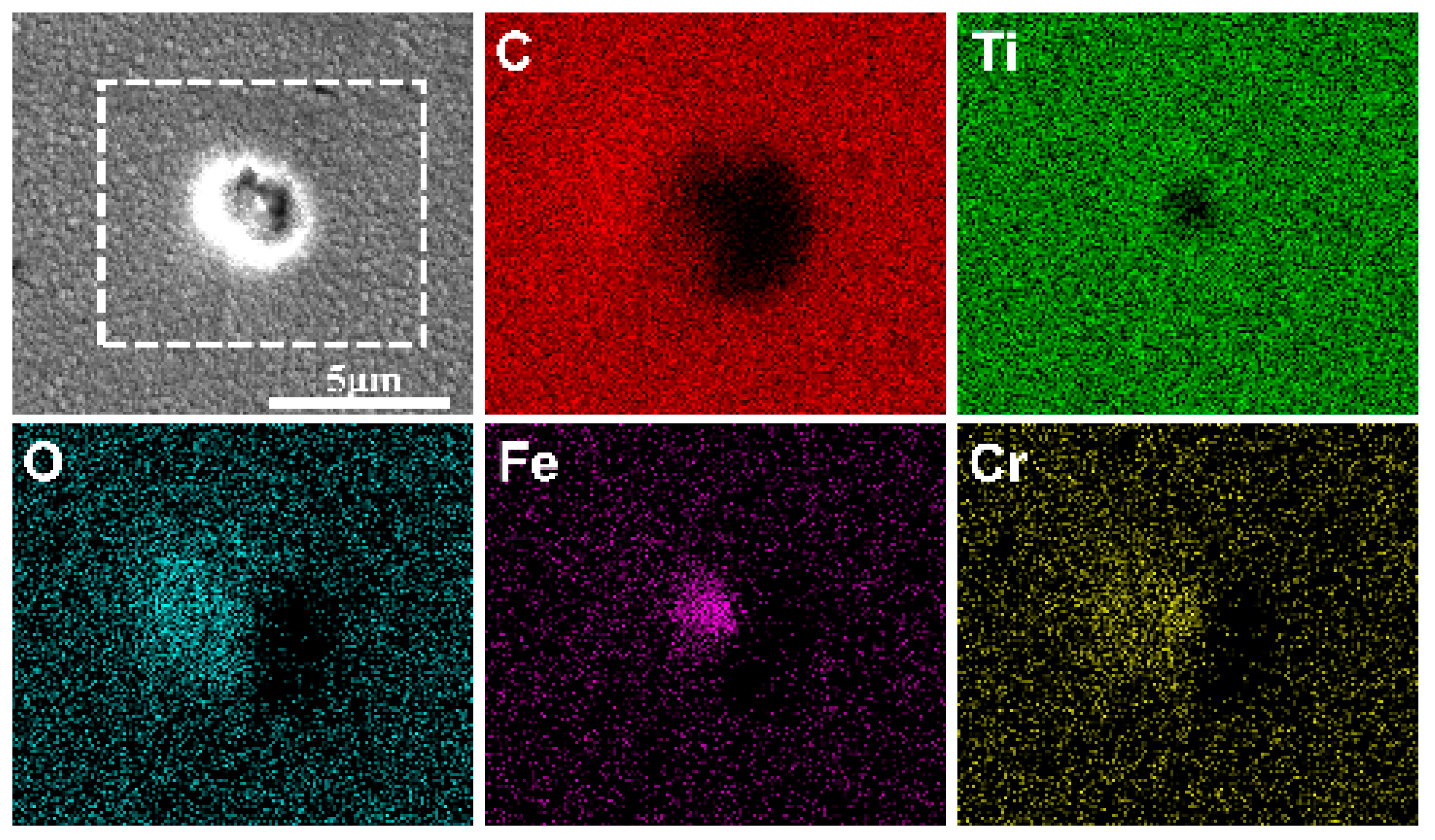
3.3. Corrosion Properties
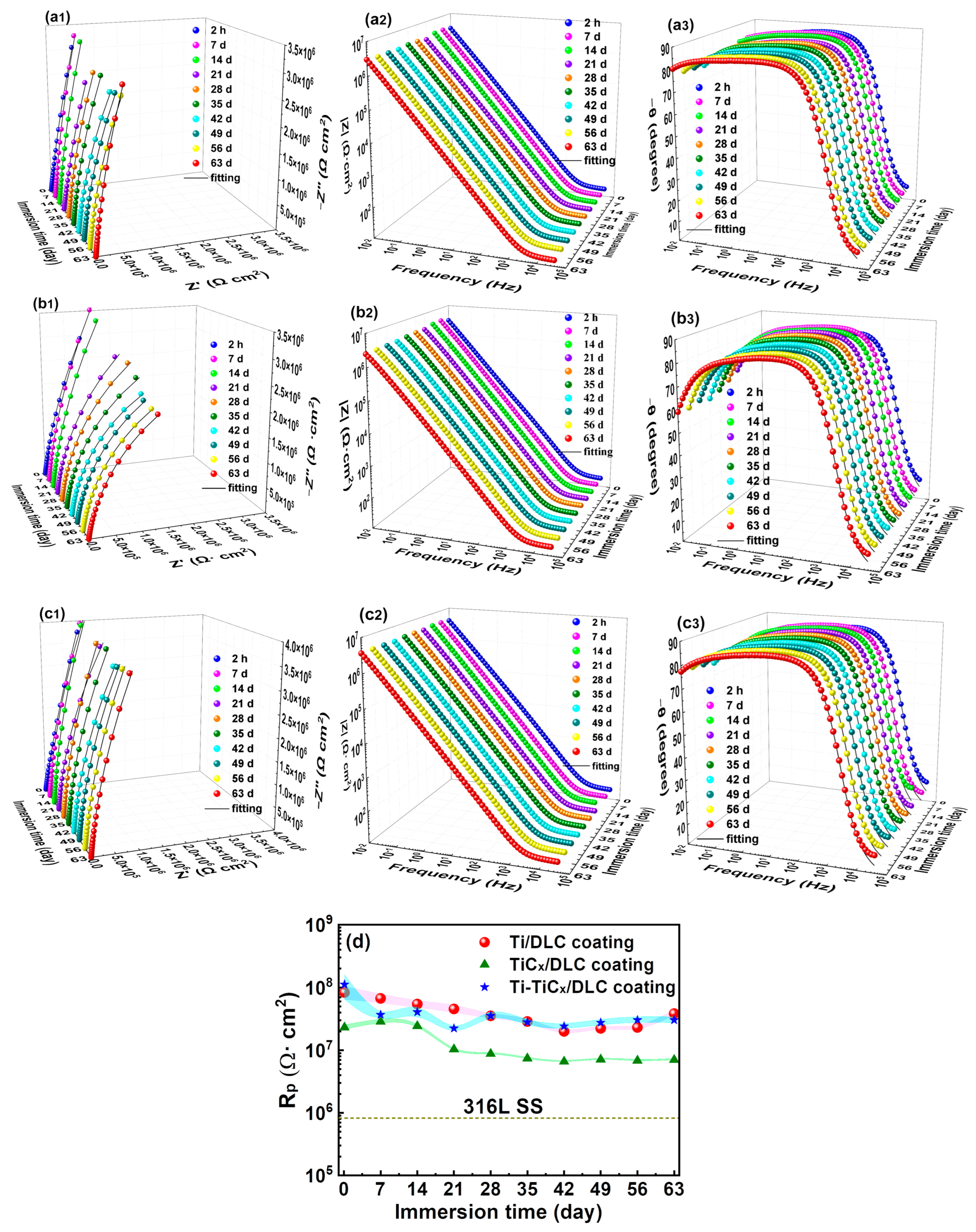
3.4. Electrochemical Corrosion Properties after Long-Term (63 Days) In-Situ Immersion
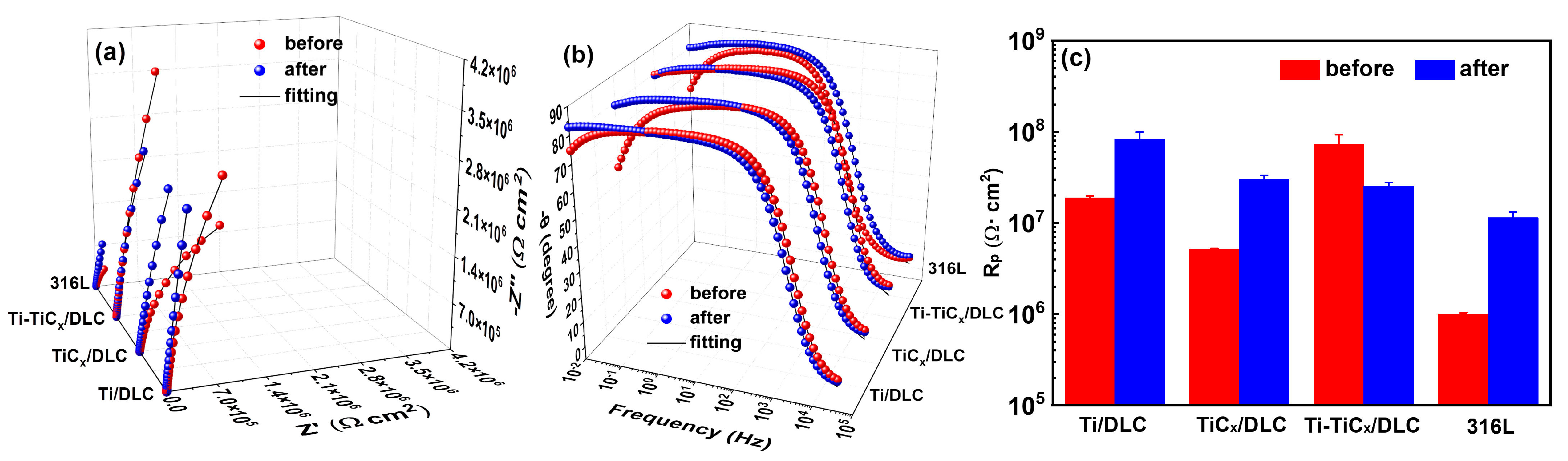
3.5. Electrochemical Corrosion Properties after a 3000 h Salt Spray Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, R.J.K. Marine wear and tribocorrosion. Wear 2017, 376, 893–910. [Google Scholar] [CrossRef]
- Litwin, W.; Dymarski, C. Experimental research on water-lubricated marine stern tube bearings in conditions of improper lubrication and cooling causing rapid bush wear. Tribol. Int. 2016, 95, 449–455. [Google Scholar] [CrossRef]
- Han, W.; Chen, S.; Campbell, J.; Zhang, X.J.; Tang, Y.H. Fracture toughness and wear properties of nanosilica/epoxy composites under marine environment. Mater. Chem. Phys. 2016, 177, 147–155. [Google Scholar] [CrossRef]
- Biswas, P.V.; Ahn, J.H.; Lee, H.R. Monitoring of bearing wear in a marine engine by change of piston position—Effect of wear and inertia. Int. J. Precis. Eng. Manuf. 2013, 14, 1697–1701. [Google Scholar] [CrossRef]
- Li, Y.; Hu, T.; Li, Q.; Wu, Y.; Wang, L.; You, Y.; Wang, B.Y. Evaluation of the stress corrosion crack growth behaviour of high-strength marine steel based on model of crack tip mechano-electrochemical effect. J. Mater. Sci. Technol. 2024, 190, 93–105. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, Z.H.; Chen, X.G.; Dong, Y.C.; Yang, Y.; Li, Y.Z.; Yan, D.R. Electrochemical impedance studies on tribocorrosion behavior of plasma-sprayed Al2O3 coatings. J. Therm. Spray Technol. 2015, 24, 878–884. [Google Scholar] [CrossRef]
- Xu, J.; Cai, L.X.; Lian, J.D.; Liao, Y.H. Corrosion-resistant behavior and structural optimization of Ti/Cr, Ti/Cu, Cr/Cu, Ti/Cr/Cu multimetallic layer coatings in simulated seawater. J. Phys. D-Appl. Phys. 2024, 57, 155501. [Google Scholar] [CrossRef]
- Zhang, S.L.; Huang, T.L.; Sun, S.D.; Wu, S.Z.; Yang, X.D.; Guo, F.; Zhang, B.; Dai, L.J. Effects of bias voltages on the tribological behaviors of DLC coatings. Coatings 2024, 14, 176. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, Q.; Ramachandran, C.S.; Guo, P.; Wang, A.Y. Microstructure andperformance of high-velocity oxygen-fuel coupled physical vapor deposition (HVOF-PVD) duplex protective coatings: A review. Coatings 2022, 12, 1395. [Google Scholar] [CrossRef]
- Sui, X.; Xu, R.; Liu, J.; Zhang, S.; Wu, Y.; Yang, J.; Hao, J. Tailoring the tribocorrosion and antifouling performance of (Cr, Cu)-GLC coatings for marine application. ACS Appl. Mater. Interfaces 2018, 10, 36531–36539. [Google Scholar] [CrossRef]
- Azzi, M.; Paquette, M.; Szpunar, J.A.; Klemberg-Sapieha, J.E.; Martinu, L. Tribocorrosion behaviour of DLC-coated 316L stainless steel. Wear 2009, 267, 860–866. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, K.R.; Yang, S.J. Wear-corrosion performance of Si-DLC coatings on Ti-6Al-4V substrate. J. Biomed. Mater. Res. Part A 2008, 86, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Q.; Yang, C.Y. Enhanced Wear and Corrosion Resistance of AZ91 Magnesium Alloy via Adherent Si-DLC Coating with Si-Interlayer: Impact of Biasing Voltage. Coatings 2024, 14, 341. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yin, P.M.; Mou, C.L.; Cao, X.Q.; Zhang, G.A.; Xue, Q.J. Corrosion behavior of Si-DLC film in simulant solutions containing CO2-H2S-Cl. Surf. Coat. Technol. 2024, 478, 130455. [Google Scholar] [CrossRef]
- Vicen, M.; Kajánek, D.; Trsko, L.; Bokuvka, O.; Buchtík, M.; Florková, Z.; Frkán, M. Improving of 100Cr6 steel corrosion and wear properties in simulated sea water environment by Tungsten-Doped DLC coating. Materials 2023, 16, 4334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Du, N.Z.; Li, X.W.; Wei, X.B.; Ding, J.Q.; Lu, S.Q.; Du, S.J.; Feng, C.A.; Chen, K.; Zhang, D.K.; et al. Atomic-scale understanding on the tribological behavior of amorphous carbon films under different contact pressures and surface textured shapes. Materials 2023, 16, 6108. [Google Scholar] [CrossRef] [PubMed]
- Totolin, V.; Pejaković, V.; Csanyi, T.; Hekele, O.; Huber, M.; Rodríguez Ripoll, M. Surface engineering of Ti6Al4V surfaces for enhanced tribocorrosion performance in artificial seawater. Mater. Des. 2016, 104, 10–18. [Google Scholar] [CrossRef]
- Wei, J.; Li, H.; Liu, L.; Guo, P.; Ke, P.; Wang, A. Enhanced tribological and corrosion properties of multilayer ta-C films via alternating sp3 content. Surf. Coat. Technol. 2019, 374, 317–326. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.L.; Li, X.; Guo, P.; Ke, P.; Wang, A. Enhanced tribocorrosion performance of Cr/GLC multilayered films for marine protective application. ACS Appl. Mater. Interfaces 2018, 10, 13187–13198. [Google Scholar] [CrossRef]
- Maguire, P.D.; McLaughlin, J.A.; Okpalugo, T.I.T.; Lemoine, P.; Papakonstantinou, P.; McAdams, E.T.; Needham, M.; Ogwu, A.A.; Ball, M.; Abbas, G.A. Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam. Relat. Mater. 2005, 14, 1277–1288. [Google Scholar] [CrossRef]
- Turcio-Ortega, D.; Rodil, S.E.; Muhl, S. Corrosion behavior of amorphous carbon deposit in 0.89% NaCl by electrochemical impedance spectroscopy. Diam. Relat. Mater. 2009, 18, 1360–1368. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Li, H.; Ma, G.; Sun, L.; Guo, P.; Ke, P.; Lee, K.-R.; Wang, A. Controllable defect engineering to enhance the corrosion resistance of Cr/GLC multilayered coating for deep-sea applications. Corros. Sci. 2022, 199, 110175. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zuo, X.; Guo, P.; Liu, L.; Lee, K.-R.; Wang, A.; Ke, P. Cr/GLC multilayered coating in simulated deep-sea environment: Corrosion behavior and growth defect evolution. Corros. Sci. 2021, 188, 109528. [Google Scholar] [CrossRef]
- Li, H.; Guo, P.; Zhang, D.; Liu, L.; Wang, Z.; Ma, G.; Xin, Y.; Ke, P.; Saito, H.; Wang, A. Interface-induced degradation of amorphous carbon films/stainless steel bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2020, 469, 228269. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Guo, P.; Sun, L.; Wei, J.; Liu, Y.; Li, S.; Wang, S.; Lee, K.-R.; Ke, P.; et al. Long-term tribocorrosion resistance and failure tolerance of multilayer carbon-based coatings. Friction 2022, 10, 1707–1721. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Sun, H.; Leng, Y.X.; Li, X.Y.; Yang, W.M.; Huang, N. Effect of modulation periods on the microstructure and mechanical properties of DLC/TiC multilayer films deposited by filtered cathodic vacuum arc method. Appl. Surf. Sci. 2015, 328, 319–324. [Google Scholar] [CrossRef]
- Ipaz, L.; Caicedo, J.C.; Esteve, J.; Espinoza-Beltran, F.J.; Zambrano, G. Improvement of mechanical and tribological properties in steel surfaces by using titanium–aluminum/titanium–aluminum nitride multilayered system. Appl. Surf. Sci. 2012, 258, 3805–3814. [Google Scholar] [CrossRef]
- Li, C.L.; Wu, F.B.; Lee, J.W.; Tsai, Y.Z.; Chang, L.C. Characteristics of Cr2N/Cu multilayered thin films with different bilayer thickness. Surf. Coat. Technol. 2009, 204, 941–946. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, F.; Liu, L.; Yang, S.; Ji, W. Investigation on cavitation erosion of diamond-like carbon films with heterogeneous multilayer structure. Surf. Coat. Technol. 2021, 405, 126682. [Google Scholar] [CrossRef]
- Taki, Y.; Takai, O. XPS structural characterization of hydrogenated amorphous carbon thin films prepared by shielded arc ion plating. Thin Solid Film. 1998, 316, 45–50. [Google Scholar] [CrossRef]
- Merel, P.; Tabbal, M.; Chaker, M.; Moisa, S.; Margot, J. Direct evaluation of the sp(3) content in diamond-like-carbon films by XPS. Appl. Surf. Sci. 1998, 136, 105–110. [Google Scholar] [CrossRef]
- Diaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys. Rev. B 1996, 54, 8064–8069. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.K.; Li, L.H. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006, 96, 253–277. [Google Scholar] [CrossRef]
- Mohagheghpour, E.; Rajabi, M.; Gholamipour, R.; Larijani, M.M.; Sheibani, S. Correlation study of structural, optical and electrical properties of amorphous carbon thin films prepared by ion beam sputtering deposition technique. Appl. Surf. Sci. 2016, 360, 52–58. [Google Scholar] [CrossRef]
- Guo, P.; Li, X.W.; Sun, L.L.; Chen, R.D.; Ke, P.L.; Wang, A.Y. Stress reduction mechanism of diamond-like carbon films incorporated with different Cu contents. Thin Solid Film. 2017, 640, 45–51. [Google Scholar] [CrossRef]
- Nakao, S.; Yukimura, K.; Nakano, S.; Ogiso, H. DLC Coating by HiPIMS: The Influence of Substrate Bias Voltage. IEEE Trans. Plasma Sci. 2013, 41, 1819–1829. [Google Scholar] [CrossRef]
- Chen, X.J.; Du, Y.; Chung, Y.W. Commentary on using H/E and H3/E2 as proxies for fracture toughness of hard coatings. Thin Solid Films 2019, 688, 137265. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yan, M.F.; Yang, Y.; Zhang, Y.X.; Yan, F.Y.; Li, H.T. Excellent mechanical, tribological and anti-corrosive performance of novel Ti-DLC nanocomposite thin films prepared via magnetron sputtering method. Carbon 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Ren, P.; Wen, M.; Zhang, K.; Du, S.; Zhang, Y.; Chen, J.; Zheng, W. Self-assembly of TaC@Ta core–shell-like nanocomposite film via solid-state dewetting: Toward superior wear and corrosion resistance. Acta Mater. 2018, 160, 72–84. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.F.; Zhang, Y.X.; Li, D.Y.; Zhang, C.S.; Zhu, Y.D.; Wang, Y.X. Catalytic growth of diamond-like carbon on Fe3C-containing carburized layer through a single-step plasma-assisted carburizing process. Carbon 2017, 122, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Bai, W.; Wang, X.; Gu, C.; Jin, G.; Tu, J. Mechanical properties and in vitro and in vivo biocompatibility of a-C/a-C:Ti nanomultilayer films on Ti6Al4V Alloy as medical implants. ACS Appl. Mater. Interfaces 2017, 9, 15933–15942. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Zhang, K.; He, X.; Du, S.X.; Yang, X.Y.; An, T.; Wen, M.; Zheng, W.T. Toughness enhancement and tribochemistry of the Nb-Ag-N films actuated by solute Ag. Acta Mater. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Mockute, A.; Palisaitis, J.; Alling, B.; Berastegui, P.; Broitman, E.; Naslund, L.A.; Nedfors, N.; Lu, J.; Jensen, J.; Hultman, L.; et al. Age hardening in (Ti1-xAlx) B2+Δ thin films. Scr. Mater. 2017, 127, 122–126. [Google Scholar] [CrossRef]
- Lin, J.; Wei, R.; Bitsis, D.C.; Lee, P.M. Development and evaluation of low friction TiSiCN nanocomposite coatings for piston ring applications. Surf. Coat. Technol. 2016, 298, 121–131. [Google Scholar] [CrossRef]
- Habibi, M.; Javadi, S.; Ghoranneviss, M. Investigation on the structural properties and corrosion inhibition of W coatings on stainless steel AISI 304 using PF device. Surf. Coat. Technol. 2014, 254, 112–120. [Google Scholar] [CrossRef]
- Choi, H.S.; Park, B.; Lee, J.J. CrB2 coatings deposited by inductively coupled plasma assisted DC magnetron sputtering. Surf. Coat. Technol. 2007, 202, 982–986. [Google Scholar] [CrossRef]
- Audronis, M.; Kelly, P.J.; Arnell, R.D.; Leyland, A.; Matthews, A. The structure and properties of chromium diboride coatings deposited by pulsed magnetron sputtering of powder targets. Surf. Coat. Technol. 2005, 200, 1366–1371. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II. EIS interpretation of corrosion behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.H.; Kim, H.G.; Kim, J.G. A study on the quantitative determination of through-coating porosity in PVD-grown coatings. Appl. Surf. Sci. 2004, 233, 105–114. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, S.; Chen, D.L.; Qasim, A.M.; Ding, K.J.; Wu, G.S.; Chu, P.K. Effects of diamond-like carbon film on the corrosion behavior of NdFeB permanent magnet. Surf. Coat. Technol. 2017, 312, 66–74. [Google Scholar] [CrossRef]
- Shanaghi, A.; Rouhaghdam, A.R.S.; Ahangarani, S.; Chu, P.K.; Farahani, T.S. Effects of duty cycle on microstructure and corrosion behavior of TiC coatings prepared by DC pulsed plasma CVD. Appl. Surf. Sci. 2012, 258, 3051–3057. [Google Scholar] [CrossRef]
- Zeng, A.; Liu, E.; Annergren, I.F.; Tan, S.N.; Zhang, S.; Hing, R.; Gao, J. EIS capacitance diagnosis of nanoporosity effect on the corrosion protection of DLC films. Diam. Relat. Mater. 2002, 11, 160–168. [Google Scholar] [CrossRef]
- Grips, V.K.W.; Selvi, V.E.; Barshilia, H.C.; Rajam, K.S. Effect of electroless nickel interlayer on the electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive dc magnetron sputtering. Electrochim. Acta 2006, 51, 3461–3468. [Google Scholar] [CrossRef]
- Manninen, N.K.; Calderon, S.; Carvalho, I.; Henriques, M.; Cavaleiro, A.; Carvalho, S. Antibacterial Ag/a-C nanocomposite coatings: The influence of nano-galvanic a-C and Ag couples on Ag ionization rates. Appl. Surf. Sci. 2016, 377, 283–291. [Google Scholar] [CrossRef]
- ASTM B117-2016; Standard Practice for Operating Salt Spray (Fog) apparatus. ASTM International: West Conshohocken, PA, USA, 2018.
- Trinh, D.; Dauphin Ducharme, P.; Mengesha Tefashe, U.; Kish, J.R.; Mauzeroll, J. Influence of edge effects on local corrosion rate of magnesium alloy/mild steel galvanic couple. Anal. Chem. 2012, 84, 9899–9906. [Google Scholar] [CrossRef]
- Guimaraes, M.C.R.; de Castilho, B.C.N.M.; Nossa, T.D.; Avila, P.R.T.; Cucatti, S.; Alvarez, F.; Garcia, J.L.; Pinto, H.C. On the effect of substrate oscillation on CrN coatings deposited by HiPIMS and dcMS. Surf. Coat. Technol. 2018, 340, 112–120. [Google Scholar] [CrossRef]
- Viswanathan, S.; Mohan, L.; Bera, P.; Kumar, V.P.; Barshilia, H.C.; Anandan, C. Corrosion and wear behaviors of Cr-Doped diamond-like carbon coatings. J. Mater. Eng. Perform. 2017, 26, 3633–3647. [Google Scholar] [CrossRef]
- Shukla, K.; Rane, R.; Alphonsa, J.; Maity, P.; Mukherjee, S. Structural, mechanical and corrosion resistance properties of Ti/TiN bilayers deposited by magnetron sputtering on AISI 316L. Surf. Coat. Technol. 2017, 324, 167–174. [Google Scholar] [CrossRef]
- Bai, W.Q.; Xie, Y.J.; Li, L.L.; Wang, X.L.; Gu, C.D.; Tu, J.P. Tribological and corrosion behaviors of Zr-doped graphite-like carbon nanostructured coatings on Ti6Al4V alloy. Surf. Coat. Technol. 2017, 320, 235–239. [Google Scholar] [CrossRef]
- Lin, J.L.; Wang, B.; Ou, Y.X.; Sproul, W.D.; Dahan, I.; Moore, J.J. Structure and properties of CrSiN nanocomposite coatings deposited by hybrid modulated pulsed power and pulsed dc magnetron sputtering. Surf. Coat. Technol. 2013, 216, 251–258. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]







| Sample | D Peak (cm−1) | G Peak (cm−1) | GFWHM (cm−1) | ID/IG |
|---|---|---|---|---|
| Ti/DLC | 1368.04 | 1545.73 | 181.14 | 0.56 |
| TiCx/DLC | 1369.25 | 1552.84 | 181.73 | 0.59 |
| Ti-TiCx/DLC | 1369.80 | 1547.26 | 179.95 | 0.56 |
| Sample | H (GPa) | E (GPa) | H/E | H3/E2 (GPa) |
|---|---|---|---|---|
| 316Lss | 5.18 ± 0.16 | 238.46 ± 7.64 | 0.022 | 0.0024 |
| Ti/DLC | 16.44 ± 0.42 | 210.37 ± 4.92 | 0.078 | 0.1004 |
| TiCx/DLC | 22.79 ± 0.33 | 255.78 ± 4.57 | 0.089 | 0.1809 |
| Ti-TiCx/DLC | 16.53 ± 0.68 | 205.78 ± 8.37 | 0.080 | 0.1067 |
| Sample | Ti/DLC | TiCx/DLC | Ti-TiCx/DLC | 316Lss |
|---|---|---|---|---|
| Ecorr (V) | −0.05 | −0.15 | 0.12 | −0.19 |
| ipass (A/cm2) | 1.12 × 10−9 | 4.27 × 10−9 | 5.68 × 10−10 | 1.86 × 10−8 |
| βa (mV/decade) | 135.00 | 177.70 | 110.70 | 61.00 |
| βc (mV/decade) | 87.92 | 66.99 | 244.50 | 46.15 |
| Rp (Ω·cm2) | 2.06 × 107 | 4.95 × 106 | 5.83 × 107 | 6.13 × 105 |
| Epit (V) | ~1.01 | ~0.98 | ~1.00 | ~0.36 |
| Sample | Ti/DLC | TiCx/DLC | Ti-TiCx/DLC | 316Lss |
|---|---|---|---|---|
| Rs (Ω·cm2) | 18.62 | 20.25 | 23.7 | 22.27 |
| Cpore (F·cm−2) | 4.00 × 10−6 | 3.87 × 10−6 | 3.05 × 10−6 | 3.24 × 10−5 |
| Rpore (Ω·cm2) | 547,820 | 62,590 | 515,040 | 103,820 |
| Qdl (Ω−1·cm−2·sn) | 3.43 × 10−7 | 8.255 × 10−7 | 3.83 × 10−7 | 2.48 × 10−6 |
| ndl | 0.651 | 0.639 | 0.65 | 0.50 |
| Rct (Ω·cm2) | 2.23 × 107 | 5.76 × 106 | 1.78 × 108 | 1.19 × 106 |
| χ2 | 3.22 × 10−4 | 2.82 × 10−4 | 9.37 × 10−4 | 4.94 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, H.; Guo, P.; Li, X.; Yang, W.; Ma, G.; Nishimura, K.; Ke, P.; Wang, A. Enhanced Long-Term Corrosion Resistance of 316L Stainless Steel by Multilayer Amorphous Carbon Coatings. Materials 2024, 17, 2129. https://doi.org/10.3390/ma17092129
Li S, Li H, Guo P, Li X, Yang W, Ma G, Nishimura K, Ke P, Wang A. Enhanced Long-Term Corrosion Resistance of 316L Stainless Steel by Multilayer Amorphous Carbon Coatings. Materials. 2024; 17(9):2129. https://doi.org/10.3390/ma17092129
Chicago/Turabian StyleLi, Shuyu, Hao Li, Peng Guo, Xiaowei Li, Wei Yang, Guanshui Ma, Kazuhito Nishimura, Peiling Ke, and Aiying Wang. 2024. "Enhanced Long-Term Corrosion Resistance of 316L Stainless Steel by Multilayer Amorphous Carbon Coatings" Materials 17, no. 9: 2129. https://doi.org/10.3390/ma17092129





