Effect of Cooling Rate on Microstructure of In Situ TiC-Reinforced Composite Surface Layers Synthesized on Ductile Cast Iron by Laser Alloying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser Processing
2.3. Examination of Thermal Conditions in the Molten Pool
2.4. Metallographic Examination
2.5. Hardness and Nanoindentation Testing
3. Results
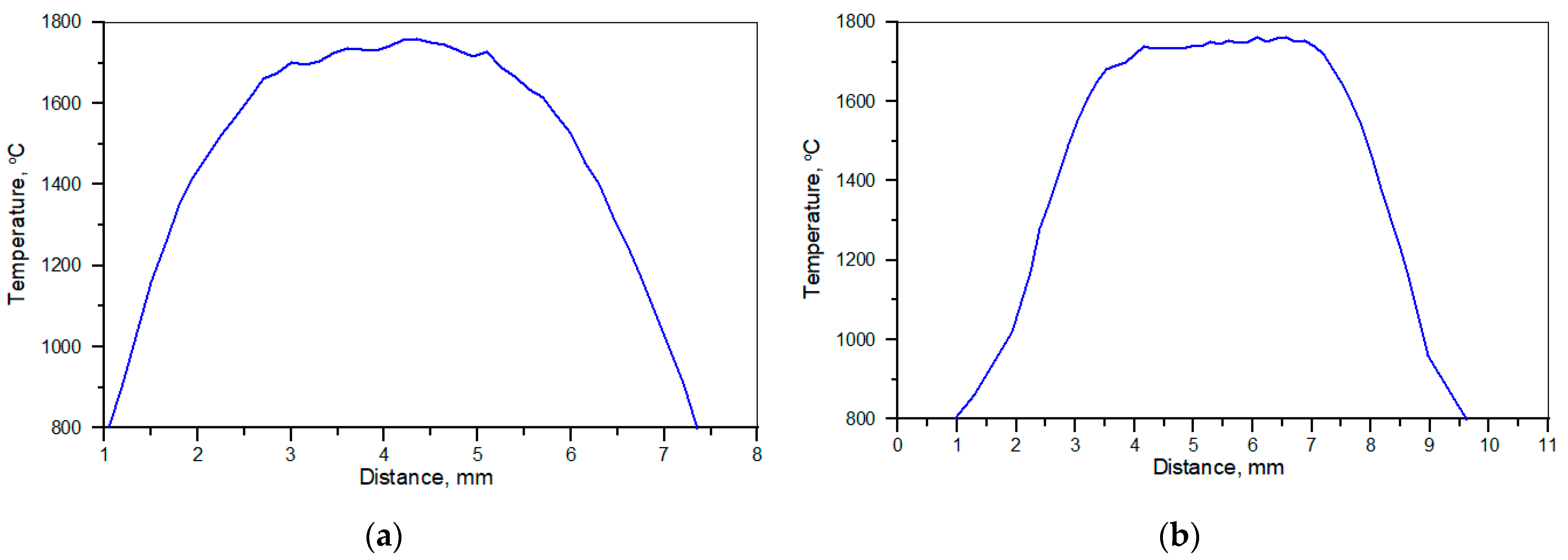
3.1. Examination of Thermal Conditions in the Molten Pool
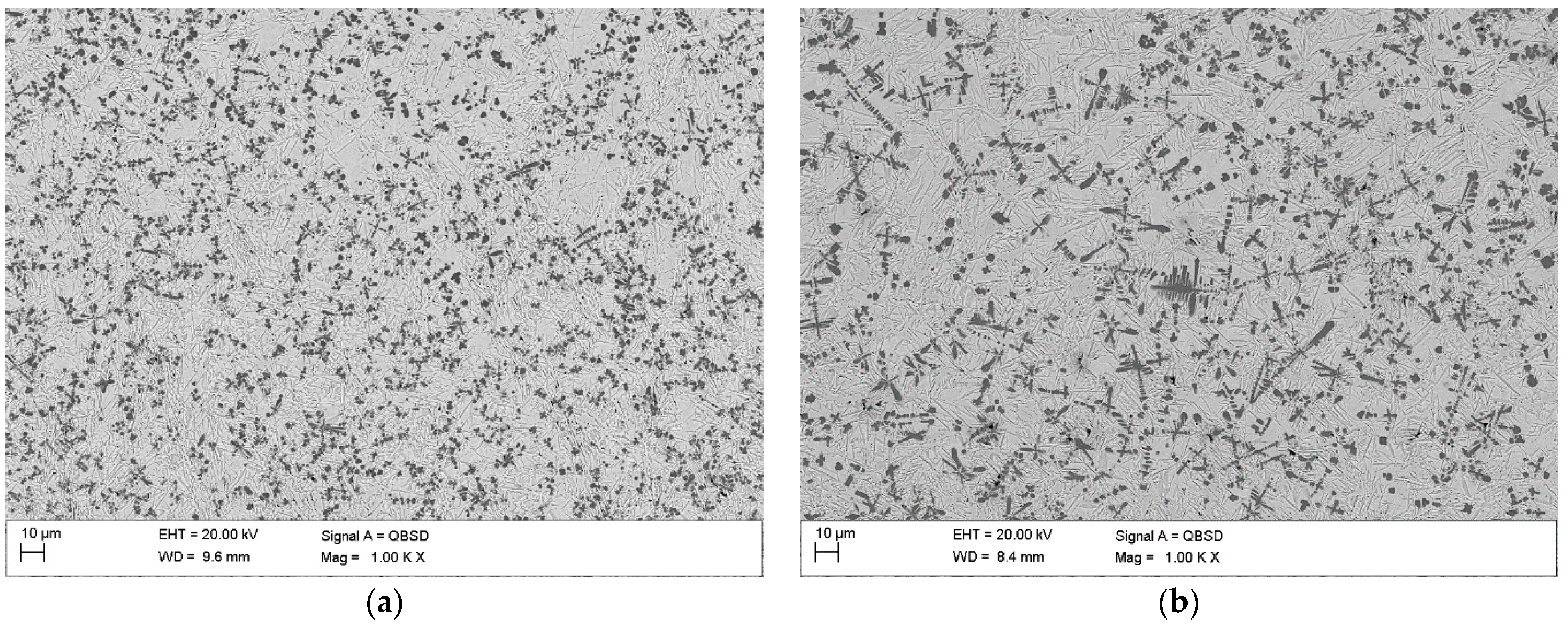
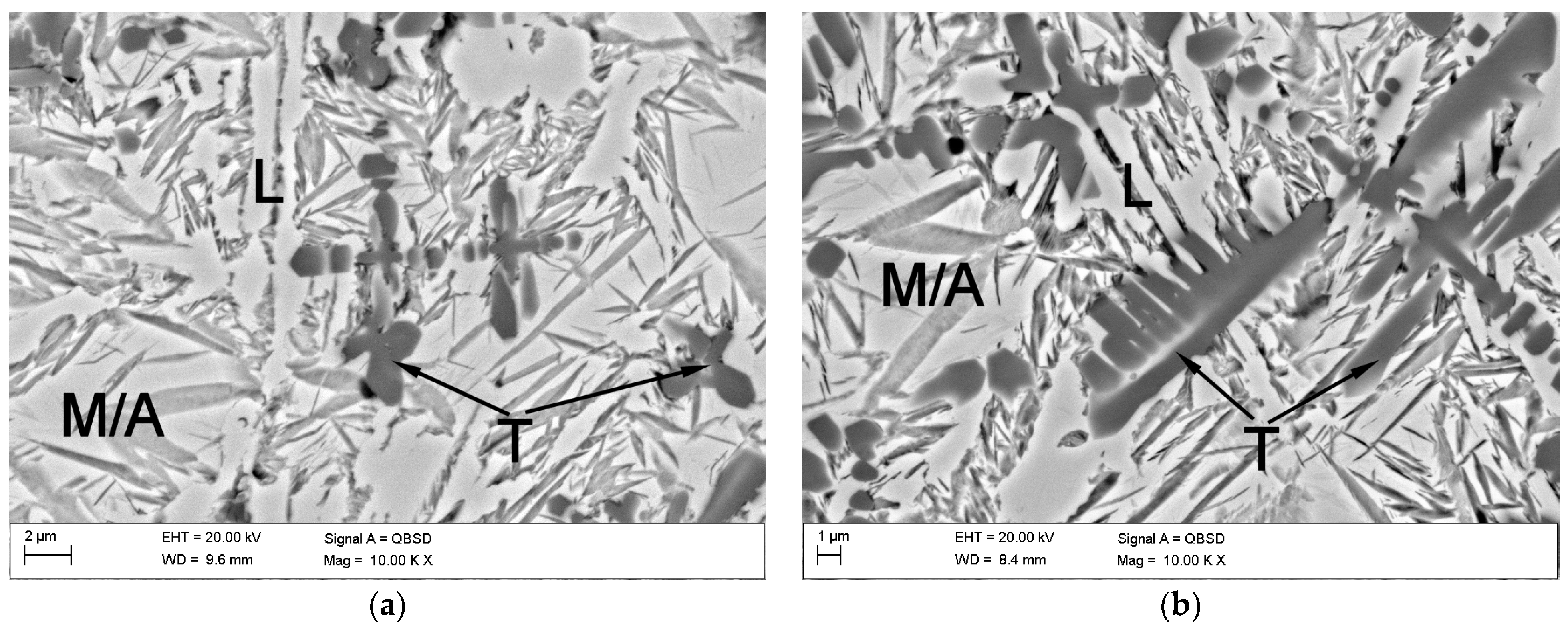
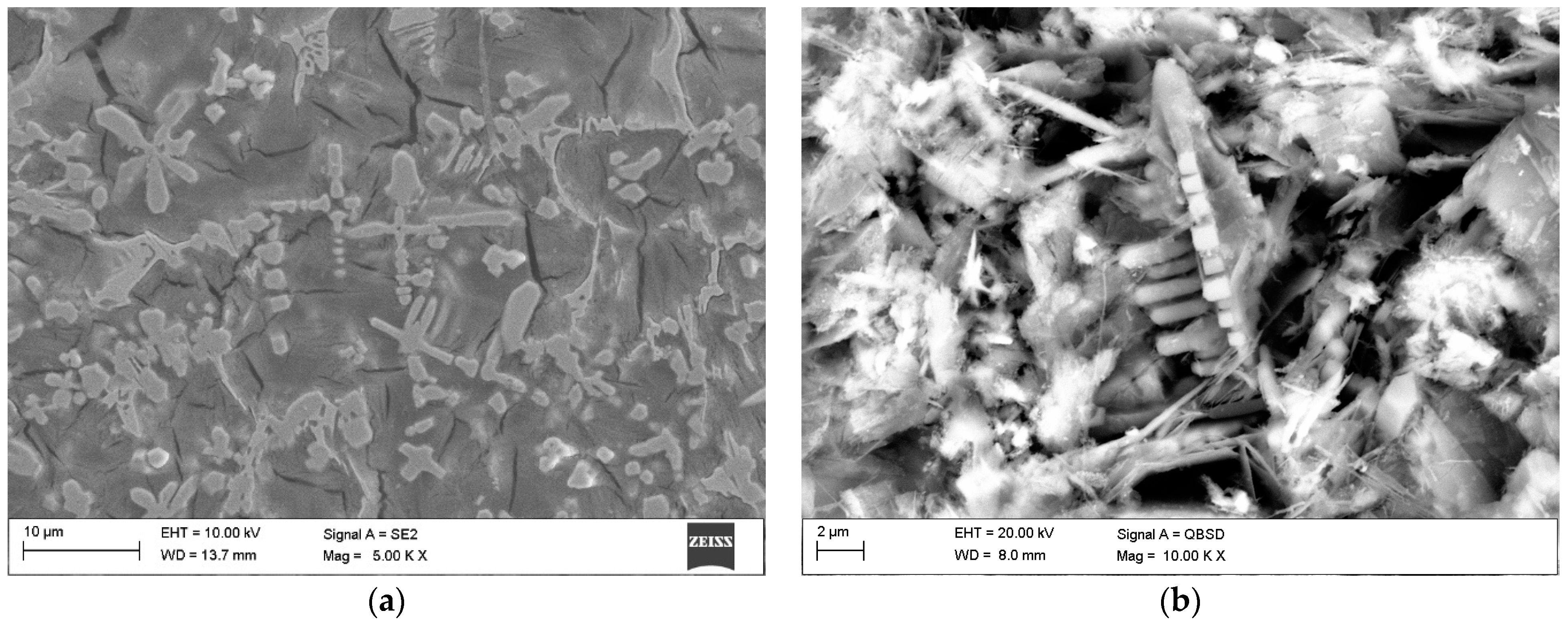
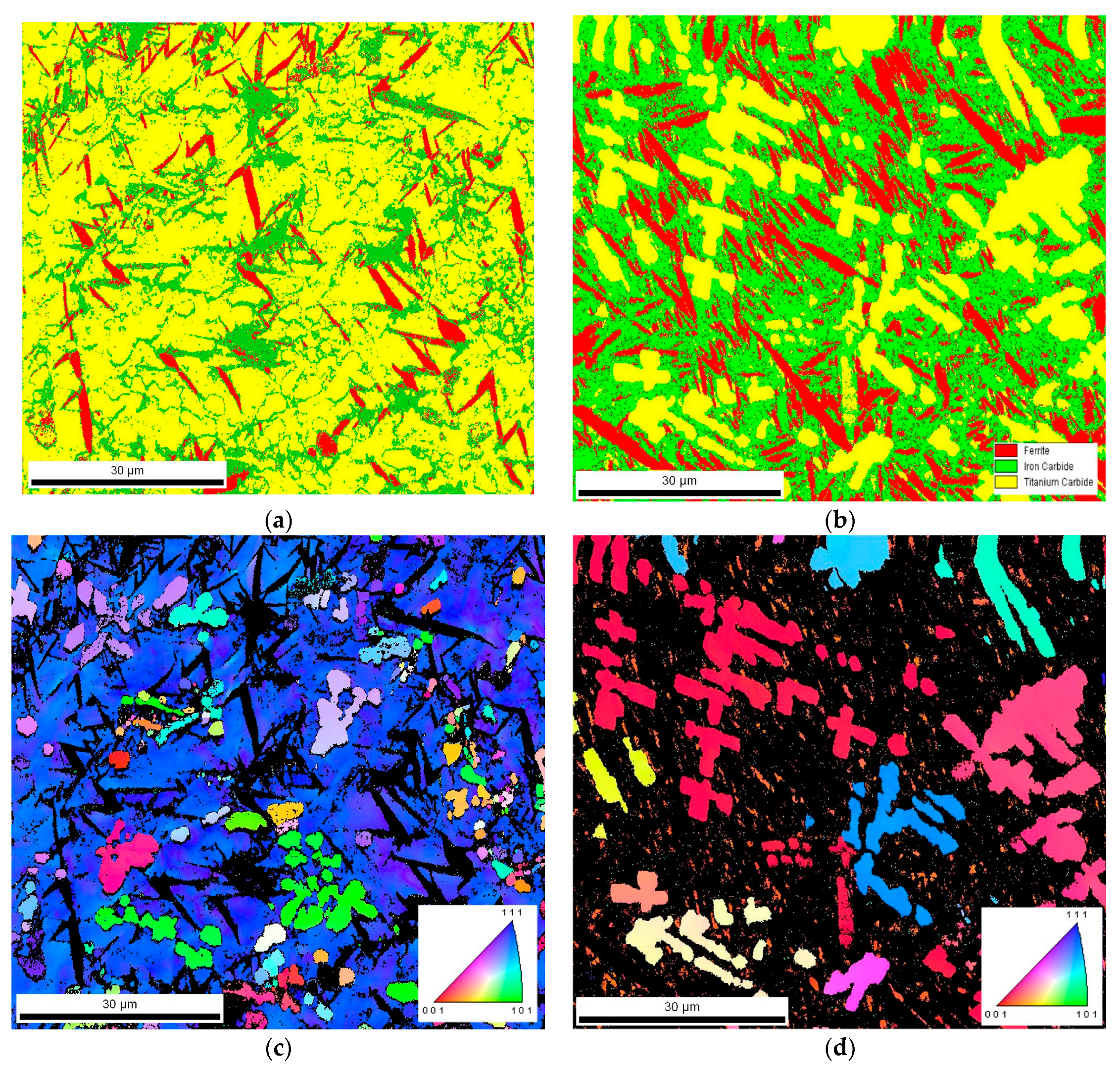
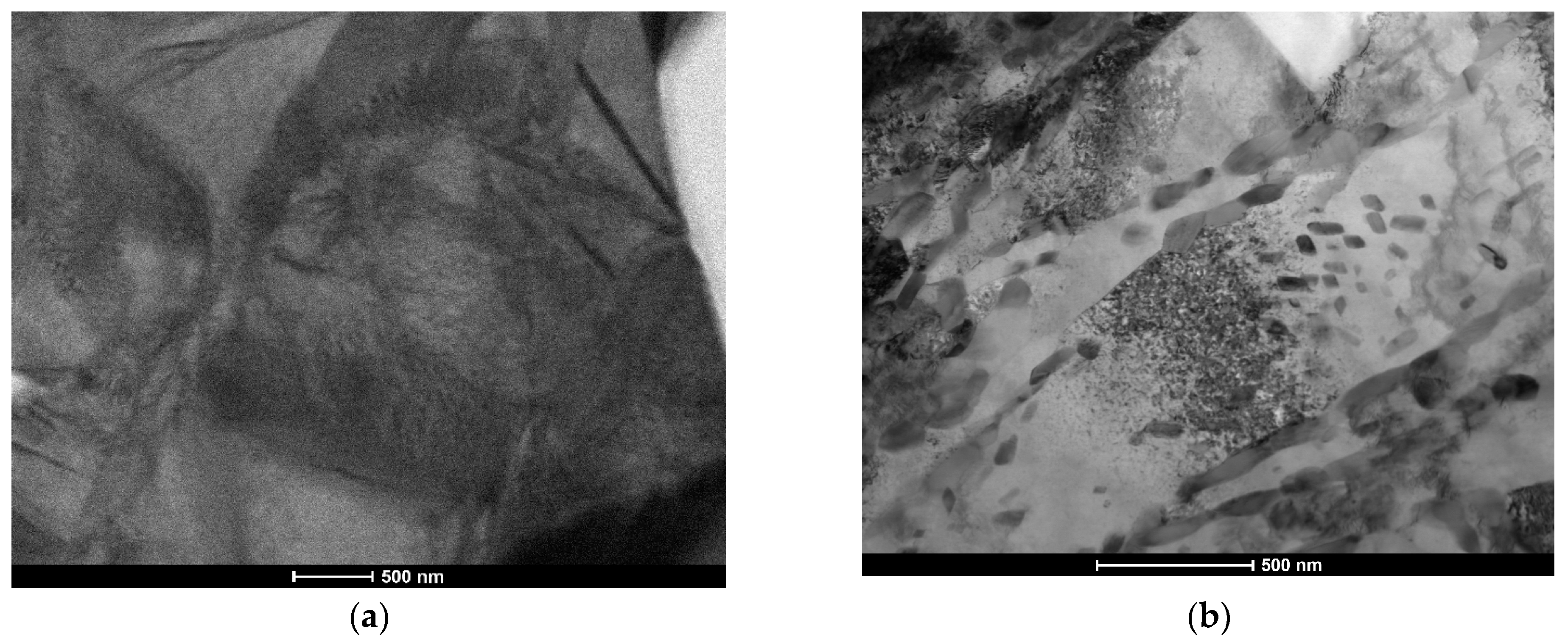
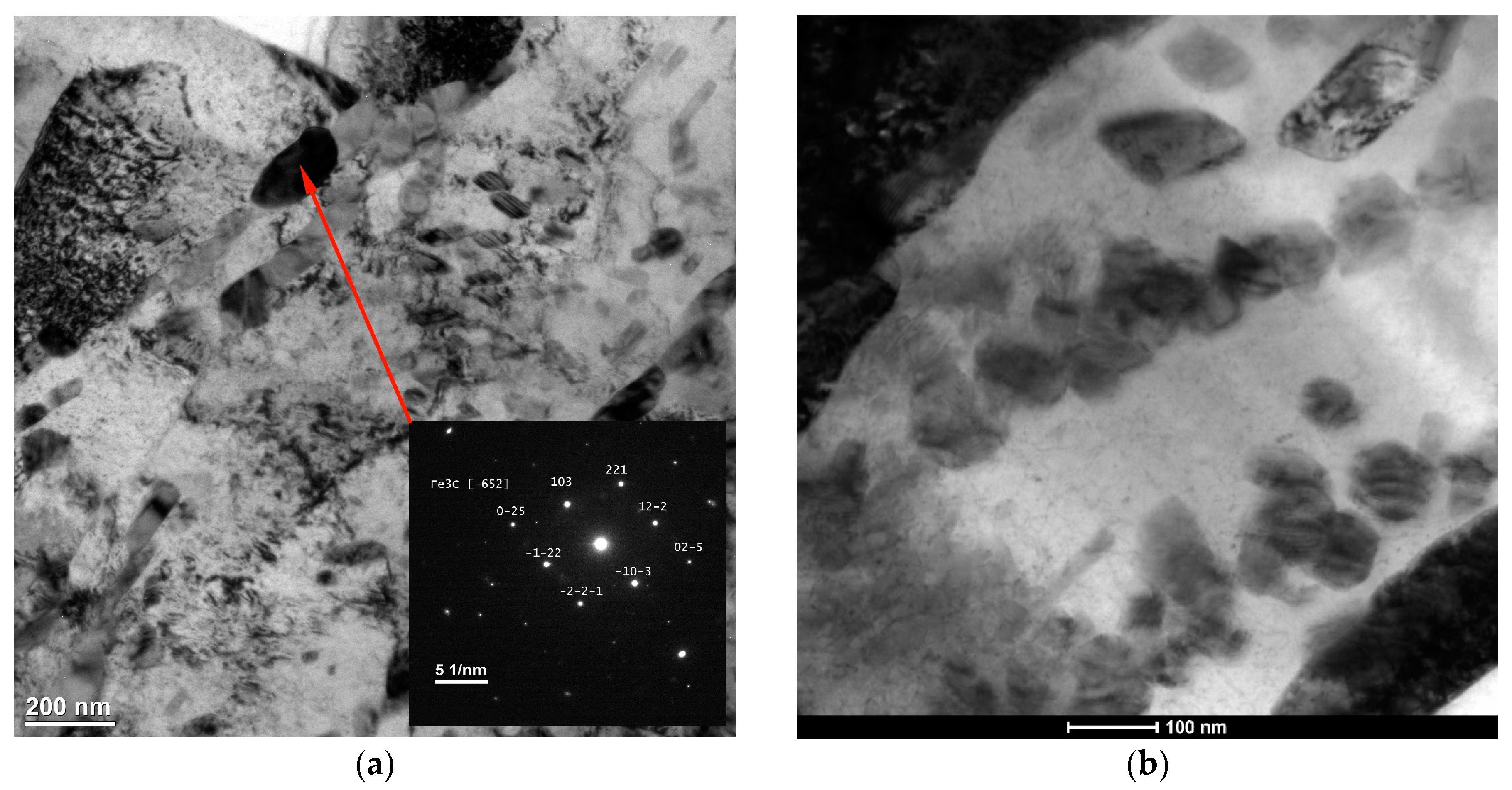
3.2. Macro- and Microstructure Analysis
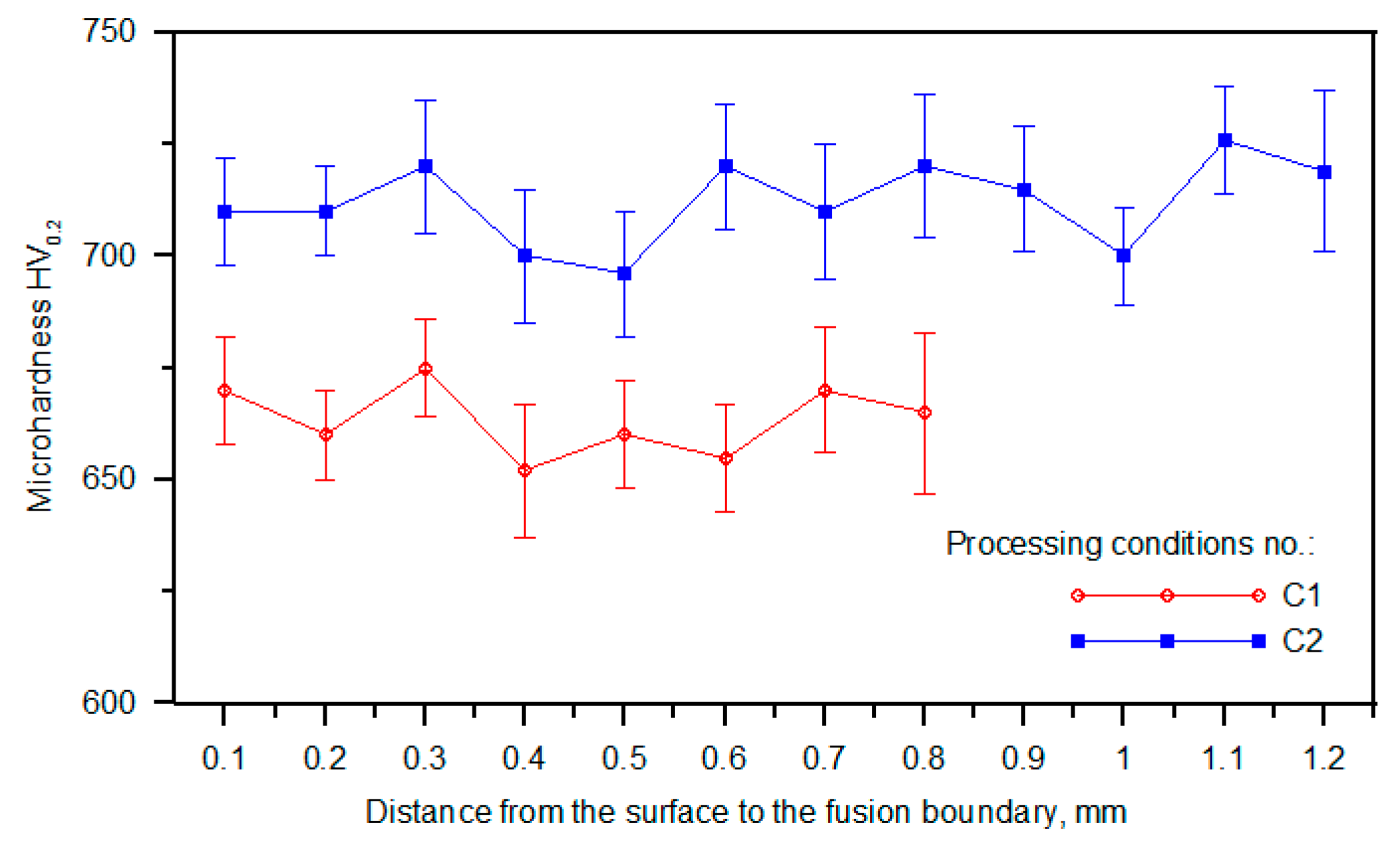
3.3. Hardness Analysis
4. Discussion
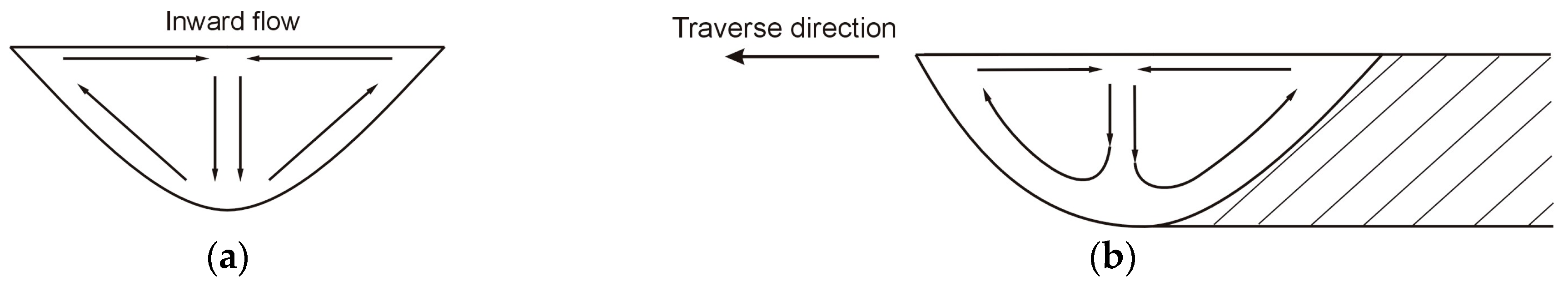
4.1. Examination of Thermal Conditions in the Molten Pool
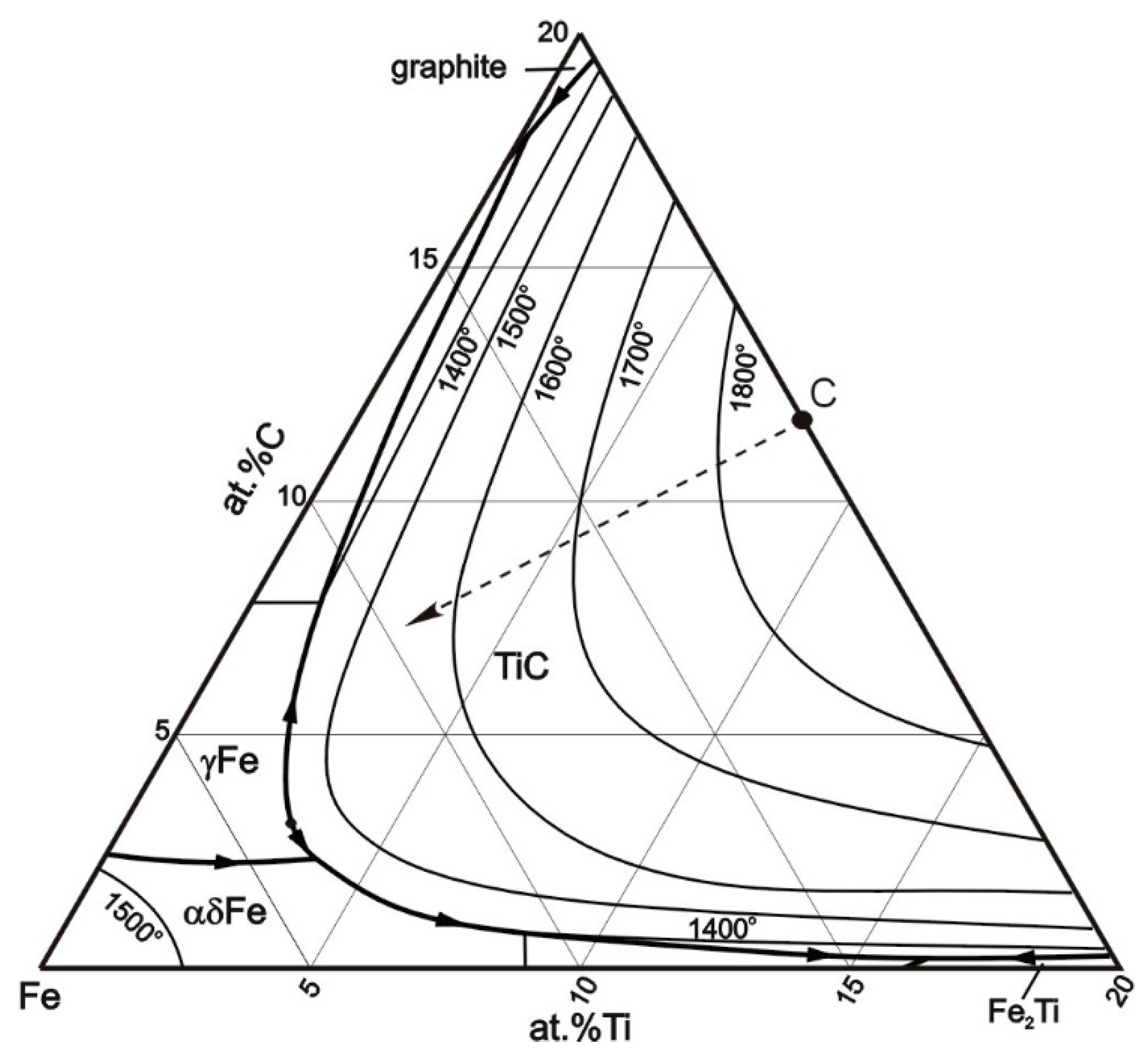
4.2. Microstructure and Hardness Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández, J.E.; Vijande, R.; Tucho, R.; Rodrıguez, J.; Martın, A. Materials selection to excavator teeth in mining industry. Wear 2001, 250, 11–18. [Google Scholar] [CrossRef]
- Singh, J.; Chatha, S.S.; Sidhu, B.S. Abrasive wear behavior of newly developed weld overlaid tillage tools in laboratory and in actual field conditions. J. Manuf. Process. 2020, 55, 142–152. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.; Yu, H.; Li, H. Characteristics of Fe-based alloy coating produced by plasma cladding process. Mater. Sci. Eng. A 2007, 457, 319–324. [Google Scholar] [CrossRef]
- Lyu, Y.; Sun, Y.; Jing, F. On the microstructure and wear resistance of Fe-based composite coatings processed by plasma cladding with B4C injection. Ceram. Int. 2015, 41, 10934–10939. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Sun, Y.; Wei, Y.; Wang, X.; Liu, D. Coarse TiC particles reinforced H13 steel matrix composites produced by laser cladding. Surf. Coat. Technol. 2020, 395, 125867. [Google Scholar] [CrossRef]
- Wang, X.H.; Song, S.L.; Qu, S.Y.; Zou, Z.D. Characterization of in situ synthesized TiC particle reinforced Fe-based composite coatings produced by multi-pass overlapping GTAW melting process. Surf. Coat. Technol. 2007, 201, 5899–5905. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Zhang, M.; Zuo, Z. Microstructure and wear properties of Fe–TiC surface composite coating by laser cladding. J. Mater. Sci. 2008, 43, 1546–1551. [Google Scholar] [CrossRef]
- Chen, L.; Yu, T.; Guan, C.; Zhao, Y. Microstructure and properties of metal parts remanufactured by laser cladding TiC and TiB2 reinforced Fe-based coatings. Ceram. Int. 2022, 48, 14127–14140. [Google Scholar] [CrossRef]
- Cui, C.; Guo, Z.; Wang, H.; Hu, J. In situ TiC particles reinforced grey cast iron composite fabricated by laser cladding of Ni–Ti–C system. J. Mater. Process. Technol. 2007, 183, 380–385. [Google Scholar] [CrossRef]
- Moreira, A.B.; Ribeiro, L.M.M.; Vieira, M.F. Production of TiC-MMCs Reinforcements in Cast Ferrous Alloys Using In Situ Methods. Materials 2021, 14, 5072. [Google Scholar] [CrossRef]
- Chung, R.J.; Tang, X.; Li, D.Y.; Hinckley, B.; Dolman, K. Effects of titanium addition on microstructure and wear resistance of hypereutectic high chromium cast iron Fe–25 wt.%Cr–4 wt.%C. Wear 2009, 267, 356–361. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zheng, Z.; Wang, A.; Huang, Q.; Zeng, D. Microstructure and high-temperature wear properties of in situ TiC composite coatings by plasma transferred arc surface alloying on gray cast iron. Int. J. Miner. Metall. Mater. 2015, 22, 12. [Google Scholar] [CrossRef]
- Zhu, M.; Jiang, Y.; Siu, Y.; Zhu, M. Study on microstructure and abrasive wear properties of in-situ TiC reinforced high chromium cast iron matrix composite. Mater. Res. Exspress. 2022, 9, 036517. [Google Scholar] [CrossRef]
- Janicki, D. The friction and wear behaviour of in-situ titanium carbide reinforced composite layers manufactured on ductile cast iron by laser surface alloying. Surf. Coat. Technol. 2021, 406, 126634. [Google Scholar] [CrossRef]
- Paczkowska, M.; Selech, J. An investigation of the influence of laser alloying of the surface layer on abrasive wear resistance of cast iron elements. Tribologia 2018, 6, 107–117. [Google Scholar] [CrossRef]
- Janicki, D. Fabrication of TiC-Reinforced Surface Layers on Ductile Cast Iron Substrate by Laser Surface Alloying. Solid. State Phenom. 2020, 308, 76–99. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Tabandeh-Khorshid, M.; Omrani, E.; Lovell, M.R.; Menezes, P.L. Tribology of metal matrix composites. In Tribology for Scientists and Engineers; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 8. [Google Scholar]
- AlMangour, B.; Grzesiak, D.; Yang, J.M. In-situ formation of novel TiC-particle reinforced 316L stainless steel bulk-form composites by selective laser melting. J. Alloys Compd. 2017, 706, 409–418. [Google Scholar] [CrossRef]
- Khalili, A.; Goodarzi, M.; Mojtahedi, M.; Torkamany, M.J. Solidification microstructure of in-situ laser-synthesized Fe-TiC hard coating. Surf. Coat. Technol. 2016, 307, 747–752. [Google Scholar] [CrossRef]
- Emamian, A.; Alimardani, M.; Khajepour, A. Correlation between temperature distribution and in situ formed microstructure of Fe–TiC deposited on carbon steel using laser cladding. Appl. Surf. Sci. 2012, 258, 9025–9031. [Google Scholar] [CrossRef]
- Janicki, D. Microstructure and sliding wear behaviour of in-situ TiC-reinforced composite surface layers fabricated on ductile cast iron by laser alloying. Materials 2018, 11, 75. [Google Scholar] [CrossRef]
- Janicki, D. Fabrication of high chromium white iron surface layers on ductile cast iron substrate by laser surface alloying. Stroj. Vestn. J. Mech. Eng. 2017, 63, 705–714. [Google Scholar]
- Janicki, D.; Górka, J.; Kwaśny, W.; Pakieła, W.; Matus, K. Influence of Solidification Conditions on the Microstructure of Laser-Surface-Melted Ductile Cast Iron. Materials 2020, 13, 1174. [Google Scholar] [CrossRef]
- Vander Voort, G.F. Metallography Principles and Practice; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Guemmaz, M.; Mosser, A.; Boudoukha, L.; Grob, J.J.; Raiser, D.; Sens, J.C. Ion beam synthesis of non-stoichiometric titanium carbide: Composition structure and nanoindentation studies. Nucl. Instrum. Methods Phys. Res. Sect. B 1996, 111, 263–270. [Google Scholar] [CrossRef]
- Sanders, D.J. Temperature distribution produced by scanning Gaussian laser beams. Appl. Opt. 1984, 23, 33–35. [Google Scholar] [CrossRef]
- Lhospitalier, S.; Bourges, P.; Bert, A.; Quesada, J.; Lambertin, M. Temperature measurement inside and near the weld pool during laser welding. J. Laser Appl. 1999, 11, 32–37. [Google Scholar] [CrossRef]
- Yuan, P.; Gu, D. Molten pool behaviour and its physical mechanism during selective laser melting of TiC/AlSi10Mg nanocomposites: Simulation and experiments. J. Phys. D Appl. Phys. 2015, 48, 035303. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Mi, H.; Deng, Z.; Liu, J.; Chen, T. Numerical simulation on evolution process of molten pool and solidification characteristics of melt track in selective laser melting of ceramic powder. Ceram. Int. 2022, 48, 18302–18315. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, X. Study on the Numerical Simulation of the SLM Molten Pool Dynamic Behavior of a Nickel-Based Superalloy on the Workpiece Scale. Materials 2019, 12, 2272. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additive manufacturing of aluminum alloy powder. Mater. Des. 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Kou, S. Fluid Flow and Solidification in Welding: Three Decades of Fundamental Research at the University of Wisconsin. Weld. J. 2012, 91, 287S–302S. [Google Scholar]
- Mills, K.C.; Keene, B.J.; Brooks, R.F.; Shirali, A. Marangoni effects in welding. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1998, 1739, 911–926. [Google Scholar] [CrossRef]
- Heiple, C.A.; Roper, J.R.; Stagner, R.T.; Aden, R.J. Surface active elements effects on shape of GTA, laser and electron beam welds. Weld. J. 1983, 62, 72. [Google Scholar]
- Le, T.N.; Lo, Y.L. Effects of sulfur concentration and Marangoni convection on melt-pool formation in transition mode of selective laser melting process. Mater. Des. 2019, 179, 107866. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Cheng, J.; Ertas, Y. Thermal behavior of the molten pool, microstructural evolution, and tribological performance during selective laser melting of TiC/316L stainless steel nanocomposites: Experimental and simulation methods. J. Mater. Process. Technol. 2018, 257, 288–301. [Google Scholar] [CrossRef]
- Jonsson, S. Assessment of the Fe-Ti-C System, Calculation of the Fe-Ti-N System, and Prediction of the Solubility Limit of Ti(C,N) in Liquid Fe. Metall Mater Trans B 1998, 29, 371–384. [Google Scholar] [CrossRef]
- Thakare, M.R.; Wharton, J.A.; Wood, R.J.K.; Menger, C. Effect of abrasive particle size and the influence of microstructure on the wear mechanisms in wear-resistant materials. Wear 2012, 276–277, 16–28. [Google Scholar] [CrossRef]
- Roger, J.; Gardiola, B.; Andrieux, J.; Viala, J.C.; Dezellus, O. Synthesis of Ti matrix composites reinforced with TiC particles: Thermodynamic equilibrium and change in microstructure. J. Mater. Sci. 2017, 52, 4129–4141. [Google Scholar] [CrossRef]





| C | Si | Cu | Mn | Cr | Ni | Ti | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 3.61 | 2.50 | 0.81 | 0.24 | 0.03 | 0.03 | 0.02 | 0.008 | 0.021 | balance |
| Processing Conditions No. | Laser Power (W) | Traverse Speed (mm/s) | Heat Input 1 (J/mm) | Powder Feed Rate (g/min) |
|---|---|---|---|---|
| C1 | 2000 | 3.33 | 600 | 1.2 |
| C2 | 1500 | 1.25 | 1200 | 0.7 |
| Processing Conditions No. | C | Ti | Si | Mn | Cu | Ni | S | P | Fe |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 3.11 | 7.9 | 2.04 | 0.160 | 0.64 | 0.03 | 0.125 | 0.020 | balance |
| C2 | 3.16 | 8.2 | 2.03 | 0.166 | 0.79 | 0.03 | 0.131 | 0.020 | balance |
| Processing Conditions No. | α-Fe (Martensite) Fraction (wt%) | Retained Austenite Fraction (wt%) | Eutectic Cementite Fraction (vol %) | TiC Fraction (vol %) |
|---|---|---|---|---|
| C1 | 37.6 ± 1.2 | 32.9 ± 1.2 | 17.2 ± 2.2 | 12.9 ± 1.0 |
| C2 | 67.8 ± 1.5 | 13.6 ± 1.0 | 16.4 ± 2.1 | 13.2 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicki, D.; Czupryński, A.; Górka, J.; Matus, K. Effect of Cooling Rate on Microstructure of In Situ TiC-Reinforced Composite Surface Layers Synthesized on Ductile Cast Iron by Laser Alloying. Materials 2024, 17, 932. https://doi.org/10.3390/ma17040932
Janicki D, Czupryński A, Górka J, Matus K. Effect of Cooling Rate on Microstructure of In Situ TiC-Reinforced Composite Surface Layers Synthesized on Ductile Cast Iron by Laser Alloying. Materials. 2024; 17(4):932. https://doi.org/10.3390/ma17040932
Chicago/Turabian StyleJanicki, Damian, Artur Czupryński, Jacek Górka, and Krzysztof Matus. 2024. "Effect of Cooling Rate on Microstructure of In Situ TiC-Reinforced Composite Surface Layers Synthesized on Ductile Cast Iron by Laser Alloying" Materials 17, no. 4: 932. https://doi.org/10.3390/ma17040932







